Abstract
Background
Chronic infections, including periodontal infections, may predispose to cardiovascular disease. We investigated the relationship between periodontal microbiota and subclinical atherosclerosis.
Methods and Results
Of 1056 persons (age 69±9 years) with no history of stroke or myocardial infarction enrolled in the Oral Infections and Vascular Disease Epidemiology Study (INVEST), we analyzed 657 dentate subjects. Among these subjects, 4561 subgingival plaque samples were collected (average of 7 samples/subject) and quantitatively assessed for 11 known periodontal bacteria by DNA-DNA checkerboard hybridization. Extensive in-person cardiovascular risk factor measurements, a carotid scan with high-resolution B-mode ultrasound, white blood cell count, and C-reactive protein values were obtained. In 3 separate analyses, mean carotid artery intima-media thickness (IMT) was regressed on tertiles of (1) burden of all bacteria assessed, (2) burden of bacteria causative of periodontal disease (etiologic bacterial burden), and (3) the relative predominance of causative/over other bacteria in the subgingival plaque. All analyses were adjusted for age, race/ethnicity, gender, education, body mass index, smoking, diabetes, systolic blood pressure, and LDL and HDL cholesterol. Overall periodontal bacterial burden was related to carotid IMT. This relationship was specific to causative bacterial burden and the dominance of etiologic bacteria in the observed microbiological niche. Adjusted mean IMT values across tertiles of etiologic bacterial dominance were 0.84, 0.85, and 0.88 (P=0.002). Similarly, white blood cell values increased across tertiles of etiologic bacterial burden from 5.57 to 6.09 and 6.03 cells × 109/L (P=0.01). C-reactive protein values were unrelated to periodontal microbial status (P=0.82).
Conclusions
Our data provide evidence of a direct relationship between periodontal microbiology and subclinical atherosclerosis. This relationship exists independent of C-reactive protein.
Keywords: infection, inflammation, atherosclerosis, epidemiology, carotid arteries
A number of epidemiological studies have reported a relationship between periodontal disease and vascular disease.1–4 The relationship is contextually consistent with other studies implicating chronic infections with vascular disease.5,6 It is also biologically plausible and supported by existing data on transient bacteremia and elevated inflammatory markers in patients with periodontitis.7–9 However, previous reports of the relationship between periodontal disease and vascular disease have relied on surrogate markers of exposure. Those indirect measures have been either clinical (such as pocket depth and attachment loss10,11 or dental indices),1,12 radiographic (bone loss),2,13 or based on extent of tooth loss4,14 or self-reported periodontal status.3 None of the studies have directly examined the microbiology of periodontal infection. Furthermore, most studies have reported on clinical events, whereas only a few4–6,11 have reported on underlying subclinical mechanisms, such as atherosclerosis.
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) was specifically designed to study the hypothesis that periodontal infections predispose to accelerated progression of carotid atherosclerosis and incidence of stroke, myocardial infarction, and cardiovascular disease death. The study assessed several microbes obtained from the subgingival environment adjacent to selected teeth, namely, all those known to be etiologically related to periodontitis and a selection of those of which the etiologic significance is neutral or not established. In this report, we investigated whether, among the microbes assayed, carotid intima-media thickness (IMT) correlated cross-sectionally with (1) the cumulative microbiological burden in the periodontium; (2) specifically, the organisms causally associated with periodontal disease; and (3) the microbial dominance of these causal organisms in relation to other organisms of the ecological niche. Our a priori hypothesis was that those bacteria causally related to periodontitis would relate to increasing IMT, whereas the others would not, thereby buttressing the specificity of the infectious component of periodontal disease in atherosclerosis. The present study also paid particular attention to the assessment of social and cardiovascular risk factors identified as potential confounders in other studies, as described previously.4
Methods
INVEST is a randomly sampled prospective, population-based cohort study investigating the relationship between oral infections, carotid atherosclerosis, and stroke. Details of this study have been published previously.4 Briefly, 1056 subjects were randomly selected from Northern Manhattan, an area between 145th Street and 218th Street bordered westward by the Hudson River and separated eastward from the Bronx by the Harlem River. Hispanics, blacks, and whites live together in this area and have similar access to medical care. The selection process was derived from the Northern Manhattan Study (NOMAS), in which patients are also enrolled.15
Eligibility criteria for INVEST are as follows: (1) Hispanic, black, or white resident (>3 months) of Northern Manhattan (zip codes 10031, 10032, 10033, 10034, and 10040); (2) contacted by random-digit dialing among households with a telephone (all eligible invited); (3) age 55 years or older at time of first in-person assessment; (4) no baseline history of stroke, myocardial infarction, or chronic inflammatory conditions such as systemic lupus erythe-matosus, Lyme disease, gonococcal arthritis, or bacterial endocarditis; and (5) ability to come to the clinic.
A total of 1056 participants were enrolled at baseline, of whom 841 were dentate. Carotid ultrasonography and subgingival plaque samples were available for 737 subjects. Another 80 patients were excluded from multivariate analyses because of missing information on body mass index (BMI; n=7), smoking (n=15), systolic blood pressure (n=2), LDL cholesterol (n=60), HDL cholesterol (n=57) or diabetes (n=2); some patients lacked several variables. Therefore, 657 patients were included in the final analyses, representing 78% of the dentate patients. This report is restricted to the dentate subjects because subgingival plaque collection requires the presence of teeth. The institutional review boards approved the study, and all subjects provided informed consent.
Dental History and Oral Examination
Details of the oral examination have been described previously.4 Briefly, subjects were interviewed and underwent a complete examination of the oral cavity administered by trained, calibrated dental examiners. Tooth brushing and flossing were recorded as times/day and times/week respectively. Assessment of periodontal status for all teeth present included presence/absence of dental plaque and bleeding on probing, probing depth, and location of the gingival margin in relation to the cementoenamel junction measured in millimeters at 6 sites per tooth (mesiobuccal, midbuccal distobuccal, mesiolingual, midlingual, and distolingual) with a UNC-15 manual probe (Hu-Friedy).
Oral Plaque Collection and Laboratory Processing
Subgingival Plaque Samples
A maximum of 8 subgingival plaque samples (mean 7; median 8) were collected from each subject. To avoid the biased collection of samples from the most diseased sites, samples were collected from the 2 most posterior teeth in each quadrant as available (mesiopalatal sites in the maxilla and mesiobuccal sites in the mandible). A total of 5369 bacterial plaque samples were collected and processed. Because of missing covariate data described previously, 4561 samples were analyzed in the present report.
Plaque samples were collected by the use of sterile Gracey curettes. The curette was inserted into the pocket until its base was reached, and subgingival plaque was collected by a single scaling stroke. The collected plaque mass from each site was transferred into an individual Eppendorf tube containing 200 µL of sterile T-E buffer (10 mmol/L Tris HCl, 1.0 mm EDTA, pH 7.6). The tubes were immediately transferred into the laboratory, and the plaque pellet was resuspended and subjected to a vigorous vortex, and 200 µL of a 0.5-mol/L NaOH solution was added. The samples were kept at 4°C until immobilization onto nylon membranes (see below), within a few days of sample collection.
Checkerboard DNA-DNA Hybridizations
Digoxigenin-labeled, whole genomic probes were prepared by random priming with the High-Prime labeling kit (Roche/Boehringer-Mannheim) from the following microbial strains selected to include species currently considered to be (1) etiologically linked with periodontal diseases or frequently encountered in pathological periodontal conditions16,17 (Porphyromonas gingivalis [ATCC 33277], Tannerella forsythensis [ATCC 43037], Actinobacillus actinomyce-temcomitans [ATCC 43718], and Treponema denticola [ATCC 35404]); (2) putatively associated with periodontal disease16 (Prevotella intermedia [ATCC 25611], Fusobacterium nucleatum [ATCC 10953], Micromonas micros [ATCC 33270], Campylobacter rectus [ATCC 33238], and Eikenella corrodens [ATCC 23834]); and (3) primarily associated with healthy periodontal conditions17 (Veillonella parvula [ATCC 10790] and Actinomyces naeslundii [ATCC 49340]).
Analysis of subgingival plaque samples was performed according to the checkerboard DNA-DNA hybridization method.16 The sensitivity and specificity of whole genomic probes constructed as above have been described previously,17 and a comparison between checkerboard hybridization and culture in the identification of subgingival microbiota has been published by our group.18 In brief, the samples were boiled for 5 minutes, neutralized, transferred onto nylon membranes by means of a Minislot device (Immunetics), and immobilized by UV light and baking at 120°C. After 2 hours of prehybridization, the DNA probes were allowed to hybridize overnight with the immobilized plaque DNA in a Miniblotter device (Immunetics) at 42°C. After a series of stringency washes, hybrids were detected by application of an anti-digoxigenin antibody conjugated with alkaline phosphatase and incubation with an appropriate chemiluminescent substrate (CSPD, Roche/Boehringer-Mannheim). Evaluation of the chemiluminescence signal was performed in a LumiImager workstation equipped with a charge-coupled device (CCD) camera (Roche/Boehringer-Mannheim) by comparing the obtained signals with the ones generated by pooled standard samples containing 106 or 105 of each of the species. Standard curves were generated for each species by means of LumiAnalyst software, and the obtained chemiluminescent signals were ultimately transformed into bacterial counts and exported into Excel files (Microsoft). The lower detection level of the method is between 103 and 104 bacterial cells.
C-Reactive Protein and White Blood Cell Measurements
C-reactive protein (CRP) measurements were performed at the University of Vermont.19 The assay range is 0.175 to 1100 mg/L. CRP was available on 538 (82%) of the analyzed patients. White blood cells (WBCs) were measured with automated cell counters via standard techniques (Coulter STK-R and Coulter STK-S, Coulter Electronics, and Sysmex SE-9500, TOA Medical Electronics).20 WBC counts were available for 611 analyzed patients (93%).
Assessment of Carotid IMT
Carotid IMT Scanning Protocol
The carotid IMT protocol consisted of scanning the carotid arteries longitudinally in the 3 segments, using the lateral extent of each carotid segment as defined relative to the tip of the flow divider, which is normally the most clearly defined anatomic reference in the proximity of the carotid bifurcation. The carotid segments were defined as follows: (1) near and far wall of the segment extending from 10 to 20 mm proximal to the tip of the flow divider into the common carotid artery; (2) near and far wall of the carotid bifurcation beginning at the tip of the flow divider and extending 10 mm proximal to the flow divider tip; and (3) near and far wall of the proximal 10 mm of the internal carotid artery. Fine transducer angulations were used to clearly display both the blood-intima and media-adventitia boundaries on both the near and far walls of the artery, and the transducer was moved toward the mandible until the lumen area increased with the appearance of the carotid bifurcation, and finally the internal carotid artery was visualized. The focus was positioned at the 40-mm depth of the near or the far wall depending on the optimized image during scanning. In the case of plaque presence, carotid IMT was measured outside the portion of plaque. Minimum IMT site criteria in INVEST specify that at least 4 carotid IMT sites must be insonated and measurable.
Carotid IMT Reading Protocol
Carotid IMT measurements were performed offline with IMAGE-Pro version 5.1 (Microsoft) image-analysis software. Recorded image sequences (on S-VHS tapes) were reviewed frame by frame to select the best-quality images for measurement. On each frame, the visualized blood-intima and media-adventitia boundaries were marked with a mouse-controlled caliper within the defined segment. Leading edges were traced with the calipers for measurement of the far walls, and trailing edges were traced for measurement of the near walls. Carotid IMT was calculated as a composite measure (mean of the 12 sites) that combined the near and the far wall of the maximal common carotid artery IMT, the maximal bifurcation IMT, and the maximal internal carotid artery IMT bilaterally.
Risk Factor Assessment
Data were collected through interview by trained research assistants, medical record reviews, physical and neurological examination by study physicians, in-person measurements, and fasting (overnight) blood specimens. All assessments were conducted in English or Spanish. Race/ethnicity was based on self-identification.15
Subjects were interviewed about sociodemographic characteristics, cardiovascular risk factors, and other medical conditions. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System as described previously.4
Anthropometric measurements of height and weight were determined with calibrated scales. Research assistants measured blood pressure using a calibrated aneroid sphygmomanometer (Omron), and the average of 2 measurements was used in analysis, as described previously.21
Blood samples were sent for complete blood count on enrollment. Fasting glucose and lipid panels were measured as described previously.21 LDL cholesterol was computed with the Friedewald equation.21 Diabetes mellitus was defined by a history of diagnosed diabetes or the use of insulin or hypoglycemic medication, or a fasting glucose ≥126 mg/dL (7.7 mmol/L). Smoking was assessed both categorically (currently smoking, former smoking, or never smoking) and continuously as total pack-years of cigarette smoking, as described previously.4 A physical activity survey was adapted from the National Health Interview Survey of the National Center for Health Statistics, which is considered reliable in the evaluation of elderly subjects.22
Statistical Analysis
All analyses were performed with SAS Windows 8.0. Laboratory analysis provided a relative quantity of bacteria per subgingival plaque sample by comparing all samples to known standards (see methods above). For each microbial species, bacterial values were then natural log transformed, averaged within mouth, and standardized by dividing these values by the log-transformed population standard deviation. As a result, 1 SD on the natural log scale was equivalent across microbes. Thus, for each person, microbial exposure was defined in 3 ways. First, summing the standardized values for the 11 microbes analyzed created a cumulative subgingival bacterial burden score. Second, we used (1) the consensus of the 1996 World Workshop in Periodontics that identified the 3 bacterial species causally related to periodontal disease (P gingivalis, T forsythensis, and A actinomycetemcomitans)23 and (2) the definition of Socransky’s red complex,24 which further identifies those bacterial species that covary with causal microbes in pathological periodontal pockets (which led to the addition of T denticola), to create an etiologic bacterial burden score, defined a priori, which comprised the 4 bacterial species (A actinomycetemcomitans, P gingivalis, T forsythensis, and T denticola). Finally, we evaluated the predominance of the etiologic bacterial group in the ecological niche by creating a dominance score. Dominance scores were computed for the etiologic microbial group by dividing the etiologic bacterial burden by the cumulative bacterial burden. Thus, the “nonetiologic” bacterial group comprised the group of bacteria deemed putatively associated with periodontal disease23 (C rectus, E corrodens, F nucleatum, M micros, P intermedia) and the “health-associated” bacterial group24 (A naeslundii, V parvula) as previously defined. 23,24 The differentiation between the etiologic and nonetiologic (putative and health-associated) bacterial groups was intended to determine the bacterial specificity of our results.
Mean carotid IMT was regressed on tertiles of these independent variables with linear regression models. All adjusted models included the following covariates: age, BMI, sex, race/ethnicity (Hispanic, black, or white), education (defined dichotomously as completed high school, yes or no), smoking (defined as never, former, and current), diabetes, systolic blood pressure, LDL cholesterol, and HDL cholesterol. Secondary analyses included prediction of CRP and WBC count from the bacterial variables.
Results
General Characteristics
Sixty percent of the 657 participants were females, and males were younger (67±8 versus 70±9 years; P<0.001). The study population was predominantly triethnic, with 57% Hispanics, 23% non-Hispanic blacks, and 18% non-Hispanic whites (the remaining 2% reported “other”). Ninety-five percent of Hispanics were foreign born, with most from the Dominican Republic.4 Additional characteristics of the study participants are given in Table 1. Mean carotid IMT was.86±0.12 mm and was thicker in males than in females after adjustment for age and BMI (0.89 versus 0.84 mm, P<0.0001).
TABLE 1.
Characteristics Across Cumulative Bacterial Burden Tertiles, Adjusted for Age and Gender (% or Mean±SE)
| Tertile I | Tertile II | Tertile III | |
|---|---|---|---|
| Variable | (n=219) | (n=219) | (n=219) |
| Sociodemographic variables | |||
| Age,* y | 70±0.6 | 70±0.6 | 67±0.6 |
| Female gender, % | 61 | 58 | 62 |
| Completed high school, % | 55 | 53 | 52 |
| Hispanic, % | 55 | 54 | 53 |
| Black, % | 22 | 19 | 28 |
| White, % | 20 | 24 | 17 |
| Other race/ethnicity, % | 3 | 3 | 2 |
| Lifestyle and behavioral variables | |||
| Former smokers, % | 36 | 38 | 34 |
| Current smokers, % | 13 | 11 | 15 |
| Pack-years of smoking | 12±1.6 | 12±1.6 | 12±1.6 |
| No physical activity,† % | 34 | 42 | 46 |
| Light physical activity,† % | 52 | 46 | 41 |
| Moderate/heavy physical activity, % | 13 | 13 | 11 |
| Brushing at least 1/d, % | 97 | 97 | 99 |
| Flossing at least 1/d* % | 55 | 45 | 35 |
P<0.05 for any difference in tertiles.
P<0.10 for any difference in tertiles.
With adjustment for age, gender, and BMI, average carotid IMT was thickest among blacks (0.89±0.009 mm), followed by whites (0.88±0.010 mm) and Hispanics (0.84±0.006 mm; P<0.0001 for any difference among ethnic groups). These comparisons were consistent in analyses across carotid segments.
An analysis of the periodontal microbiology showed an unequal distribution, with a predominance of A naeslundii (34%), followed by P intermedia (20%), those 2 bacteria accounting for 54% of the subgingival microbiota assessed. The etiologic cluster of 4 bacteria accounted for 23% of the microbiota assessed in absolute numbers and 35% after natural log transformation (Table 2).
TABLE 2.
Microbe Specific Dominance±SD per Mouth Before and After Natural Log Transformation (Among Measured Oral Microbes)
| No Ln | Ln | |
|---|---|---|
| Microbe | Transformation, % | Transformation, % |
| Etiologic* | ||
| A actinomycetemcomitans | 2±2 | 8±1 |
| P gingivalis | 9±12 | 9±1 |
| T denticola | 8±8 | 9±1 |
| T forsythensis | 4±4 | 9±1 |
| Putative* | ||
| C rectus | 1±1 | 8±0.5 |
| E corrodens | 3±4 | 9±1 |
| F nucleatum | 6±5 | 9±1 |
| M micros | 9±10 | 9±1 |
| P intermedia | 20±19 | 10±1 |
| Health associated* | ||
| A naeslundii | 34±24 | 11±1 |
| V parvula | 4±4 | 9±1 |
Validation of A Priori Concepts About Microbial Groups
Correlations between groups were 0.8 between the etiologic and putative pathogen groups, 0.39 between health-associated and etiologic bacteria, and 0.46 between health-associated and putative bacteria. In an effort to validate the a priori definition of the 3 groups of etiologic, putative, and health-associated bacteria, we ran a multiple regression model that related clinical periodontal characteristics to the 3 standardized burden scores (Table 3). Across tertiles, higher etiologic bacterial burden was associated with a higher extent of periodontitis, the putative burden was associated with an intermediate increase in the extent of periodontitis, and the group of bacteria defined as health associated were associated with a progressively lower extent of periodontitis. The findings were similar for pocket depth (Table 3). Thus, we considered our choice of microbes to be validated by their expected relations with clinical periodontal disease.
TABLE 3.
Mean±SE Clinical Periodontal Measures Across Tertiles of Microbial Burden
| Variable | Tertile I | Tertile II | Tertile III | P |
|---|---|---|---|---|
| Etiologic burden | ||||
| Percent of sites with PD ≥3 mm | 31±0.02 | 38±0.02 | 63±0.02 | <0.0001 |
| Percent of sites with AL ≥3 mm | 44±0.02 | 53±0.02 | 71±0.02 | <0.0001 |
| Putative burden | ||||
| Percent of sites with PD ≥3 mm | 37±0.02 | 47±0.02 | 48±0.02 | 0.0002 |
| Percent of sites with AL ≥3 mm | 48±0.02 | 57±0.02 | 63±0.02 | 0.001 |
| Health-associated burden | ||||
| Percent of sites with PD ≥3 mm | 49±0.02 | 43±0.02 | 39±0.02 | <0.0001 |
| Percent of sites with AL ≥3 mm | 60±0.02 | 56±0.02 | 52±0.02 | 0.03 |
PD indicates pocket depth; AL, attachment loss. n=644 for AL and n=654 for PD. Some participants had missing clinical attachment loss (n=13) and pocket depth (n=1) measures, respectively, owing to crowns, restorations, or other reasons. The table presents 2 models (dependent variables: percent of sites with PD ≥3 mm or percent of sites with AL ≥3 mm), each adjusted for age and gender, plus etiologic, putative, and health-associated microbe burdens.
Cumulative Periodontal Bacterial Burden and Carotid IMT
After adjustment for conventional risk factors, mean carotid IMT increased across tertiles of cumulative bacterial burden from 0.84 to 0.86 to 0.87 mm (P for trend=0.04). In secondary analyses (n=506) with additional adjustments for both WBC count and CRP, mean carotid IMT values were 0.83, 0.85, and 0.86 mm across tertiles of increasing microbial burden (P for trend=0.02). In a fully adjusted model, WBC and CRP values remained stable across tertiles of cumulative bacterial burden (5.81, 5.89, and 6.01 cells × 109/L, P for trend=0.29, and 3.89, 4.34, and 4.15 mg/L, P for trend=0.76, respectively).
Etiologic Bacterial Burden and Carotid IMT
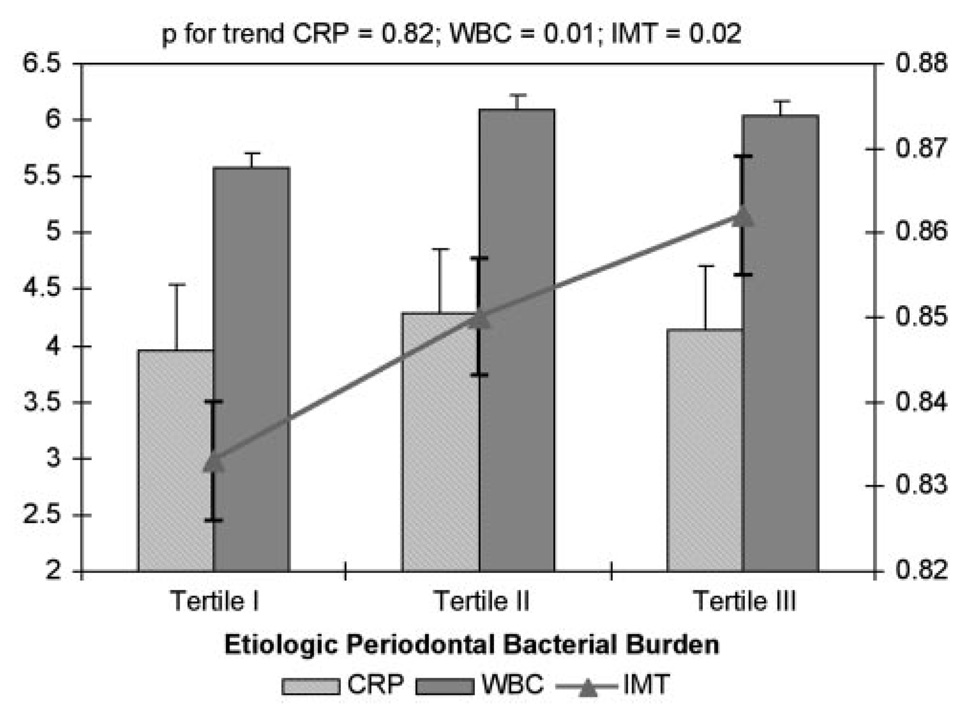
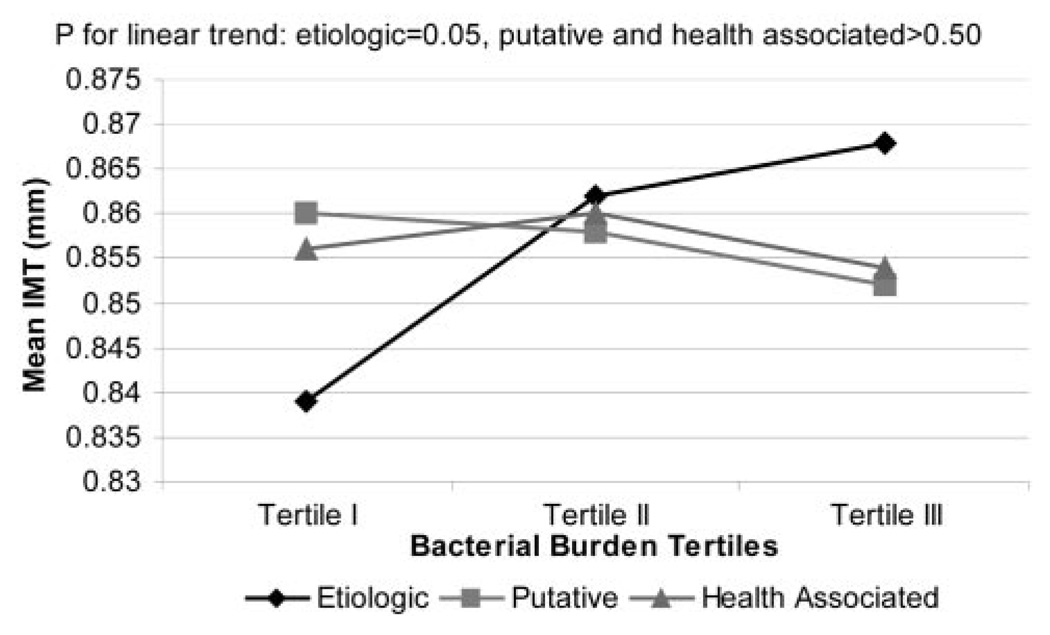
Adjusted mean carotid IMT increased across tertiles of etiologic bacterial burden from 0.84, 0.86, to 0.87 mm (P for trend=0.03) (Table 4). After additional adjustment for CRP and WBC values, the relationship remained present, with mean carotid IMT increasing from 0.83 and 0.85 to 0.86 mm (P for trend=0.02; Figure 1). However, although WBC values increased across tertiles of etiologic bacterial burden (5.57, 6.09, and 6.03 cells ×109/L, P for trend=0.01), CRP values remained stable (3.96, 4.29, and 4.14 mg/L, P for trend=0.82; Figure 1). In an additional model that further included health-associated and putative bacterial groups as independent variables of interest, the relationship of the etiologic bacterial group with carotid IMT thickness remained, whereas no relationship existed with the other 2 bacterial groups (Figure 2).
TABLE 4.
Adjusted Mean±SE Carotid Artery IMT Across Increasing Tertiles of Periodontal Bacterial Exposure Definitions
| Exposure | Tertile I | Tertile II | Tertile III | P for |
|---|---|---|---|---|
| Definitions | (n=219) | (n=219) | (n=219) | Linear Trend |
| Cumulative burden | 0.84±0.008 | 0.86±0.008 | 0.87±0.008 | 0.04 |
| Etiologic burden | 0.84±0.010 | 0.86±0.008 | 0.87±0.010 | 0.03 |
| Etiologic Dominance | 0.84±0.008 | 0.85±0.007 | 0.88±0.008 | 0.002 |
The table presents 3 models, the first with IMT predicted from cumulative burden, the second with IMT predicted by etiologic microbe burden, and the third with IMT predicted by etiologic dominance. Burden is a measure of the size of the microbial population (see Methods) cumulated over 4 etiologic, or all 11 (cumulative) microbes assessed. Etiologic dominance is the etiologic burden divided by the cumulative burden. All models adjusted for age, body mass index, sex, race/ethnicity, education, smoking, diabetes, systolic blood pressure, LDL-cholesterol, and HDL-cholesterol.
Figure 1.
Mean carotid IMT, CRP, and WBC across tertiles of etiologic bacterial burden. WBC count and CRP values adjusted for age, BMI, gender, race/ethnicity, smoking, systolic blood pressure, education, diabetes, HDL cholesterol, and LDL cholesterol; IMT values additionally adjusted for WBC count and CRP. Some participants were missing data on WBC (n=46) or CRP (n=119).
Figure 2.
Mean carotid IMT across tertiles of bacteria burden: etiologic, putative, and health-associated bacterial burdens, adjusted for age, BMI, smoking, systolic blood pressure, race/ethnicity, gender, diabetes, education, LDL cholesterol, and HDL cholesterol. The standard error for each of the 9 mean IMT values is ≈0.009 mm.
Bacterial Dominance and Carotid IMT
We further conducted an analysis to examine the dominance of etiologic bacteria in the studied ecological niche, that is, controlling for other bacteria by division rather than by including them as covariates. After adjustment for conventional risk factors, mean carotid IMT increased with increasing dominance of the etiologic bacterial group (Table 4). This relationship of carotid IMT with the ecological dominance of etiologic bacteria was present irrespective of the level of statistical adjustment (Table 4). Again, WBC, but not CRP, increased across tertiles of bacterial dominance (5.67, 6.01, and 6.00 cells × 109/L, P for trend=0.09) and 4.05, 4.20, and 4.19 mg/L, P for trend=0.86). All results above were unchanged in analyses that adjusted for brushing, flossing, and physical activity.
Exploratory Analysis of Other Bacterial Species in Relation to Carotid IMT
We performed exploratory analysis to examine whether any of the bacteria that were not labeled a priori as etiologic contributors to periodontitis could enhance the relationship of the etiologic group with carotid IMT. We ran several analyses in which we (1) systematically removed each of the 4 etiologic bacteria from the a priori etiologic burden score and (2) systematically added each of the nonetiologic bacteria to the a priori etiologic burden score. In each analysis, we studied carotid IMT in relation to the test set of bacteria in that analysis. All microbial species in the set labeled a priori as etiologic contributed to prediction of carotid IMT, and the relationship weakened with removal of any of the 4 microbes. However, the relationship with carotid IMT thickness improved to a difference across tertiles of 0.05 mm when M micros was added to the set of 4, and the new set was expressed as “dominance” among the set of measured microbes. No other bacterial species improved the relationship with carotid IMT. Thus, for the set of bacteria that included P gingivalis, A actinomycetemcomitans, T forsythensis, T denticola, and M micros, the mean carotid IMT across tertiles was 0.83, 0.87, and 0.88 mm (P for trend <0.0001); however, M micros was unrelated to worsening clinical periodontal status.
Discussion
We report a positive independent relationship between carotid IMT and cumulative periodontal bacterial burden. Furthsermore, we have shown that the observed relationship with carotid IMT reflects both the burden and dominance of those pathogens etiologically related to periodontal disease in the subgingival microbial niche. These findings strengthen the hypothesis that oral infections may contribute to cardiovascular disease morbidity and bolster the supposition that accelerated atherosclerotic development is a possible mechanism connecting chronic infections and cardiovascular disease.
In the present study, we collected and analyzed an average of 7 subgingival plaque samples per mouth and were careful not to simply select the most periodontally diseased sites for bacterial sampling. Although the latter might have increased our likelihood of positive findings, we opted instead for a protocol that would give us a representative picture of the subject’s periodontal microbiota, under the assumption that it would likely be most relevant to systemic health.
Furthermore, because we were concerned that overall pathogen burden might simply reflect poor oral and perhaps general health, we performed additional analyses focused on the burden of those specific bacteria admitted as causally related to periodontal disease.23,24 Both the validity and robustness of the present findings were enhanced, because these etiologic pathogens were the ones driving the relationship with increasing carotid IMT. This specificity argues against an overall health effect. Moreover, these findings could not be accounted for by lifestyle variables such as brushing, flossing, and physical activity, which adds further support to the concept of a relationship independent of healthy behaviors. Additionally, by design, subjects were enrolled from 5 specific zip codes in northern Manhattan, minimizing the possibility of socioeconomic bias. Interestingly, although these periodontally etiologic pathogens were not the largest contributors to the measured absolute bacterial burden (representing only 23% of bacterial distribution), only 1 additional bacterial species (M micros) added to the relationship with carotid IMT.
Neither WBC nor CRP values increased with overall bacterial burden; however, WBC values, but not CRP values, tended to rise with both increasing levels of etiologic bacterial burden and carotid IMT, which suggests a possible direct role of certain, but not all, infections. Although these findings add to the evidence that microbiological burden may be important in atherosclerosis,5,6 they also demonstrate that a level of specificity exists. In the present study, the implicated bacteria share a common pathogenicity toward the periodontium, which may extend to the vasculature, via release of cytokines,25 molecular mimicry,26 repeated bacteremias, or a hyper-inflammatory response to a distant aggression.7–9
Because CRP values in this population were relatively elevated (mean 4.1±7.6 mg/L; median 1.9 mg/L), it is possible that the contributions of CRP were masked; CRP might discriminate better at lower values. Nevertheless, even after removal of the extreme CRP values or natural log transformation, we consistently found no relationship between CRP and either IMT or etiologic burden. This would be consistent with the findings by Folsom et al27 of no cross-sectional relationship between CRP and carotid IMT in the Atherosclerosis Risk In Communities (ARIC) study. This does not, however, exclude an indirect inflammatory role, because other studies have found positive associations between increasing extent of periodontal disease and systemic markers of inflammation.7,8 Slade et al7 noted that the relationship between periodontal disease and CRP was only present among individuals with low BMI, with the strongest findings among participants with BMI=20 kg/m2. If BMI is truly a modifying factor, the higher average BMI in the INVEST population (>28 kg/m2) might explain the differing findings.
DNA-DNA hybridization is a reliable method that has been directly compared with culture.18 It generally yields higher values than culture because, unlike culture, it does not require preservation of bacterial viability, thus allowing for identification of dead bacteria whose DNA was preserved in the subgingival plaque. Thus, in the context of the hypothesis of chronic infections influencing carotid IMT, we believe this method to be preferable, because it allows an investigation of a larger window of past exposure. It is also particularly suitable for epidemiological studies, because it allows the relatively rapid analysis of large numbers of plaque samples with respect to multiple species.
Direct assessment of bacterial load by means of DNA-DNA hybridization may be preferable over the serological study of antibody titers in dentate subjects in that it quantifies the microbial burden in a manner unregulated by the host immune status. Conversely, bacterial DNA recovery does not discriminate between states of colonization, with or without disease. Unlike the presence of bacteria in the bloodstream, which generally signifies pathology, the oral cavity naturally harbors multiple bacterial species, some of them associated with good health.23,24 This underscores the importance of taking into account the relative burden and dominance of specific bacteria.
Nevertheless, we make no assumptions about the precise number of microbes present but only as to the relative ranking of proportional burden between individuals. Thus, we refrained from reporting exact bacterial level values but rather ranked bacterial burden across tertiles. The present findings should be seen in the context of both the advantages and limitations of the hybridization method used.
The magnitude of carotid IMT changes reported here is in keeping with values that have been deemed significant in prior observational and interventional studies. The Etude du Vieillssement Arteriel (EVA) reported that a cross-sectional difference of 0.03 mm in carotid IMT was associated with a 15-mm Hg increase in systolic blood pressure,28 and the Longitudinal Study of Aging reported that a cross-sectional difference of 0.04 mm in carotid IMT thickness was equivalent to a 10-year age difference in subjects without bulbar plaque.29 In progression studies, Hodis et al30 reported that a 0.03-mm/y increase in carotid IMT is associated with a 2.3-increased relative risk for nonfatal myocardial infarction or coronary death. Interventional studies assessing the effect of statins report as clinically significant a difference of 0.0082 mm/y in mean maximum carotid IMT between annualized progression rates with placebo (0.067 mm) and pravastatin (0.059 mm).31 Thus, notwithstanding obvious design differences, the present finding of a 0.03- to 0.04-mm mean difference between the highest and lowest tertiles of bacterial burden/dominance appears to fall within the range of clinically relevant differences.
The present study shares with others the limitations of cross-sectional data. Because both carotid IMT and periodontal microbiology were measured concurrently, the time sequence cannot be established, and causal inferences cannot be made. We must await the prospective results of INVEST and other studies to make firmer conclusions. It is also possible that the bacterial changes noted here might reflect other risk factors not properly measured or identified; however, we adjusted extensively for confounders, and the relationship strengthened after statistical adjustment, which renders this possibility less likely.
Admittedly, there are hundreds of bacterial species that colonize the oral cavity.32 Although we opted for a selection of microorganisms originating from different bacterial complexes that are relevant to population studies,9,27 additional pathogens might have been of value and could conceivably modify the overall relationship.
Further studies are needed to confirm those findings. However, in INVEST, patients with a dominance of oral pathogens causally related to periodontal disease had thicker carotid IMT after adjustment for conventional risk factors. This study provides the first direct evidence of a possible role of periodontal bacteria in atherosclerosis. This relationship appears to be independent of CRP. If confirmed, these findings could be of public health importance because they raise the possibility that atherosclerotic damage possibly could be reduced and perhaps reversed through selective control of pathogenic periodontal bacteria by antibacterial or immunologic means.
Acknowledgments
This research is supported by NIH grants R01 DE-13094 (Dr Desvarieux) and NS-29993 (Dr Sacco). R. Demmer is supported by T32 HL-07779. Dr Rundek is supported by the Hazel K. Goddess Fund for Stroke Research in Women. We thank George Loo, Drs Mariana Cukier and Shantanu Lal, and Janet DeRosa for their devoted patient care; the INVEST staff; Drs Romel Ramas, Sam Trocio, and Oscar Ramos for performing the ultrasound scans; Miriam Herrera-Abreu, Romi Celenti, and Jun Yang for laboratory analysis of the dental plaque samples; and most importantly, the patients. Patients were seen at the Columbia University General Clinical Research Center, NIH grant RR-00645.
References
- 1.Mattila KJ, Valle MS, Nieminen MS, Valtonen VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103:205–211. doi: 10.1016/0021-9150(93)90263-t. [DOI] [PubMed] [Google Scholar]
- 2.Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, Dorfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- 3.Joshipura KJ, Hung H-C, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 4.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DRJ, Papapanou PN, Sacco RL. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34:2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 6.Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F, Muggeo M, Xu Q, Wick G, Poewe W, Willeit J. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 7.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163:1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlen G. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 2000;71:885–897. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the First National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 11.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:1816–1822. doi: 10.1161/hq1101.097803. [DOI] [PubMed] [Google Scholar]
- 12.Janket SJ, Qvarnstrom M, Meurman JH, Baird AE, Nuutinen P, Jones JA. Asymptotic dental score and prevalent coronary heart disease. Circulation. 2004;109:1095–1100. doi: 10.1161/01.CIR.0000118497.44961.1E. [DOI] [PubMed] [Google Scholar]
- 13.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67 suppl:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 14.Loesche WJ, Schork A, Terpenning MS, Chen YM, Kerr C, Dominguez BL. The relationship between dental disease and cerebral vascular accident in elderly United States veterans. Ann Periodontol. 1998;3:161–174. doi: 10.1902/annals.1998.3.1.161. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 16.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 17.Gunaratnam M, Smith GL, Socransky SS, Smith CM, Haffajee AD. Enumeration of subgingival species on primary isolation plates using colony lifts. Oral Microbiol Immunol. 1992;7:14–18. doi: 10.1111/j.1399-302x.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 18.Papapanou PN, Madianos PN, Dahlen G, Sandros J. “Checkerboard” versus culture: a comparison between two methods for identification of subgingival microbiota. Eur J Oral Sci. 1997;105:389–396. doi: 10.1111/j.1600-0722.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 19.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 20.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL Northern Manhattan Stroke Study. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 22.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 23.Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 24.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 25.Kesavalu L, Chandrasekar B, Ebersole JL. In vivo induction of proinflammatory cytokines in mouse tissue by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002;17:177–180. doi: 10.1034/j.1399-302x.2002.170307.x. [DOI] [PubMed] [Google Scholar]
- 26.Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti G, Hajjar D. Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000;20:1417–1420. doi: 10.1161/01.atv.20.6.1417. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 28.Zureik M, Touboul PJ, Bonithon-Kopp C, Courbon D, Berr C, Leroux C, Ducimetiere P. Cross-sectional and 4-year longitudinal associations between brachial pulse pressure and common carotid intima-media thickness in a general population: the EVA study. Stroke. 1999;30:550–555. doi: 10.1161/01.str.30.3.550. [DOI] [PubMed] [Google Scholar]
- 29.Ando F, Takekuma K, Niino N, Shimokata H. Ultrasonic evaluation of common carotid intima-media thickness (IMT): influence of local plaque on the relationship between IMT and age. J Epidemiol. 2000;10:S10–S17. doi: 10.2188/jea.10.1sup_10. [DOI] [PubMed] [Google Scholar]
- 30.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Crouse JR, III, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, McGovern ME, Furberg CD. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II) Am J Cardiol. 1995;75:455–459. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 32.Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]