Abstract
Peloruside B (2), a natural congener of peloruside A (1), was isolated in sub-milligram quantities from the New Zealand marine sponge Mycale hentscheli. Peloruside B promotes microtubule polymerization and arrests cells in the G2M phase of mitosis similar to paclitaxel, and its bioactivity was comparable to that of peloruside A. NMR-directed isolation, structure elucidation, structure confirmation by total synthesis and bioactivity of peloruside B are described in this article. The synthesis features Sharpless dihydroxylation, Brown's asymmetric allylboration reaction, reductive aldol coupling, Yamaguchi macrolactonization and selective methylation.
Keywords: Peloruside B, NMR directed isolation, Microtubule stabilization, Reductive aldol, Yamaguchi macrolactonization, Sharpless dihydroxylation, Brown's allylboration
Introduction
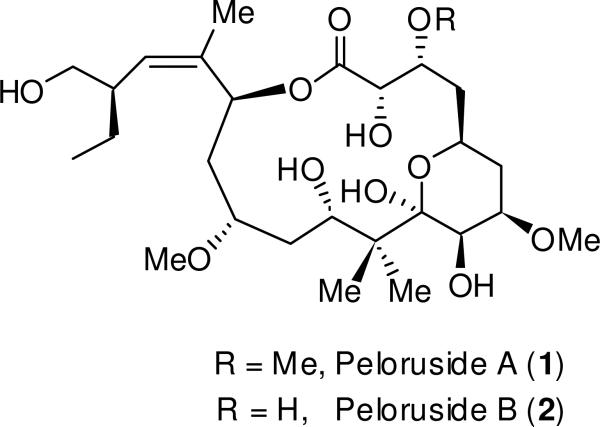
The New Zealand marine sponge Mycale hentscheli (Poecilosclerida, Demospongiae) is the source of three classes of biologically active secondary metabolites, each with a different activity profile. Heterocyclic amides mycalamides A, B, D and the macrodiolide pateamine are protein synthesis inhibitors acting at different stages of translation.1-6 In 2000, the polyoxygenated, 16-membered macrolide peloruside A (1, Figure 1) was reported from a Pelorus Sound collection of M. hentscheli.7 Peloruside A has shown potent cytotoxic activity against P388 murine leukemia cells, with an IC50 value of 10 ng/mL (18 nM). Furthermore, it causes cells to accumulate in the G2/M phase of mitosis by promoting microtubule polymerization.8 This activity is similar to the highly successful antitumor drug paclitaxel (Taxol®), and thus peloruside A has become a promising pharmaceutical candidate and a popular synthetic target. To date, a number of total syntheses9-13 as well as synthetic studies14 of peloruside A have been reported. Recently, a review on the synthesis and biological activity of peloruside A has appeared.15 Currently, quantities required for biological studies are still presently achieved mainly by collection from the wild and aquaculture of the sponge.16,17 Recently, it was shown that like laulimalide, peloruside A occupies a different tubulin binding site than paclitaxel,18-21 and acts synergistically with taxoid site drugs (e.g. paclitaxel, discodermolide, epothilones) in an in vitro assay using isolated tubulin,22 as well as in cultured cells.23 Further studies have shown that peloruside A does not, in contrast to paclitaxel, induce the production of pro-inflammatory mediators in murine macrophages.
Figure 1.
Structures of peloruside A (1) and peloruside B (2).
Our continuing interest in M. hentscheli and NMR-directed isolation has resulted in the isolation of a new compound, peloruside B (2), possessing the 3-des-O-methyl variant of peloruside A. Peloruside B was isolated in sub-milligram quantities from a wild M. hentscheli specimen collected from Kapiti Island off the southwestern coast of the North Island of New Zealand. Its bioactivity was comparable to peloruside A, and it promotes microtubule polymerization and arrests cells in the G2/M phase of mitosis as does paclitaxel. The structure of peloruside B was confirmed unequivocally through an enantioselective total synthesis and comparison of bioactivity with the natural product. Herein, we report the isolation of peloruside B, structure elucidation, structure confirmation by total synthesis and evaluation of its bioactivity.
Results/Discussion
Isolation and Structure
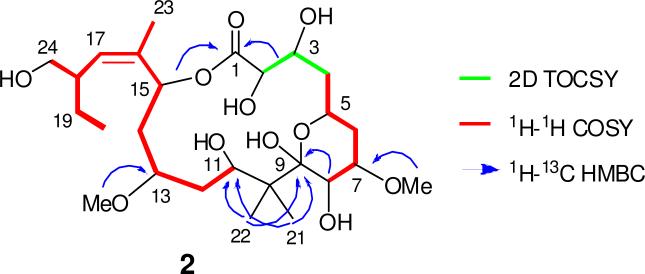
Methanolic extracts of a 230 g wild Kapiti Island M. hentscheli specimen were fractionated using reversed- (PSDVB, Me2CO/H2O and MeOH/H2O gradients) and normal-phase (DIOL, MeCN/CH2Cl2 and i-PrOH/n-hexane) column chromatography, yielding the known compounds mycalamides A (7.0 mg) and D (3.0 mg), peloruside A (1) (1.1 mg) and the new compound peloruside B (2) (327 μg). Positive-ion HRESIMS of 2 afforded a molecular formula of C26H46O11 (m/z 557.2935, [M + Na]+, calcd 557.2932), which like 1, required four degrees of unsaturation. The 1H NMR spectrum of 2 contained several resonances similar to the parent structure 1, except for with the observation of two methyl ether resonances (δH 3.38; 3.48) rather than three, which was consistent with the difference of 14 mass units between 1 and 2. Detailed inspection of the 1D and 2D NMR spectra (COSY, HSQC, HMBC) for 1 and 2 revealed the very similar nature of the two compounds, which allowed most 13C and 1H NMR resonances to be assigned by direct comparison (Table 1), resulting in the same pelorusane carbon skeleton as represented by 1. HMBC correlations (Figure 2) from 7-OMe (δH 3.38) to C-7 (δC 75.9), and 13-OMe (δH 3.48) to C-13 (δC 77.9), established the connection of the two oxymethyls. Changes in chemical shift (Δδ2–1) for the CH-3 oxymethine (ΔδC-3 + 8.5 ppm; ΔδH-3 + 0.35 ppm) clearly showed a change in substitution at this center, confirming the absence of an oxymethyl at this position. Near identical chemical shifts, 1H–1H coupling constants and diagnostic NOESY correlations (all within experimental error) between 1 and 2 strongly suggested the retention of relative configuration. Hence, peloruside B (2) is established here as the 3-des-O-methyl congener of peloruside A (1).
Table 1.
13C (150 MHz) and 1H (600 MHz) NMR Data for Natural Peloruside B (2) in CDCl3.
| 13C | 1H | COSY | HMBC | NOESY | ||
|---|---|---|---|---|---|---|
| position | δC | mult. | δH (mult., J, Hz) | |||
| 1 | 174.5 | C | - | - | - | 2-OH, 4a (w), 5, 11, 15 |
| 2 | 69.8 | CH | 4.49 (s) | - | 1 | 2, 5, 7, 8, 11, 21 |
| 2-OH | - | - | 6.74 (br s) | - | - | 4b, 11, 15 (w), 23 |
| 3 | 69.9 | CH | 4.57 (br s) | 4a, 4b | - | 2 (w), 4b, 5 |
| 4a | 38.4 | CH2 | 1.82 (td, 11.7, 3.8) | 3, 4b, 5 | 3 | 3, 4a, 6a |
| 4b | 1.98 (m) | 3, 4a, 5 | 3 | 2, 2-OH, 4a, 6b, 7 | ||
| 5 | 63.7 | CH | 4.15 (tdd, 14.5, 4.4, 2.4) | 4a, 4b, 6a | - | 4b, 6b, 7 |
| 6a | 31.4 | CH2 | 1.51 (q, 12.0) | 5, 6b, 7 | 7 | 5, 6a, 7 |
| 6b | 1.75 (ddd, 12.6, 5.0, 2.4) | 6a, 7 | 7, 8 | 2-OH, 5, 6a, 6b, 7-OMe, 8 | ||
| 7 | 75.9 | CH | 3.79 (ddd, 11.4, 5.0, 3.2) | 6a, 6b, 8 | 7-OMe | 7, 8 |
| 7-OMe | 55.7 | CH3 | 3.38 (s) | - | 7, 7-OMe | 2-OH, 7, 7-OMe, 21 |
| 8 | 66.9 | CH | 4.00 (d, 3.0) | 7 | 6, 7, 9 | - |
| 9 | 101.9 | C | - | - | - | - |
| 10 | 43.6 | C | - | - | - | 2, 2-OH, 3, 12a, 14a, 22 |
| 11 | 73.8 | CH | 4.91 (br d, 12.0) | 12b | - | 11, 12b, 13, 22 |
| 12a | 33.9 | CH2 | 1.42 (m) | 12b, 13 | - | 12a, 21 |
| 12b | 2.07 (m) | 11, 12a, 13 | 12a, 12b, 13-OMe, 14b | |||
| 13 | 77.9 | CH | 3.98 (m) | 12a, 12b, 14a, 14b | - | 13, 15 |
| 13-OMe | 59.1 | CH3 | 3.48 (s) | - | 13, 13-OMe | 11, 14b, 15, 23 |
| 14a | 35.6 | CH2 | 2.05 (dd, 15.0, 10.8) | 13, 14b, 15 | 12, 13, 15, 16 | 14a, 15, 20 |
| 14b | 2.15 (dd, 15.0, 10.2) | 13, 14a, 15 | 12, 13 | 2, 3 (w), 13-OMe, 14a, 14b, 17, 18, 20, 23 | ||
| 15 | 71.7 | CH | 5.69 (d, 11.2) | 14a, 14b, 17, 23 | 1, 13, 14, 16, 17, 23 | - |
| 16 | 136.7 | C | - | - | - | 15, 18, 19a, 19b, 20, 23, 24a |
| 17 | 130.9 | CH | 5.02 (d, 10.6) | 15, 18, 23 | 15, 18, 19, 23, 24 | 15, 17, 19b, 20, 24b |
| 18 | 43.3 | CH | 2.56 (m) | 17, 19a, 19b, 24a, 24b | 16, 17, 19, 20, 24 | 17, 19b, 20 |
| 19a | 24.6 | CH2 | 1.14 (m) | 18, 19b, 20 | 17, 18, 20, 24 | 17 (w), 18, 19a, 20, 24b |
| 19b | 1.42 (m) | 18, 19a, 20 | 17, 18, 20, 24 | 14b, 15, 17, 18, 19a, 19b, 24b | ||
| 20 | 12.3 | CH3 | 0.85 (t, 7.5) | 19a, 19b | 18, 19, 20 | 8, 12b |
| 21 | 15.7 | CH3 | 1.08 (s) | - | 9, 10, 11, 21, 22 | 11, 12a |
| 22 | 20.8 | CH3 | 1.10 (s) | - | 9, 10, 11, 21, 22 | 3, 15, 17 |
| 23 | 17.6 | CH3 | 1.71 (d, 1.2) | 15, 17 | 15, 16, 17, 23 | 17, 19a, 19b, 24b |
| 24a | 67.0 | CH2 | 3.34 (t, 10.3) | 18, 24b | 17, 18, 19 | 18, 19b, 20, 24a |
| 24b | 3.62 (br d, 10.3) | 18, 24a | 17, 18, 19 | |||
Figure 2.
Key COSY, 2D TOCSY and HMBC correlations for peloruside B (2).
Enantioselective Synthesis of Peloruside B
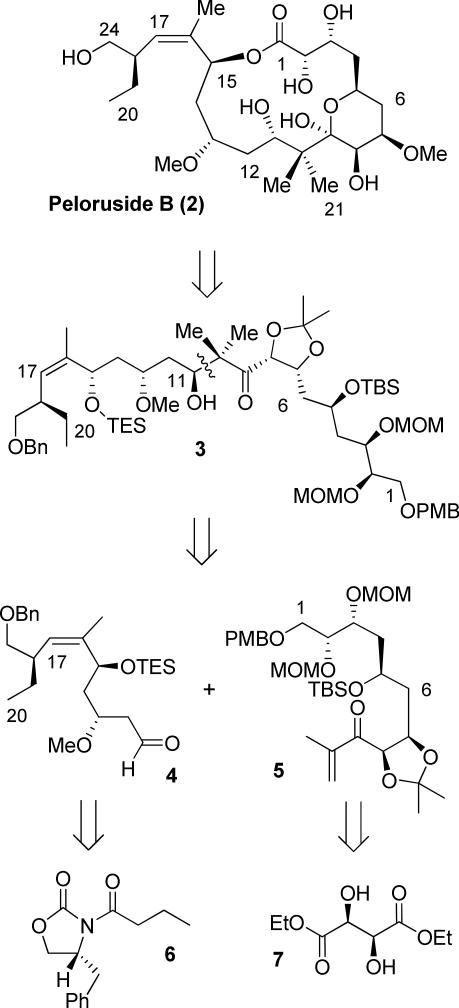
Peloruside B has been isolated in miniscule quantity and to confirm relative and absolute configuration of peloruside B, as well as to compare it's bioactivity, we have undertaken an enantioselective synthesis of the proposed structure of peloruside B. Our retrosynthetic analysis of peloruside B is shown in Figure 3. Strategic bond disconnection of the macrolactone bond provided acyclic intermediate 3. We envisioned that the presence of a gem-dimethyl at C-10 and the resulting lactol formed between C-5 to C-9 makes the 16-membered ring very sterically hindered for macrolactonization. Therefore, we planned to protect the hydroxyl group at C-5 and carry out a 16-membered ring macrolization. Further disconnection of the C-10 to C-11 bond provided segments 4 and 5, which can be assembled by a stereoselective aldol reaction to provide 3. Due to the steric hindrance of the gem-dimethyl group adjacent to the C-9 ketone, selective enolization may be problematic. We planned the same reductive aldol coupling protocol that was utilized during the synthesis of (+)-peloruside A.11 Segment 4 has been synthesized by us previously,11 which features asymmetric alkylation of oxazolidinone 6, Horner-Emmons olefination and Brown's asymmetric allylboration. The synthesis of segment 5 can be derived from diethyl tartrate 7.
Figure 3.
Retrosynthetic analysis of peloruside B (2).
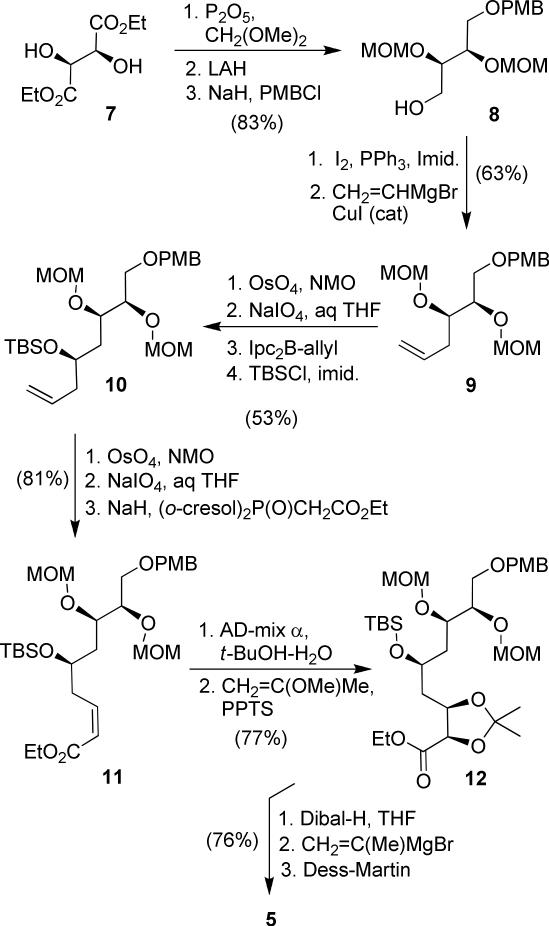
The synthesis of C-1 to C-10 fragment 5 commenced with commercially available diethyl d-tartrate as shown in Scheme 1. Both hydroxyl groups were protected with MOM ether, and the resulting diester was reduced by LAH reduction to give the corresponding diol.24 Treatment of the diol with NaH and PMBCl gave mono-protected PMB ether 8 (83% yield over three steps). It was converted to alkene 9 by transformation of the alcohol into an iodide, using iodine and triphenylphosphine in THF at 0 °C, followed by substitution of the corresponding iodide with vinyl cuprate (63% yield for 2 steps).25 The terminal olefin was converted to an aldehyde using oxidative cleavage.
Scheme 1.
Synthesis of fragment 5.
This was accomplished by dihydroxylation with OsO4 followed by treatment of the resulting diol with NaIO4.26 The aldehyde was subjected to Brown's asymmetric allylboration27 to afford the corresponding homoallylic alcohol. The resulting alcohol was protected as TBS ether 10. The allylboration proceeded with high diastereoselectivity (95:5 dr), and the diastereomers were separated at this step (53% over 4 steps, 95:5 dr). Conversion of terminal alkene to the corresponding aldehyde, using the above oxidative cleavage followed by Ando's modified Horner-Emmons reaction,28 provided the Z-olefin 11 (Z:E 7:1, 81% yield over three steps). Sharpless asymmetric dihydroxylation of the pure Z-olefin 11 furnished the corresponding diol in 77% yield (9:1 dr).29 The resulting diol was protected as an isopropylidene derivative 12 in near quantitative yield. Reduction of ester 12 to the aldehyde, followed by reaction with isopropenylmagnesium bromide and Dess-Martin oxidation, provided the enone 5 in 76% yield over three steps. The other coupling fragment 4 was synthesized following the reaction schemes reported by us in the context of the synthesis of (+)-peloruside A.11
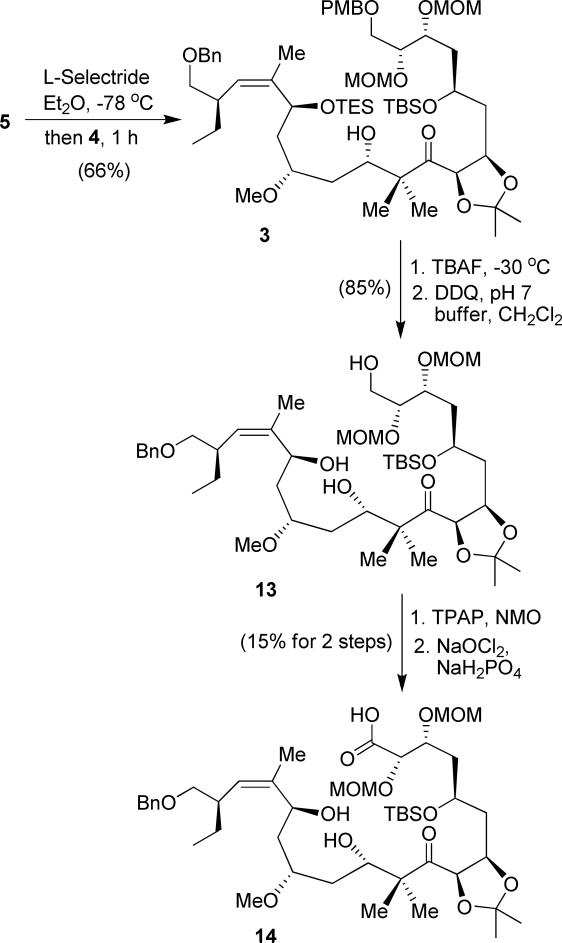
With segments 4 and 5 in hand, our subsequent plan was to use a reductive aldol reaction to couple these segments and set the C-11 hydroxyl stereocenter. As shown in Scheme 2, enone 5 was treated with L-selectride to generate the corresponding enolate, which was reacted with aldehyde 4 to afford the aldol product 3 as the major desired isomer (66% yield, 6.5:1 dr by 1H-NMR). This reductive coupling reaction provided a lower yield and slightly improved diastereoselectivity compared to C3-OMe derivative during the synthesis of peloruside A.11 The stereochemical outcome and diastereomeric ratios were consistent with our previous observation.11,30 The TES protecting group was selectively removed by treatment with dilute TBAF at –30 °C. DDQ oxidation effected the removal of the PMB group to provide alcohol 13 in 85% yield. Conversion of the primary alcohol to the seco-acid 14 was carried out in a two-step sequence involving: (1) TPAP oxidation of the alcohol to the corresponding aldehyde and (2) oxidation of the aldehyde to the acid with NaClO2. However, the yield was extremely low (15%). The reason for the low yield is possibly due to the presence of the C-15 allylic alcohol, which gets readily oxidized. Attempts with other oxidizing reagents were unsuccessful.
Scheme 2.
Reductive aldol reaction and synthesis of seco-acid 14.
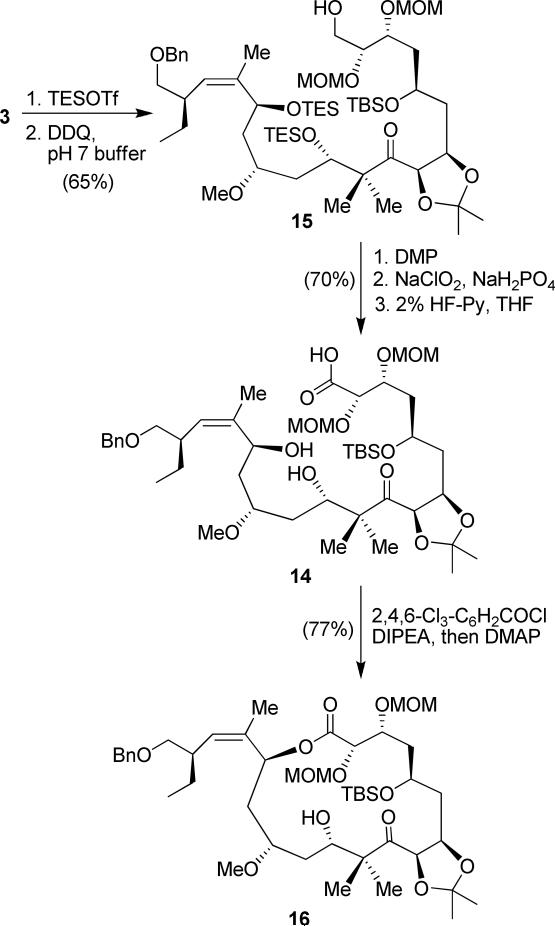
We then envisaged that selective oxidation of the primary alcohol in the presence of two other hydroxyl groups would be very difficult due to the C-15 allylic alcohol. While we achieved the selective oxidation during the synthesis of (+)-peloruside A, for peloruside B synthesis, it was difficult to selectively oxidize the primary alcohol in good yield. We decided to protect both C-11 and C-17 secondary hydroxyl groups as TES-ethers before oxidizing the primary alcohol. Reductive aldol product 3 was treated with TESOTf and pyridine, as shown in Scheme 3. Subsequent removal of the PMB protecting group with DDQ gave the corresponding primary alcohol 15 in 65% yield for two steps. The primary alcohol 15 was oxidized to the corresponding aldehyde, and without purification further oxidized to the corresponding acid in 88% yield over two steps. Both TES protecting groups at the C-11 and C-14 positions were selectively removed with dilute HF/pyridine in 80% yield to afford the corresponding seco-acid 14. The seco-acid 14 was subjected to the Yamaguchi protocol31 using 2,4,6-trichlorobenzoyl chloride in the presence of DMAP to generate macrolactone 16 in 77% yield.
Scheme 3.
Revised route to macrolactone 16.
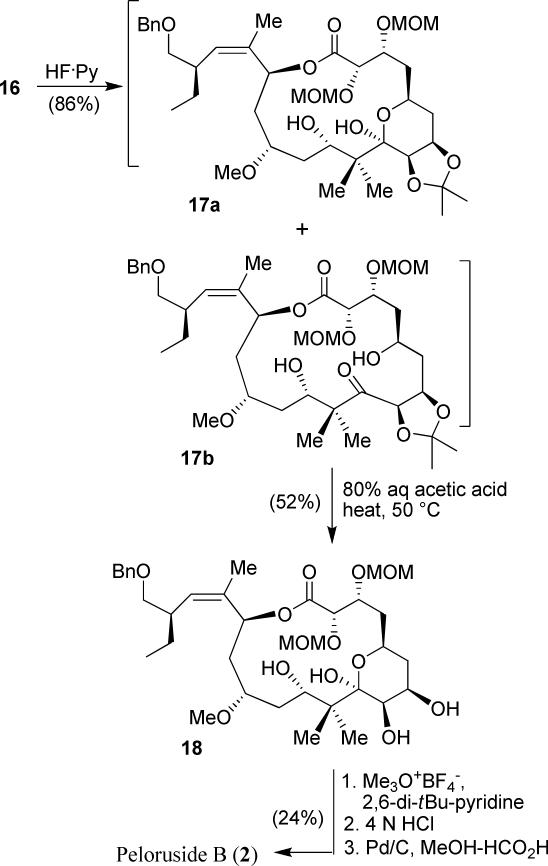
The synthesis of the proposed structure of peloruside B is shown in Scheme 4. We initially attempted the deprotection of both TBS ethers and acetonide groups by treatment of 16 with 1 N HCl. However, the reaction was not very satisfactory, yielding a number of undesired products. We then planned to remove the TBS ethers of macrolactone 16 by treatment with HF/pyridine. This resulted in a mixture (6:1) of hemi-ketal 17a and the corresponding hydroxyketone 17b in 86% combined yield. The isopropylidene group from both hemi-ketal 17a and hydroxyketone 17b was deprotected with 80% aqueous acetic acid at 50 °C for 3 h to afford the corresponding diol 18 in 52% isolated yield. Selective methylation of the diol was carried out with Meerwein's reagent at –5 °C to afford the corresponding C-7 monomethylated product. The product was then exposed to 4 N HCl in THF, and this effected the removal of both MOM groups. Catalytic transfer hydrogenation with Pd/C in the presence of formic acid selectively removed the benzyl group in the presence of the C-17-trisubtituted olefin to generate the synthetic peloruside B in 24% yield over three steps. The 1D and 2D NMR data of synthetic peloruside B is in complete agreement with that of natural peloruside B.
Scheme 4.
Completion of total synthesis of peloruside B (2).
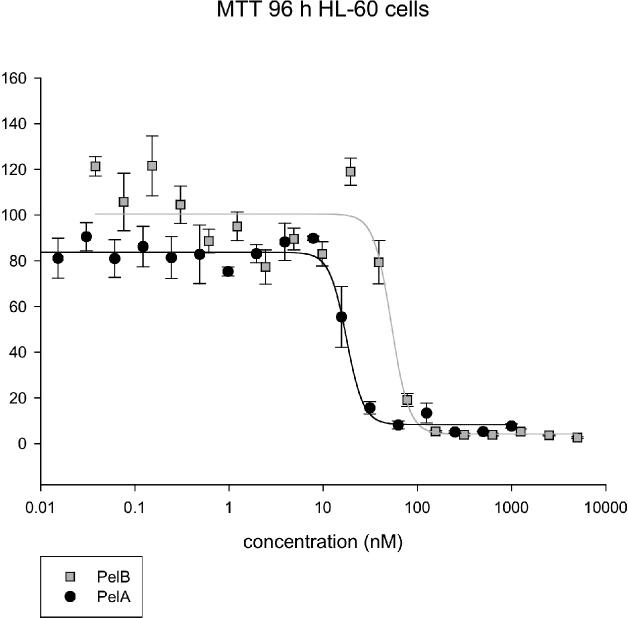
Furthermore, using synthetic and natural peloruside B, we have performed MTT cell proliferation assays (96 h incubation) and flow cytometric measurements (16 h incubation) as previously described with human myeloid leukemic cells (HL-60 cells).23 Natural peloruside B (2) gave an IC50 of 33 ± 10 nM, a value approximately one-third of that obtained with peloruside A (1) tested at the same time (10 ± 4 nM) (n = 3 independent experiments, Figure 4). Natural peloruside B was also slightly less active than peloruside A against arresting cells in the G2/M phase of the cell cycle, with 56 ± 1% of cells in G2/M for 2 (n = 6 replicates from 3 experiments) compared to 67 ± 3% in G2/M for 1 (200 nM concentration of 1 or 2; n = 5 replicates in 3 experiments). Control, untreated HL-60 cells had 23 ± 2% of the cells in the G2/M phase of the cell cycle.
Figure 4.
Typical MTT cell proliferation assay for pelorusides A (1) and B (2). HL-60 cells were treated for 96 h (n = 3 replicates from a single preparation).
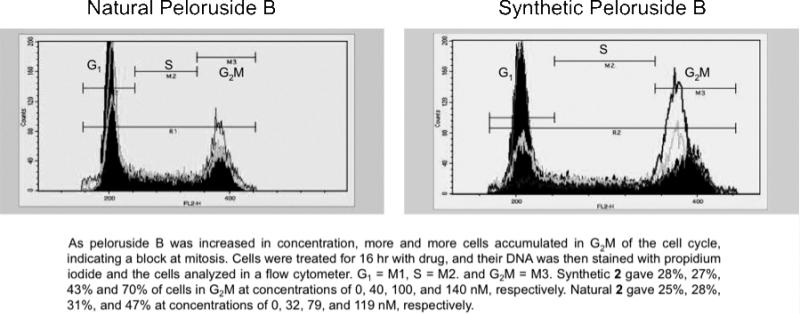
Synthetic 2 and natural 2 were compared in paired MTT cell proliferation assays in human ovarian carcinoma 1A9 cells. 1A9 cells were treated for 72 h with drug. The synthetic 2 (48 ± 11 nM IC50) was slightly more potent than the natural 2 (71 ± 6 nM) in this cell line (n = 3 experiments). The G2/M arrest results for the synthetic product were also slightly higher than those obtained with the natural congener. Synthetic 2 gave 28%, 27%, 43%, and 70% of cells in G2/M at concentrations of 0, 40, 100, and 140 nM, respectively (Figure 5). Natural 2 gave 25%, 28%, 31%, and 47% at concentrations of 0, 32, 79, and 119 nM, respectively. The slight differences in biological activity between natural and synthetic 2 are well within the normal uncertainties of the assays performed. Considering the fact that the synthetic enantiomer of peloruside A has no bioactivity, the observed potent activity of synthetic peloruside B further confirms the absolute configuration of natural peloruside B. 9
Figure 5.
G2/M arrest of 1A9 cells by natural and synthetic peloruside B (2).
Conclusion
We have isolated a sub-milligram amount of a new natural product, peloruside B (2), a natural congener of peloruside A (1), from the New Zealand marine sponge Mycale hentscheli. The structure of peloruside B was elucidated based upon extensive 1D and 2D NMR studies. Like peloruside A, peloruside B promotes microtubule polymerization and arrests cells in the G2M phase of the cell cycle, as does paclitaxel. The bioactivity of natural peloruside B was comparable to that of peloruside A. We have accomplished an enantioselective and convergent synthesis of peloruside B. The synthesis involved Sharpless dihydroxylation, Brown's asymmetric allylboration reaction, reductive aldol coupling and Yamaguchi macrolactonization as the key reactions. The relative and absolute configuration of 2 was confirmed by total synthesis and comparison of its bioactivity with natural peloruside B. Synthesis and biological evaluation of structural variants of peloruside B are the subjects of ongoing research in our laboratories.
Experimental Section
Natural Peloruside B
General
600 MHz NMR spectra for both natural and synthetic peloruside B (2) were obtained using the same spectrometer equipped with a triple resonance HCN cryogenic probe, operating at 600 MHz or 150 MHz for 1H and 13C nuclei respectively. Chemical shifts δ (ppm) were referenced to the residual solvent peak (δH 7.26 ppm, δC 77.16 ppm for CDCl3). Catalytic amounts (1–2 μL) of d5-pyridine were added to each NMR sample performed in CDCl3 to prevent compound degradation due to trace acidity associated with the solvent. High-resolution positive-ion mass spectra were recorded on a TOF electrospray mass spectrometer. Normal-phase column chromatography was carried out using 2,3-dihydroxypropoxypropyl-derivatized silica (DIOL). Reversed-phase column chromatography was achieved using HP20 or Amberchrom poly(styrene divinylbenzene) (PSDVB) chromatographic resin. HPLC was performed using a solvent delivery module equipped with 25 mL pump heads. Solvents used for flash normal- and reversed-phase column chromatography were of HPLC or analytical grade quality. All other solvents were purified by glass-distillation. Solvent mixtures are reported as % vol/vol unless otherwise stated. Specimens were stored at –20 °C until required.
Isolation of Natural Peloruside B
Mycale hentscheli, (230 g, NIWA no. MNP 0026), collected at a depth of 23 m from Kapiti Island, New Zealand, was cut into small segments and extracted with MeOH (2 × 700 mL) for 24 h. The combined extracts were loaded on to HP20 PSDVB beads, washed with H2O and eluted with i) 20% Me2CO/H2O, ii) 55% Me2CO/H2O, iii) 55% Me2CO/0.2 M NH4OH and iv) 55% Me2CO/0.2 M NH4OH adjusted to pH 4 with AcOH. Fraction ii) was concentrated to dryness to yield 82.0 mg of a viscous brown oil. The resulting oil was dissolved in MeOH, loaded onto Amberchrom PSDVB and eluted with increasing concentrations of MeOH in H2O (10–100%). The 48–54% MeOH/H2O fractions were concentrated to dryness to yield mycalamide D (3 mg). The 56–60% MeOH/H2O fractions were concentrated to dryness to yield peloruside A (1) (1.1 mg), and concentration of the 68–72% MeOH/H2O fractions gave mycalamide A (7.0 mg). The 54–56% MeOH/H2O fractions were concentrated to dryness to yield 5.0 mg of brown oil. A portion of this oil (3.0 mg) was recycled twice on DIOL with increasing concentrations of MeCN in CH2Cl2 (1–10%), and 50% MeOH/CH2Cl2. The 10% MeCN/CH2Cl2 and 50% MeOH/CH2Cl2 fractions were concentrated to dryness to yield a pale yellow oil (1.5 mg). This oil was then purified using HPLC (DIOL, 5 μm, 4 mm × 250 mm), with 20% i-PrOH/n-hexane as the mobile phase, collecting fractions at 1 min intervals to give 13.9 μg of mycalamide D, 9.4 μg of peloruside A (1) and 327 μg of peloruside B (2).
Peloruside A (1)
Colorless film; all other data as previously published. 7
Peloruside B (2)
Colorless film; [α]D25 could not be determined accurately because of very small quantity of natural sample and magnitude of rotation was very small; NMR data see Table 1; HRESIMS, [M + Na]+, observed m/z 557.29356, calculated 557.29323 for C26H46O11Na, Δ = 0.58 ppm.
Synthetic Peloruside B
General experimental details are provided as Supporting Information.
Aldol product 3
To the enone 5 (521 mg, 0.81 mmol) dissolved in anhydrous ethyl ether (132 mL) was added L-selectride (1 M in THF, 0.85 mL, 0.85 mmol) slowly at –78 °C. The solution was stirred at –78 °C for 0.5 h to form the corresponding enolate. The formation of the enolate was monitored by TLC. Then the aldehyde 4 (500 mg, 1.12 mmol) dissolved in Et2O (10 mL) was added dropwise over 15 min at –78 °C. The solution was stirred at –78 °C for 1 h and quenched with saturated ammonium chloride solution. The aqueous layer was washed and extracted with ethyl ether. The organic extracts were washed with brine and dried over anhydrous sodium sulfate. The solvent was removed under vacuum, and the residue was purified by flash chromatography on silica gel (hexane/ethyl acetate, 85/15) to afford the alcohol 3 (503.3 mg, 57% yield) and the other C-11 diastereomer (77 mg, 9% yield; total yield 66%, 6.5:1 dr). Alcohol 3: 1H NMR (400 MHz, CDCl3) δ 0.06 (s, 3H), 0.07 (s, 3H), 0.55 (q, J = 7.9 Hz, 6H), 0.83 (t, J = 7.5 Hz, 3H), 0.87 (s, 9H), 0.89 (t, J = 8.9 Hz, 9H), 1.10 (s, 3H), 1.17 (s, 3H), 1.17–1.26 (m, 3H), 1.33 (s, 3H), 1.42–1.49 (m, 2H), 1.52 (s, 3H), 1.59–1.74 (m, 6H), 1.70 (s, 3H), 2.06 (m, 1H), 2.54 (m, 1H), 3.26–3.39 (m, 3H), 3.29 (s, 3H), 3.35 (s, 3H), 3.36 (s, 3H), 3.52 (dd, J = 10.0, 7.1 Hz, 1H), 3.57 (m, 1H), 3.63 (dd, J = 10.0, 3.6 Hz, 1H), 3.71 (m, 1H), 3.79 (s, 3H), 3.79 (m, 1H), 3.98 (m, 1H), 4.06 (d, J = 10.5 Hz, 1H), 4.45 (s, 2H), 4.48 (s, 2H), 4.55 (m, 1H), 4.62 (s, 2H), 4.65 (d, J = 6.8 Hz, 1H), 4.77 (d, J = 6.8 Hz, 1H), 4.93 (d, J = 10.2 Hz, 1H), 5.09 (d, J = 6.8 Hz, 1H), 6.86 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.6 Hz, 2H), 7.26 (m, 5H); 13C NMR (100 MHz, CDCl3) δ –4.8, –4.3, 4.7, 6.9, 11.6, 17.9, 18.0, 20.9, 25.3, 25.6, 25.8, 27.4, 33.5, 38.9, 39.1, 39.3, 51.5, 55.1, 55.7, 55.9, 56.2, 66.4, 67.3, 69.8, 72.7, 72.9, 73.0, 73.2, 74.2, 74.8, 76.4, 77.6, 79.2, 96.8, 109.2, 113.6, 127.2, 127.37, 127.45, 128.2, 129.2, 130.3, 138.5, 138.9, 159.0, 210.5; FT-IR (film, NaCl) 1039.5, 1082.5, 1249.4, 1379.1, 1463.4, 1514.0, 1613.3, 1715.8 cm−1; (c 1.37, CHCl3); MS, m/z (MALDI), 1113 [M + Na]+.
Primary alcohol 15
To the alcohol 3 (435 mg, 0.4 mmol) dissolved in CH2Cl2 (8 mL) was added anhydrous pyridine (0.32 mL, 4 mmol) and triethylsilyl trifluoromethanesulfonate (0.18 mL, 0.8 mmol) sequentially at 0 °C. The mixture was stirred at 0 °C for 2 h and quenched with a saturated NaHCO3 solution. The aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic extracts were washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum, and the residue was purified by flash chromatography on silica gel (hexanes/ethyl acetate: 90/10) to afford the TES protected product (425 mg, 88% yield).
1H NMR (400 MHz, CDCl3) δ 0.06 (s, 3H), 0.08 (s, 3H), 0.58 (q, J = 7.9 Hz, 6H), 0.68 (q, J = 7.9 Hz, 6H), 0.82 (t, J = 7.4 Hz, 3H), 0.88 (s, 9H), 0.91 (t, J = 7.9 Hz, 9H), 1.00 (t, J = 7.9 Hz, 9H), 1.09 (s, 3H), 1.13 (s, 3H), 1.23–1.38 (m, 2H), 1.49 (s, 3H), 1.51-1.73 (m, 4H), 1.70 (s, 3H), 1.98 (m, 1H), 2.58 (m, 1H), 3.21 (s, 3H), 3.27–3.40 (m, 2H), 3.34 (s, 3H), 3.37 (s, 3H), 3.47 (m, 1H), 3.53 (dd, J = 10.0, 6.9 Hz, 1H), 3.64 (dd, J = 10.0, 3.7 Hz, 1H), 3.68 (m, 1H), 3.80 (s, 3H), 3.81 (m, 1H), 3.97 (m, 1H), 4.07 (d, J = 9.2 Hz, 1H), 4.46 (s, 2H), 4.50 (s, 2H), 4.51 (m, 1H), 4.57 (m, 1H), 4.62 (s, 2H), 4.65 (d, J = 6.8 Hz, 1H), 4.78 (d, J = 6.8 Hz, 1H), 4.97 (d, J = 10.2 Hz, 1H), 5.09 (d, J = 6.7 Hz, 1H), 6.86 (d, J =8.6 Hz, 2H), 7.24 (d, J = 8.6 Hz, 2H), 7.32 (m, 5H); 13C NMR (100 MHz, CDCl3) δ –4.9, –4.3, 4.8, 5.5, 6.9, 7.1, 11.4, 17.89, 17.93, 19.7, 21.7, 25.2, 25.5, 25.8, 27.7, 38.4, 38.9, 39.4, 39.8, 52.7, 55.1, 55.7, 55.8, 66.3, 67.1, 69.7, 72.90, 72.93, 73.0, 73.2, 74.1, 74.4, 74.9, 77.6, 80.7, 96.9, 108.7, 113.6, 127.3, 127.5, 127.6, 128.2, 129.2, 130.3, 138.5, 138.6, 159.0, 209.2; FT-IR (film, NaCl) 1039.8, 1100.9, 1248.5, 1379.2, 1462.3, 1513.8, 1612.4, 1718.3 cm−1; (c 1.1, CHCl3); MS, m/z (ESI), 1228 [M + Na]+.
The TES protected product from above (360 mg, 0.3 mmol) was dissolved in CH2Cl2 (60 mL), and pH 7 buffer (12 mL) was added. DDQ (136 mg, 0.6 mmol) was added while the mixture was vigorously stirred. The mixture was stirred for 4 h before another portion of DDQ (136 mg, 0.6 mmol) and pH 7 buffer (12 mL) was added. The mixture was further stirred for 4 h. Then, a saturated aqueous NaHCO3 solution was added, the aqueous layer was extracted with CH2Cl2 (3 × 50 mL) and the combined extracts were washed with saturated NaHCO3 solution, brine and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum and the residue was purified by column chromatography on silica gel (hexanes/ethyl acetate: 2/1) to afford the primary alcohol 15 (240 mg, 74% yield) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 0.10 (s, 3H), 0.11 (s, 3H), 0.58 (q, J = 7.9 Hz, 6H), 0.70 (q, J = 7.9 Hz, 6H), 0.84 (t, J = 7.4 Hz, 3H), 0.91 (s, 9 H), 1.01 (t, J = 7.9 Hz, 9H), 1.11 (s, 3H), 1.15 (S, 3H), 1.27-1.41 (m, 3H), 1.32 (s, 3H), 1.51 (s, 3H), 1.53-1.65 (m, 2H), 1.69 (s, 3H), 1.75 (m, 2H), 2.02 (m, 1H), 2.59 (m, 1H), 3.17 (m, 1H), 3.24 (s, 3H), 3.31 (dd, J = 16.0, 5.6 Hz, 1H), 3.38 (dd, J = 9.3, 5.6 Hz, 1H), 3.41 (s, 3H), 3.43 (s, 3H), 3.49 (m, 1H), 3.65 (m, 2H), 3.75 (m, 2H), 3.97 (m, 1H), 4.09 (d, J = 9.3 Hz, 1H), 4.49 (m, 1H), 4.50 (s, 2H), 4.60 (dd, J = 8.0, 4.7 Hz, 1H), 4.64 (d, J = 6.8 Hz, 1H), 4.68 (d, J = 7.9 Hz, 2H), 4.73 (d, J = 6.8 Hz, 1H), 4.98 (d, J = 10.2 Hz, 1H), 5.12 (d, J = 6.7 Hz, 1H), 7.28 (m, 5H); 13C NMR (125 MHz, CDCl3) δ –4.9, –4.3, 4.8, 5.5, 6.9, 7.0, 11.4, 17.88, 17.93, 19.7, 21.6, 25.2, 25.5, 25.7, 27.7, 38.4, 39.0, 39.1, 39.5, 39.8, 52.7, 52.8, 55.8, 55.9, 62.1, 66.2, 67.1, 73.0, 73.2, 74.1, 74.4, 75.4, 80.5, 82.4, 97.1, 97.6, 108.7, 127.3, 127.5, 127.6, 128.2, 138.5, 138.6, 209.2; FT-IR (film, NaCl) 1037.5, 1082.4, 1249.1, 1379.4, 1462.8, 1716.7 cm−1; (c 1.02, CHCl3); MS, m/z (MALDI), 1108 [M + Na]+.
Macrolactone 16
To the primary alcohol 15 from above (245 mg, 0.226 mmol) dissolved in CH2Cl2 (12 mL) was added NaHCO3 (38 mg, 0.45 mmol) and Dess-Martin periodinane (144 mg, 0.34 mmol). The mixture was stirred at 23 °C for 1 h and quenched with a saturated aqueous NaHCO3 solution (10 mL) and a saturated aqueous sodium thiosulfate solution (10 mL). The mixture was stirred until the two layers became clear. The aqueous layer was extracted with CH2Cl2 (3 × 10 mL), and the combined extracts were washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum, and the crude aldehyde was used for the next step without purification.
The crude aldehyde from above was dissolved in t-BuOH (8 mL) and H2O (2 mL). To this solution was added 2-methyl-2-butene (2 mL) followed by sodium chlorite (213 mg) and sodium phosphate monobasic monohydrate (213 mg) in H2O (2.2 mL) dropwise. The mixture was stirred at 23 °C for 25 min. Then, ethyl acetate was added, the organic layer was washed with brine and dried over anhydrous Na2SO4. The solvent was removed under vacuum. The crude acid was passed through a short column to afford the crude acid (219 mg, 88% yield, over two steps).
To the acid from above dissolved in THF (15 mL) was added 10% HF.Py in THF solution (1 mL HF.Py complex dissolved in 9 mL THF, 3 mL) at 0 °C. The solution was allowed to warm to 23 °C for 2 h. Then, more 10% HF.Py (3 mL) was added at 23 °C. The mixture was kept stirring at 23 °C for another 9 h. TLC evidence showed that the two TES groups were removed. The mixture was cooled to 0 °C, and 1 mL of H2O was added. Lithium carbonate was used to quench the reaction, and the mixture was dried over anhydrous Na2SO4. The solid was filtered and washed with ethyl ether. The solvent was evaporated under vacuum, and the crude product was passed through a short silica gel column to afford the crude acid 14 (138 mg, 80% yield) as a white solid.
To the seco-acid 14 (115 mg, 0.132 mmol) from above dissolved in THF (4 mL) was added diisopropylethyl amine (172 μL, 1 mmol) and 2,4,6-trichlorobenzoyl chloride (103 μL, 0.66 mmol). The mixture was stirred at 23 °C for 4 h. Then most solvent was removed under vacuum followed by addition of dry toluene (25 mL). The mixed anhydride toluene solution was added to the DMAP (403 mg, 3.3 mmol) solution in toluene (200 mL) via a syringe pump over 13 h at 23 °C. White precipitate was formed during the addition. The mixture was stirred for an additional 36 h at 23 °C. Then, 0.1 N HCl was added to quench the reaction at 0 °C, the aqueous layer was extracted with ethyl acetate and the combined extracts were washed with saturated NaHCO3 solution and brine and dried over Na2SO4. The solvent was evaporated under vacuum, and the crude product was purified by column chromatography on silica gel (hexanes/ethyl acetate: 85/15) to afford macrolactone 16 (87 mg, 77% yield) as a colorless oil.
1H NMR (500 MHz, CDCl3) δ 0.09 (s, 3H), 0.10 (s, 3H), 0.86 (t, J = 7.5 Hz, 3H), 0.89 (s, 9H), 1.20 (s, 3H), 1.23–1.33 (m, 2H), 1.36 (s, 3H), 1.43 (s, 3H), 1.59 (s, 3H), 1.59–1.71 (m, 4H), 1.70 (s, 3H), 1.91 (m, 1H), 2.04 (m, 2H), 2.81 (m, 1H), 3.24 (d, J = 11.4 Hz, 1H), 3.31 (s, 3H), 3.34 (s, 3H), 3.36 (s, 3H), 3.55 (dd, J = 9.2, 4.2 Hz, 1H), 3.73–3.79 (m, 2H), 3.99 (d, J = 6.4 Hz, 1H), 4.09 (s, 1H), 4.51 (dd, J = 25.2, 12.2 Hz, 2H), 4.61-4.68 (m, 4H), 4.79 (d, J = 6.9 Hz, 1H), 4.92 (d, J = 7.3 Hz, 1H), 5.10 (d, J = 10.0 Hz, 1H), 5.89 (dd, J = 6.8, 3.1 Hz, 1H), 7.33 (m, 5H); 13C NMR (125 MHz, CDCl3) δ –5.0, –4.2, 11.7, 18.0, 18.2, 21.1, 24.9, 25.2, 25.4, 25.9, 26.7, 29.7, 35.9, 38.1, 38.2, 39.3, 41.4, 50.4, 56.2, 57.5, 65.7, 70.4, 72.8, 72.9, 73.9, 75.6, 80.2, 96.2, 97.3, 110.2, 127.2, 127.5, 128.2, 130.7, 133.8, 139.0, 169.2, 215.5; FT-IR (film, NaCl) 1024.8, 1102.2, 1257.9, 1378.4, 1456.7, 1733.0 cm−1; (c, 0.8, CHCl3); HRMS (ESI) [M + Na]+ calcd for C45H76O13SiNa 875.4953, found 875.4951.
Peloruside B (2)
To the macrolactone 16 (64.7 mg, 0.08 mmol) dissolved in THF (0.5 mL) in a small plastic vial was added HF.Py complex (HF.Py:Py:THF = 1:2:4, 2 mL) at 0 °C. The mixture was allowed to warm to 23 °C and stirred overnight. Then, THF (10 mL) was added and the solution was cooled to 0 °C. The diluted solution was added to a chilled saturated NaHCO3 solution dropwise. The aqueous layer was extracted with ethyl acetate (3 × 15 mL). The combined extracts were washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum, and the crude product was purified by flash column chromatography on silica gel (hexanes/ethyl acetate: 8/2 to 3/2 to 1/1 to 2/8) to afford hemi-ketal 17a (41.2 mg) and a hydroxyl ketone 17b (7 mg, total 48.2 mg, 86% yield).
The hemi-ketal 17a (31 mg, 0.042 mmol) was dissolved in acetic acid (v/v: 8/2, 2 mL) and heated to 50 °C for 3 h. After cooling to 23 °C, the solvent was removed under vacuum. The crude product was purified by column chromatography on silica gel (CHCl3/MeOH: 95/5) to afford the diol (15.7 mg, 52% yield) along with the recovered starting material (10 mg).
To the diol from above (15.7 mg, 0.02 mmol) dissolved in CH2Cl2 (6 mL) were added molecular sieves (400 mg), 2,6-di-t-butylpyridine (100 μL, 0.45 mmol) and Me3O+BF4– (33.3 mg, 0.22 mmol) at –5 °C. The mixture was stirred at –5 °C for 18 h. The molecular sieves was filtered and washed with CH2Cl2. The filtrate was washed with saturated NaHCO3 solution, brine and dried over anhydrous Na2SO4. The solvent was evaporated under vacuum, and the crude product was purified by flash column chromatography on silica gel (CHCl3/MeOH: 98/2) to afford the crude mono-methylated product (6.1 mg) along with the recovered starting material (8.7 mg).
The crude mono-methylated product was dissolved in THF (1.5 mL) and cooled to 0 °C, followed by dropwise addition of 4 N HCl (1.5 mL) dropwise. The solution was warmed to 23 °C and stirred at this temperature for 4 h. The solution was cooled to 0 °C, and ethyl acetate was added. Solid NaHCO3 was added to quench the reaction until no bubbles evolved. Then anhydrous Na2SO4 was added, and the solid was filtered and washed with ethyl acetate. The solvent was removed under vacuum, and the crude product was purified by flash chromatography on silica gel (CHCl3/CH3OH: 95/5) to afford the tetraol (2.1 mg).
The crude product (2.1 mg) was dissolved in ethyl acetate (1 mL) and methanol (1 mL) followed by addition of 10% Pd/C (7 mg) and formic acid (50 μL). The mixture was stirred at 23 °C for 1 h. Then Pd/C was filtered with celite, washed with ethyl acetate and concentrated under vacuum. The crude product was purified by flash column chromatography on silica gel (CHCl3/MeOH: 93/7 to 9/1) to afford peloruside B (2) (1.3 mg, 24% yield over three steps). The synthetic peloruside B was identical to the natural peloruside B by comparison of 1D and 2D NMR data. [α]D25 = 0 (c 0.09, CH2Cl2); 1H NMR (600 MHz, CDCl3) δ 0.86 (t, J = 7.5 Hz, 3H, H3-20), 1.09 (s, 3H, H3-21), 1.11 (s, 3H, H3-22), 1.17 (m, 1H, H-19a), 1.40–1.46 (m, 2H,H-12a, H-19b), 1.53 (m, 1H, H-6a), 1.72 (s, 3H, H3-23), 1.77 (ddd, J = 2.4, 5.1, 12.5 Hz, 1H, H-6b), 1.84 (m, 1H, H-4a), 1.98–2.22 (m, 3H, H-4b, H-12b, H-14a), 2.56 (m, 1H, H-18), 2.93 (br s, 1H, OH), 3.13 (br s, 1H, OH), 3.35 (m, 1H, H-24a), 3.38 (s, 3H, 7-OMe), 3.48 (s, 3H, 13-OMe), 3.63 (dd, J = 3.6, 10.8 Hz,1H, H-24b), 3.79 (ddd, J = 3.0, 5.0, 11.5 Hz, 1H, H-7), 3.97–4.01 (m, 2H, H-8, H-13), 4.15 (m, 1H, H-5), 4.41 (br s, 1H, OH), 4.50 (br s, 1H, H-2), 4.58 (br s, 1H, H-3), 4.91 (br d, J = 10.6 Hz, 1H, H-11), 5.02 (d, J = 10.3 Hz, 1H, H-17), 5.70 (d, J = 10.6 Hz, 1H, H-15), 6.76 (br s, 1H, 2-OH); 13C NMR (150 MHz, CDCl3) δ 12.4 (C-20), 15.8 (C-21), 17.8 (C-23), 21.0 (C-22), 24.7 (C-19), 31.6 (C-6), 33.9 (C-12), 35.7 (C-14), 38.6 (C-4), 43.6 (C-18), 43.8 (C-10), 55.8 (C-7-OMe), 59.3 (C-13-OMe), 63.8 (C-5), 67.0 (C-8), 67.1 (C-24), 69.91 (C-2), 69.94 (C-3), 71.8 (C-15), 74.0 (C-11), 76.1 (C-7), 78.1 (C-13), 102.1 (C-9), 131.0 (C-17), 136.9 (C-16), 174.7 (C-1); see Supporting Information for 2D NMR spectra; FT-IR (film, NaCl) 1084, 1462, 1736, 2850, 2919 cm−1.
Supplementary Material
Acknowledgment
Financial support of this work was provided in part by the National Institutes of Health and Purdue University. The NZ Foundation for Research, Science & Technology (FRST), Cancer Society of New Zealand and Curtis-Gordon Research Scholarship in Chemistry (VUW) are acknowledged for funding (A. J. S.). We also thank the Wellington Medical Research Foundation and a NZ Genesis Oncology Postgraduate Scholarship (A. C.). The authors thank Dr. John Ryan (VUW), and Dr. John Harwood (Purdue University) for assistance with the NMR structure analysis.
Footnotes
Supporting Information Available: Full experimental details for compounds 5, 9-12 and characterization of selected compounds; copies of 1H and 13C NMR spectra of selected compounds; comparison of 1H and 13C NMR spectra of synthetic and natural peloruside B. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Perry NB, Blunt JW, Munro MHG. J. Am. Chem. Soc. 1988;110:4851–4853. [Google Scholar]
- 2.Perry NB, Blunt JW, Munro MHG, Thompson AM. J. Org. Chem. 1990;55:223–227. [Google Scholar]
- 3.West LM, Northcote PT, Hood KA, Miller JH, Page MJ. J. Nat. Prod. 2000;63:707–709. doi: 10.1021/np9904511. [DOI] [PubMed] [Google Scholar]
- 4.Northcote PT, Blunt JW, Munro MHG. Tetrahedron Lett. 1991;32:6411–6414. [Google Scholar]
- 5.Burres NS, Clement JJ. Cancer Res. 1989;49:2935–2940. [PubMed] [Google Scholar]
- 6.Bordeleau M-E, Matthews J, Wojnar JM, Lindqvist L, Novac O, Jankowsky E, Sonenberg N, Northcote P, Teesdale-Spittle P, Pelletier J. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10460–10465. doi: 10.1073/pnas.0504249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West LM, Northcote PT, Battershill CN. J. Org. Chem. 2000;65:445–449. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]
- 8.Hood KA, West LM, Rouw\'e B, Northcote PT, Berridge MV, Wakefield SJ, Miller JH. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- 9.Liao X, Wu Y, De Brabander JK. Angew. Chem., Int. Ed. 2003;42:1648–1652. doi: 10.1002/anie.200351145. [DOI] [PubMed] [Google Scholar]
- 10.Jin M, Taylor RE. Org. Lett. 2005;7:1303–1305. doi: 10.1021/ol050070g. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh AK, Xu X, Kim J-H, Xu C-X. Org. Lett. 2008;10:1001–1004. doi: 10.1021/ol703091b. [DOI] [PubMed] [Google Scholar]
- 12.Evans DA, Welch DS, Speed AWH, Moniz GA, Reichelt A, Ho S. J. Am. Chem. Soc. 2009;131:3840–3841. doi: 10.1021/ja900020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AB, Cox JM, Furuichi N, Kenesky CS, Zheng J, Atasoylu O, Wuest WM. Org. Lett. 2008;10:5501–5504. doi: 10.1021/ol8019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For the studies towards total syntheses of peloruside A: Prantz K, Mulzer Johann. Angew. Chem. Int. Ed. 2009;48:5030–5033. doi: 10.1002/anie.200901740.Williams D, Walsh MJ, Claeboe CD, Zorn N. Pure Appl. Chem. 2009;81:181–194. doi: 10.1351/PAC-CON-08-07-23.Casey EM, Teesdale-Spittle P, Harvey JE. Tetrahedron Lett. 2008;49:7021–7023.Chen Z-L, Zhou W-S. Tetrahedron Lett. 2006;47:5289–5292.Owen RM, Roush WR. Org. Lett. 2005;7:3941–3944. doi: 10.1021/ol0514303.;Roulland E, Ermolenko MS. Org. Lett. 2005;7:2225–2228. doi: 10.1021/ol050588k.Engers DW, Bassindale MJ, Pagenkopf BL. Org. Lett. 2004;6:663–666. doi: 10.1021/ol036393z.Gurjar MK, Pedduri Y, Ramana CV, Puranik VG, Gonnade RG. Tetrahedron Lett. 2004;45:387–390.Liu B, Zhou WS. Org. Lett. 2004;6:71–74. doi: 10.1021/ol036058a.Taylor RE, Jin M. Org. Lett. 2003;5:4959–4961. doi: 10.1021/ol0358814.Ghosh AK, Kim J-H. Tetrahedron Lett. 2003;44:7659–7661. doi: 10.1016/j.tetlet.2003.08.023.Ghosh AK, Kim J-H. Tetrahedron Lett. 2003;44:3967–3969. doi: 10.1016/S0040-4039(03)00744-5.Paterson I, Di Francesco ME, Kuehn T. Org. Lett. 2003;5:599–602. doi: 10.1021/ol034035q.
- 15.Williams DR, Nag PP, Zorn N. Curr. Opin. Drug Discovery Dev. 2008;11:251–271. [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, Northcote PT, Webb VL, Mackey S, Handley SJ. Aquaculture. 2005;250:256–269. [Google Scholar]
- 17.Page M, West L, Northcote P, Battershill C, Kelly M. J. Chem. Ecol. 2005;31:1161–1174. doi: 10.1007/s10886-005-4254-0. [DOI] [PubMed] [Google Scholar]
- 18.Gaitanos TN, Buey R. e. M., D\'iaz JF, Northcote PT, Teesdale-Spittle P, Andreu J. e. M., Miller JH. Cancer Res. 2004;64:5063–5067. doi: 10.1158/0008-5472.CAN-04-0771. [DOI] [PubMed] [Google Scholar]
- 19.Jim\'enez-Barbero J. u., Canales A, Northcote PT, Buey R. e. M., Andreu J. e. M., D\'iaz JF. J. Am. Chem. Soc. 2006;128:8757–8765. doi: 10.1021/ja0580237. [DOI] [PubMed] [Google Scholar]
- 20.Huzil JT, Chik JK, Slysz GW, Freedman H, Tuszynski J, Taylor RE, Sackett DL, Schriemer DC. J. Mol. Biol. 2008;378:1016–1030. doi: 10.1016/j.jmb.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huzil JT, Chik JK, Slysz GW, Freedman H, Tuszynski J, Taylor RE, Sackett DL, Schriemer DC. J. Mol. Biol. 2008;378:1016–1030. doi: 10.1016/j.jmb.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, Curran DP, Cushman M, Nicolaou KC, Paterson I, Sorensen EJ. Mol. Pharmacol. 2006;70:1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 23.Wilmes A, Bargh K, Kelly C, Northcote PT, Miller JH. Mol. Pharm. 2007;4:269–280. doi: 10.1021/mp060101p. [DOI] [PubMed] [Google Scholar]
- 24.Iida H, Yamazaki N, Kibayashi C. J. Org. Chem. 1986;51:1069–1073. [Google Scholar]
- 25.Lipshutz BH, Sengupta S. Org. React. 1992;41:135–631. [Google Scholar]
- 26.Yu W, Mei Y, Kang Y, Hua Z, Jin Z. Org. Lett. 2004;6:3217–3219. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 27.Jadhav PK, Bhat KS, Perumal T, Brown HC. J. Org. Chem. 1986;51:432–439. [Google Scholar]
- 28.Ando K. J. Org. Chem. 1998;63:8411–8416. [Google Scholar]
- 29.Kolb H, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 30.Ghosh AK, Kass J, Anderson DD, Xu X, Marian C. Org. Lett. 2008;10:4811–4814. doi: 10.1021/ol801971t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.