Abstract
A silencing element has been previously located upstream of the human ε-globin gene promoter using transient assays and transgenic mice carrying plasmid constructs in which the element has been deleted or its transcriptional motifs have been mutated. To investigate whether this element functions in the context of the whole β-globin locus, we analyzed ε-globin gene expression in transgenic mice carrying a deletion of the silencing element in the context of a 213-kilobase human β-globin yeast artificial chromosome (β-YAC). ε-Globin gene expression was measured during embryonic and fetal development and in adult mice. ε-mRNA levels in embryonic cells in Day 12 blood were as high as those measured in wild-type β-YAC controls, indicating that the deletion does not affect ε gene promoter function. ε-Globin gene expression was confined to the embryonic cells, indicating that deletion of this silencing element did not affect ε-globin developmental expression in the context of the β-YAC. These results suggest that in the context of the whole β-globin locus, other proximal and upstream ε gene promoter elements as well as competition by the downstream globin genes contribute to the silencing of the ε-globin gene in the cells of definitive erythropoiesis.
Keywords: locus control region, globin, developmental regulation, hemoglobin switching, RNAse Protection Analysis, erythropoiesis, yeast artificial chromosome, transgenic mice, gene silencer, regulatory elements
Introduction
Switches of gene expression during development are characteristic of the globin genes of all species that use hemoglobin for oxygen transport. These switches are controlled by proximal as well as by distal regulatory elements. The major distal regulatory element is the locus control region (LCR) located 6–22 kilobases (kb) upstream of the β-globin gene cluster. This powerful element is comprised of five developmentally stable DNase I–hypersensitive sites (HS1–HS5) (1–4), it is necessary for enhancing globin gene expression (3, 5, 6), and it is involved in the developmental control of the globin genes (7, 8). The proximal regulatory elements controlling γ and β gene expression are located predominantly in the promoters of the γ and β genes (9–12). Proximal silencing elements have also been identified in the promoter of the human embryonic ε-globin gene (13–22). Thus, transgenic mice carrying a construct consisting of a 3.7-kb human ε gene sequence containing a 2-kb promoter linked to a 2.5-kb microlocus control region (µLCR) fail to express the ε gene in the adult stage of development (19). These results indicated that all elements involved in developmental regulation of the embryonic gene are located in the 3.7-kb ε genomic sequence contained in the transgenic construct. Subsequent studies showed that deletion of a 285-base-pair (bp) sequence (from −182 to −467) from the 2.0-kb ε-globin gene promoter resulted in ε-globin gene expression in the adult stage of development (13), providing in vivo evidence that this sequence contains a silencer. Other transgenic mouse studies have identified negative regulatory sequences between 2000 bp and 463 bp upstream of the ε-globin gene cap site (20). Silencing elements are also located in the direct repeats (DR) adjacent to the ε-globin gene CCAAT box (21, 22).
In the present study, we investigated the functional role of the ε gene silencer in the context of a 213-kb β human globin locus YAC (β-YAC). We deleted this element from the ε gene of β-YAC and examined globin gene expression in transgenic mice carrying the mutant YAC (Δεsil β-YAC). The Δεsil β-YAC transgenic lines showed normal ε-globin gene expression in embryonic cells, but in contrast to the results obtained with the µLCRε transgenic constructs mentioned previously, ε gene expression was silenced in the fetal and adult erythroid cells of definitive erythropoiesis. These results suggest that in the context of an intact β-globin locus, other cis elements of the proximal and the distal ε gene promoter, together with competition by the downstream globin genes, are sufficient to downregulate ε-globin gene expression in definitive erythropoiesis.
Materials and Methods
Construction of the Δεsil β-YAC Transgene
Plasmid pε3.7 is a pBluscript plasmid (Stratagene, La Jolla, CA) containing the 3.7-kb EcoRI fragment containing the ε-globin gene, 2.0 kb of 5′ flanking sequence, and 280 bp of 3′ non-translated sequences (GenBank accesssion no. U01317; coordinates 17482–21251). Digestion with HindII and BamHI released a 224-bp fragment containing the putative ε-silencer region (coordinates 19101–19325), and the digested plasmid was made blunt with Klenow DNA polymerase and ligated with T4 DNA polymerase to produce pε3.7(ΔHindII/BamHI). The BamHI is located at position −182 upstream of the ε cap site; the same site has also been identified as −177 in previous studies (14, 17), including a study in which a 155-kb β YAC was used (23). pε3.7(ΔHindII/BamHI) was digested with EcoRI to release the insert and subcloned into pRS406, a yeast integrating plasmid (YIP; Stratagene), to produce pRSε3.7(ΔHindII/BamHI). pRSε3.7(ΔHindII/BamHI) was linearized with the restriction enzyme NheI and transformed into spheroplasted Saccharomyces cerevisiae strain AB1380 strain AB1380 initially reported as a 248-kb β-YAC. Transformants were selected for uracil prototrophy on complete medium and correct recombination was determined by Southern blot analysis. Spontaneous excision of the YIP was induced by overnight growth in nonselective rich medium (yeast-peptone-dextrose). The yeast cells were plated on 5-fluoroorotic acid plates to select for loss of the URA3 gene residing on the YIP vector, resulting in 5-fluroorotic acid resistance. The deletion of the ε-silencer region was confirmed by Southern blot analysis and PCR, and the mutant β-YAC was designated Δεsil β-YAC.
YAC Purification and Production of Transgenic Mice
The yeast strain containing the Δεsil β-YAC was grown and isolated as previously described (8). The purified and filtered YAC was injected into fertilized mouse eggs (B6/C3F1) and then transferred to pseudopregnant foster mothers (B6/D2F1). Transgenic founder animals were identified by Southern hybridization slot blot of tail DNA. The transgenic founders were bred with nontransgenic mice (B6/D2F1) to produce F1 progeny, and the F1 males were subsequently bred for staged pregnancies that were interrupted at postconception Days −10, −12, and −14 and to produce F2 adults.
Determination of Transgene Copy Number
For the Δεsil β-YAC transgenic lines, agarose plugs containing high-molecular-weight liver DNA was digested with restriction enzymes overnight, and the resultant fragments were fractionated by agarose gel electrophoresis and blotted onto zeta-probe positive charged nylon membrane (BioRad, Hercules, CA). Transgene copy number was determined by comparing HS4, HS3, HS2, ε-, Aγ-, and β-globin gene hybridization signals to the endogenous murine Thy1.1 signals by Southern blot hybridization analyses as previously described (8, 24). The radioactive signals were measured using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and the ratios of human globin sequences to Thy1.1 (corrected for a haploid genome) were calculated to determine transgene copy number.
Structural Analysis of Δεsil β-YAC Transgenic Mice
High-molecular-weight DNA embedded in agarose was prepared as previously described (24). The 32P-radiolabeled probes used in the Southern hybridization analyses for the structural analyses are as follows: 0.7-kb PstI HS3, 1.9-kb HindIII HS2, 1.8-kb XbaI HS1, 3.7-kb EcoRI ε-globin gene, 2.4-kb EcoRI fragment 3′ of the Aγ-globin gene, 1.0-kb EcoRV ψβ region, 2.1-kb PstI fragment upstream of the δ-globin gene, 0.9-kb EcoRI-BamHI fragment 3′ of the β-globin gene, 1.4-kb XbaI DF10 (3′ HS1), 1.9-kb BglII HPFH3, 0.5-kb HindIII H500, and 1.5-kb EcoRI-BglII HPFH6.
Measurement of Globin mRNA Synthesis
Total RNA was isolated from yolk sac, fetal liver, and blood from F2 transgenic embryos, fetuses, and adults using the RNAgents total RNA Isolation System following the manufacturer’s instructions (Promega, Madison, WI). Human and murine globin mRNAs were detected by RNase protection analysis and the signals quantified using a PhosphorImager (Molecular Dynamics). Template DNAs used to prepare riboprobes to measure human ε-, γ-, and β-globin mRNA were pT7Huε(188), pT7Aγm(170), and pT7 βm, respectively, and to measure endogenous murine α- and ζ-globin, we used plasmids pT7 Moα and pT7 Moζ, respectively. The source and amount (in parentheses) of isolated RNAs used in RNase protection assays are as follows: Day 10 yolk sac (1000 ng), Day 12 liver (500 ng), Day 12 blood (80 ng), Day 14 liver (500 ng), and adult blood (50 ng).
Polymerase Chain Reactions
The 5′ flanking region of the ε-globin gene was amplified using the following ε-globin specific primers: Δε#3 (proximal) 5′ GGCTTCTCAGCTCCCTTCCCAGTG 3′ and Δε#4 (distal) 5′ GTCAGGTCCGGAGAGGGTCAGC 3′. One hundred nanograms of genomic DNA were PCR amplified using Taq DNA polymerase in Storage Buffer B following the manufacturer’s instructions (Promega). The PCR conditions were the following: 30 secs at 95°C, 45 secs at 58°C, 40 secs at 72°C for 30 cycles.
Results
Production of Δεsil β-YAC Transgenic Mice
To examine the effects of deletion of the ε-globin gene silencer in the context of the whole human β-globin locus, a 224-bp HindII- BamHI fragment encompassing the −406- to −182- bp region upstream of the ε-globin cap site was deleted from a 213-kb β-YAC harboring the human β-globin locus (25) (Fig. 1). The deletion of this element was done by homologous recombination of a mutant ε-promoter sequence following transformation of a yeast strain containing a wild-type 213-kb β-YAC (26). The resulting mutant Δεsil β-YAC was purified and microinjected into pronuclei of fertilized mouse oocytes. An assessment of transgene integration and globin gene expression was done in F2 progeny of transgenic mouse lines.
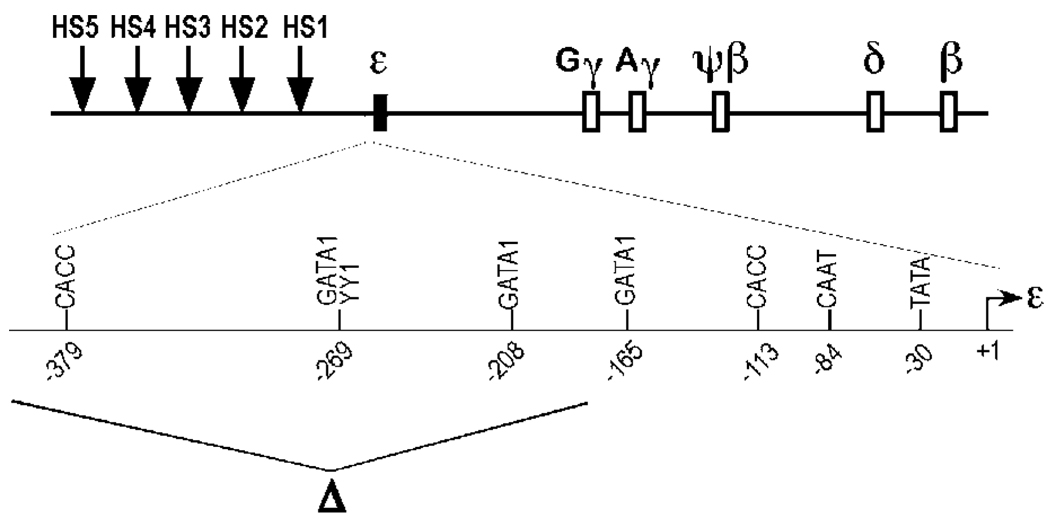
Figure 1.
Δεsil β-YAC transgene construct used for the production of transgenic mice. A diagram of the human β-globin locus is shown on the top of the panel. The expanded diagram below depicts the proximal and distal region of the ε-globin gene promoter with the regulatory motifs labeled as follows: the minimal promoter, GATA-1 sites at positions −165 and −208, two overlapping motifs for YY1 and GATA-1 at position −269, and CACC box at position −379. The deletion of the ε-globin silencer region between −406 bp to −182 bp upstream of the cap site (filled arrow) is shown below the ε-globin promoter diagram. The deletion includes the CACC box at position −379.
Confirmation of the Deletion of the ε-Gene Silencer in Δεsil β-YAC Transgenic Mice
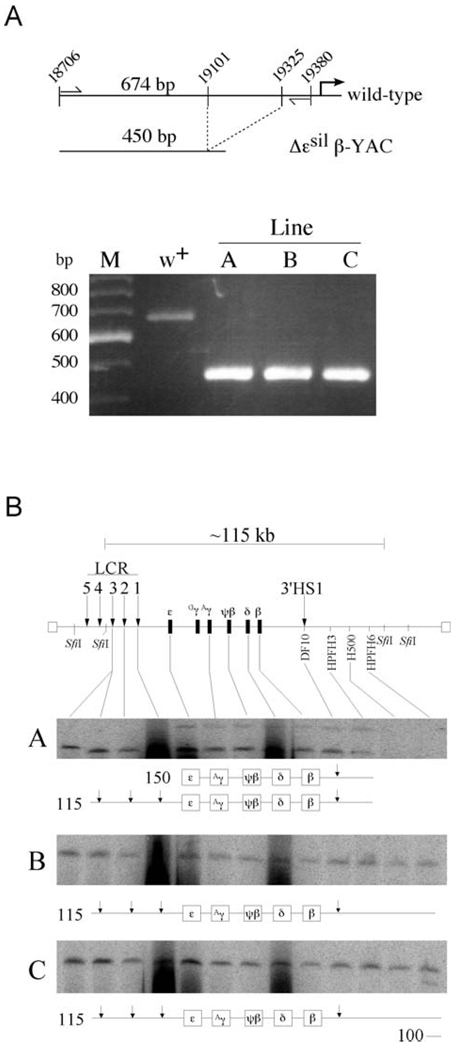
Three Δεsil β-YAC transgenic lines A, B, and C were established, and the deletion of the 224-bp fragment was confirmed by polymerase chain reaction (PCR) assay. To confirm the size of the deletion, we designed two oligonucleotides that PCR amplify the region upstream of the minimal ε-globin promoter. In the wild-type β-globin locus, the two primers are located at position −824 and −150 relative to the start of transcription, resulting in a 674-bp PCR amplicon (Fig. 2A, top panel). Because of the 224-bp deletion of the ε-silencer element, the PCR reaction performed on DNA isolated from Δεsil β-YAC transgenic lines results in a 450-bp amplicon. Notice in Figure 2A that the agarose gel shows only the 450-bp fragment in each of the three mutant β-YAC lines A, B, and C, while the 674-bp fragment was amplified from DNA isolated from the wild-type β-YAC (lane w+). These fragments were sequenced to ensure that no other mutations were introduced (data not shown).
Figure 2.
Confirmation of the ε-silencer deletion by PCR analysis and structural analysis of Δεsil β-YAC transgenic lines. (A) PCR analysis followed by gel electrophoresis to detect the ε-globin silencer deletion. A map of the wild-type region showing the location of the primers (thin vertical arrows) used to amplify the distal ε-globin promoter region. The wild-type ε-globin sequence amplified a 674-bp fragment, and the mutant ε-globin promoter amplifies a 450-bp fragment. As seen in the agarose gel stained with ethidium bromide, the PCR amplification in the wild-type lane showed a single fragment of 674 bp in length, while a 450-bp fragment was PCR amplified from each of the Δεsil β-YAC transgenic lines. M is a 100-bp marker with the fragment lengths labeled to the left of the picture of the agarose gel. (B) We used pulsed-field gel electrophoresis followed by Southern blot hybridization analysis to determine the structural integrity of the integrated β-YAC transgene. At the top of the panel is a schematic diagram of the β-globin locus that includes the LCR (vertical arrow), the globin gene cluster, and 3′HS1. Above the locus is a depiction of the 115-kb SfiI fragment encompassing most of the β-globin locus from 5′HS3 to the breakpoint of HPFH6 approximately 53 kb downstream of the β-globin gene. The probes used to determine the relative intactness of the β-YACs are labeled along the locus and are listed in Experimental Procedures. The resulting autoradiograms of transgenic lines A, B, and C are shown; note that each line contains at least one intact β-globin locus. A representation of the structures of the SfiI fragments is drawn below each autoradiogram. The first lane in each autoradiogram contains DNA from a mouse erythroleukemia cell line containing a single intact β-YAC, as determined by structural analysis and fluorescent in situ hybridization, used as a control (27).
Structural Analysis of Δεsil β-YAC Transgenic Lines
Most of the β-globin locus of the Δεsil β-YAC is contained on a 115-kb SfiI restriction enzyme fragment, having a 5′ boundary between HS3 and HS4 of the LCR and a 3′ boundary downstream of the hereditary persistence of fetal hemoglobin (HPFH) 6 breakpoint, approximately 60 kb downstream of the β-globin gene. To examine the structural integrity of the individual YACs integrated into the murine genome, β-YAC trangenic mouse DNA embedded in agarose was digested with SfiI, and the DNA fragments were fractionated by pulsed-field gel electrophoresis and subjected to Southern hybridization analysis. The integrity of an individual β-YAC copy was determined by the hybridization of a series of probes that reside along the β-globin locus. Presence of these hybridization fragments determines the continuity of the globin locus sequences. The 12 probes used to determine β-YAC intactness are listed in Materials and Methods and are shown on the schematic drawing of the β-globin locus in Figure 2B.
Line A has two SfiI fragments: an intact 115-kb SfiI fragment containing two copies of the β-globin locus from the HS3 of the LCR to the HPFH3 breakpoint and a 150-kb fragment containing portion of the β-globin cluster but missing the LCR; because of the deletion of the LCR, this 150-kb fragment is not expected to contribute to the globin gene expression.
Lines B and C have a single intact 115-kb β-YAC. Copy number analysis determined that these transgenic lines contain a single copy of the Δεsil β-YAC.
Analysis of ε-Globin Gene Expression in Δεsil β-YAC Transgenic Mice
To determine the contribution of the ε-gene silencer on the temporal regulation of the human globin genes in the context of the intact β-globin locus, we measured the expression of human globin genes as well the endogenous murine α- and ζ-globin genes by RNase protection analysis. The amount of human messenger RNA was measured by phosphorimaging analysis, and the expression levels were calculated as a percentage of endogenous murine α + ζ globin per gene copy.
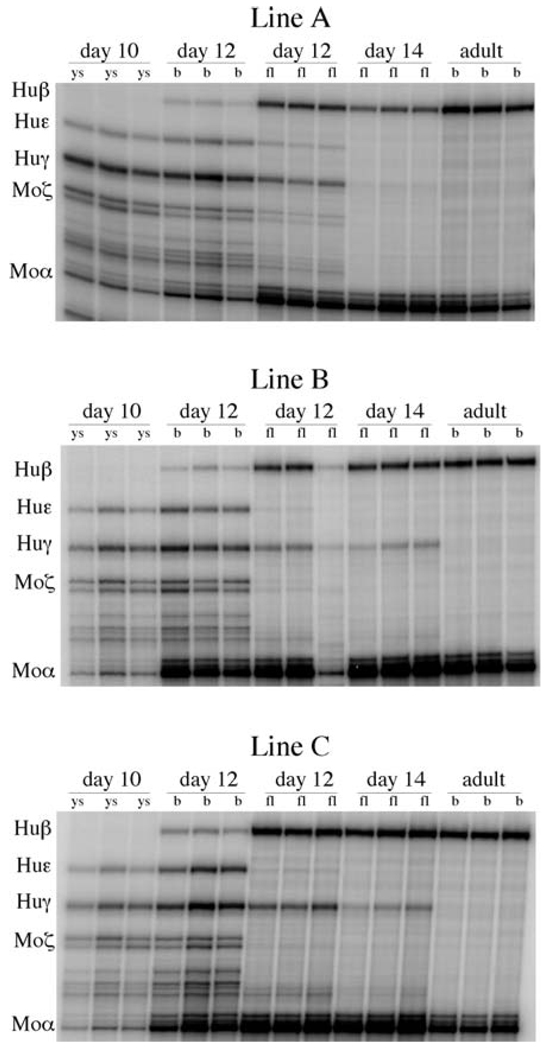
To assess globin gene expression during development, we isolated total RNA from yolk sac, liver, and blood samples from Day −10, Day −12, and Day −14 F2 fetuses from the same litter. In wild-type β-YAC transgenic mice, the human ε-globin gene is exclusively expressed in the embryonic cells of yolk sac origin. The mean level of ε-globin gene expression was 12.1% ± 1.3% in Day 10 yolk sac and 20.4% ± 1.4% in Day 12 blood, which is composed almost exclusively from yolk sac origin erythroblasts and nucleated red cells. In Δεsil β-YAC transgenic lines, ε-globin gene expression in Day 10 yolk sac ranged from 13.6%–16.9% (mean 15.4% ± 1.7%) and increased to 19.1%–21.9% (mean 20.3% ± 1.4%) in Day 12 blood, suggesting that the deletion of the silencer element had no impact on ε-globin gene expression during embryonic erythropoiesis (Fig. 3 and Table 1).
Figure 3.
RNase protection analysis of F2 progeny of the Δεsil β-YAC transgenic mice. The locations of the protected fragments are shown to the left of each autoradiogram. The protected fragments sizes are human β, 205 bases; human γ, 170 bases; human ε, 188 bases; murine α, 128 base; and murine ζ, 151 bases. The days of gestation of the tissue samples are labeled above the autoradiogram, and the tissue sources are indicated above each lane (ys, yolk sac; fl, fetal liver; b, blood).
Table 1.
Human ε-Globin mRNA Levels in Δεsil Transgenic Mice and Wild Type β-YAC Control Mice
| Human ε-globin mRNA levels as a % of murine α- plus ζ-globin mRNA (mean ± SD) | ||||
|---|---|---|---|---|
| Embryonic erythropoiesis | Definitive erythropoiesis | |||
| Line (Copy #) | Day 10 yolk sac | Day 12 blood | Day 12 liver | Day 14 liver |
| A(2) | 13.6 ± 1.4 | 19.9 ± 2.7 | 2.0 ± 0.7 | 0 |
| B(1) | 16.9 ± 3.7 | 19.1 ± 1.7 | 1.1 ± 0.3 | 0 |
| C(1) | 15.9 ± 3.1 | 21.9 ± 1.0 | 1.2 ± 0.4 | 0 |
| Mean of Δεsil β-YAC lines | 15.4 ± 1.7 | 20.3 ± 1.4 | 1.4 ± 0.5 | 0 |
| Mean of wild-type line | 12.8 ± 0.3 | 21.4 ± 0.4 | 1.9 ± 0.7 | 0 |
The definitive stage of erythropoiesis begins in the murine fetal liver on Day 10.5 and is characterized by exclusive transcription the adult globin genes, β-major and β-minor. In the wild β-YAC fetuses, mean ε expression in the Day 12 fetal liver was 1.9 ± 0.7%; as previously shown (8), these amounts of ε mRNA are contributed by primitive erythroblasts and nucleated erythrocytes of yolk sac origin that contaminate the fetal liver. By Day 14, ε-globin mRNA transcript is undetectable in the wild β-YAC mice by RNase protection (Fig. 3 and Table 1). The mean level ε mRNA among the Δεsil β-YAC lines was 1.2% ± 0.8% in Day 12 fetal liver, and by Day 14, ε expression was undetectable. To confirm that the ε mRNA detected in the Day 12 Δεsil β-YAC lines was due to contaminating embryonic erythroblasts, we stained the fetal liver preparations with anti-ε-globin monoclonal antibodies; only embryonic erythroblasts characterized by their large cytoplasmic/nucleus volume ratio and their pycnotic nucleus stained positive (data not shown). We therefore conclude that despite the absence of the −406 to −182 sequence, there was no detectable ε-globin gene expression by the erythroid cells of the definitive erythropoiesis of the fetal liver of the Δεsil β-YAC lines.
Analysis of γ and β Gene Expression in the Δεsil β-YAC Mice
In wild-type β-YAC mice, human β expression is restricted to cells of definitive erythropoiesis, while human γ-globin gene expression occurs during the embryonic erythopoiesis of the yolk sac as well as during the definitive erythropoiesis of the fetal liver. γ-Globin gene expression in the Δεsil β-YAC ranged from 11.7%–13.2% (mean 12.6% ± 0.8%) in Day 10 yolk sac and from 20.2%– 21.3% (mean 21.8% ± 2.1%) in Day 12 blood; these values are statistically indistinguishable from those of the wild-type control (Fig. 3 and Table 2).
Table 2.
Human γ- and β-Globin mRNA Levels in Δεsil Transgenic Mice and Wild Type β-YAC Control Mice
| % of murine α- plus ζ-globin mRNA (mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|
| γ-globin mRNA during embryonic erythropoiesis |
γ-globin mRNA during definitive erythropoiesis |
β-globin mRNA during definitive erythropoiesis | |||||
| Line | Day 10 yolk sac | Day 12 blood | Day 12 liver | Day 14 liver | Day 12 liver | Day 14 liver | Adult blood |
| A | 23.4 ± 2.6 | 21.3 ± 1.5 | 14.2 ± 1.5 | 1.1 ± 0.1 | 42.7 ± 1.7 | 62.9 ± 10.6 | 94.6 ± 13.9 |
| B | 25.6 ± 1.6 | 20.2 ± 0.6 | 6.9 ± 0.8 | 2.9 ± 0.9 | 28.9 ± 9.4 | 33.1 ± 2.1 | 62.7 ± 13.2 |
| C | 26.4 ± 3.5 | 20.8 ± 2.0 | 15.0 ± 2.6 | 4.8 ± 1.5 | 50.5 ± 5.4 | 53.5 ± 3.7 | 105.3 ± 15.5 |
| Mean of Δεsil β-YAC lines |
25.1 ± 1.6 | 21.8 ± 2.1 | 12.0 ± 4.4 | 2.9 ± 1.9 | 40.7 ± 10.9 | 49.8 ± 15.2 | 87.5 ± 22.2 |
| Mean of wild-type line |
22.5 ± 2.4 | 24.5 ± 6.4 | 17.7 ± 5.1 | 4.7 ± 1.2 | 34.9 ± 2.5 | 52.4 ± 6.1 | 99.8 ± 12.3 |
During definitive erythropoiesis, γ- and β-globin gene expression levels in Δεsil β-YAC transgenic mice were similar to those measured in the wild-type controls (Fig. 3 and Table 2). The mean γ-globin mRNA levels in Day 12 and Day 14 fetal liver were 12.0% ± 4.4% and 2.9% ± 1.9%, respectively. A modest decline of 1.5-fold and 1.3-fold in Day 12 and Day 14 fetal liver, respectively, was statistically insignificant compared to the wild-type controls. The mean β-globin mRNA level during definitive erythropoiesis was 40.7% ± 10.9%, 49.8% ± 15.8%, and 87.5% ± 22.2% at Fetal Days 12 and 14 and in the adult stage of development, respectively. We conclude that the 224-bp deletion upstream of the ε-globin minimal promoter did not affect gene expression of the downstream γ- and β-globin genes during definitive erythropoiesis.
Discussion
Two regulatory components are required for proper human embryonic ε-globin gene expression in transgenic mice: the LCR and the ε-globin promoter. Previous studies have demonstrated that DNA sequences upstream of the ε-globin gene promoter contribute to silencing of ε-globin gene expression in definitive cells of the fetal liver (13, 18, 19). A small transgenic construct consisting of 2.5-kb microLCR (µLCR) linked to the ε-globin gene resulted in ε gene expression in primitive erythroblasts of yolk sac origin but failed to express in the cells of the definitive erythropoiesis of the fetal liver or adult bone marrow (19). The simplest and most probable explanation for this observation is that cis-elements located upstream of the ε-globin gene are responsible for the confinement of ε-globin gene expression to embryonic erythropoiesis. Studies in transgenic mice have identified multiple elements involved in gene silencing that are located both proximally and distally to the ε-globin gene promoter (13, 20–22). Deletion of 285 bp, from −467 to −182, of the ε gene promoter affects developmental silencing, resulting in continuation of ε gene expression in adult erythropoiesis (13). This −467 to −182 ε sequence contains three GATA sites in positions −208, −267, and −278, one YY1 site in position −269, and one SP1 site in position −379. In a previous study (28), these sites were mutated individually and in combination to abolish protein binding, and transgenic mice were produced after linking the mutated ε genes with a 2.5-kb µLCR cassette. Mutation of either the YY1 site in position −269 or of the GATA site in position −208 resulted in continuation of ε gene expression in the adult erythropoiesis (28). In contrast, mutation of the two tandemly placed GATA-1 sites in positions −267 and −278 either individually or in combination failed to affect ε gene silencing. These results suggested that the silencing of the ε gene is combinatorial and predicted that more than one DNA binding protein participates in the formation of the silencing complex. In the present study, deletion of a 224-bp sequence containing all the known motifs of the ε-globin gene silencer region from the 213-kb β-YAC did not affect ε gene silencing in primitive erythroblasts, but ε gene expression was not detectable in the cells of definitive erythropoiesis of fetal liver. How can we reconcile the different phenotypes obtained when the silencer is deleted in the context of the 2.5-kb µLCR:: ε gene construct versus the 213-kb β locus YAC?
First, the deletion of the silencer element done in the context of the β-YAC does not replicate exactly the deletion done in the context of the µLCRε construct. Thus, a 61-bp 5′ sequence has been deleted from the µLCRε construct but not from the Δεsil β-YAC (the 5′ of the β-YAC deletion is at −406ε, while the 5′ of the µLCR ε deletion is at −467ε). It is therefore possible that the 61-bp ε promoter sequence contains elements that are involved in ε gene silencing, and these elements were left intact in the Δεsil β-YAC. This possibility cannot be excluded unless ε gene expression is tested in β-YAC mice containing an ε gene promoter deletion identical to that contained in the µLCR::ε gene construct. This interpretation also applies to the findings of a previous study in which 125 bp containing several motifs of the silencer were deleted from the ε gene promoter in the context of a 155-kb β-YAC (23). This deletion had no effect on ε gene silencing in definitive erythropoiesis (23). Second, there is evidence that not only the silencing element in the −467 to −182 region of the ε gene promoter but also other elements located between −467 and −2025 of the ε promoter contribute in turning off the ε gene in definitive erythropoiesis (20, 29). Third, sequences of the proximal promoter are also involved in ε gene silencing. Thus, when the CACCC box of the γ gene is replaced by the ε gene CACCC box, there is striking reduction of γ gene expression in definitive erythropoiesis, suggesting that the ε gene CACCC box participates in gene silencing (30). Filipe et al. (21) mutated two direct repeats located near the CAAT box in the proximal ε-globin promoter and showed that these DR elements are required to silence ε-globin gene expression during definitive erythropoiesis. Tanimoto et al. (22) have further shown that mutation of the DR elements in the context of a 150-kb β-locus YAC resulted in a continued ε-globin transcription during the definitive erythropoiesis of the fetal liver and adult spleen.
It is therefore likely that a large repressor complex or a multitude of cooperating small repressor complexes interacts with sequences of the upstream as well as the proximal promoter, resulting in ε gene silencing in definitive erythropoiesis. It is also likely that this complex(es) silence ε gene expression by disrupting the interaction between the ε-globin gene and the LCR. We postulate that mutations affecting promoter sequences with which the putative complex interacts decrease the stability of the interaction between the LCR and the ε gene promoter. In the context of the µLCR::ε transgenic constructs, the deletion of the −467 to −182 silencer or mutations of its motifs produce a phenotype because the postulated repression complex is incomplete, it binds weakly on the ε promoter, and it cannot interrupt the interaction between the promoter and the LCR. Deletion of the silencer sequence in the context of the β-locus YAC results in an incomplete silencing complex that binds weakly in the ε gene promoter; however, in the context of the β-locus YAC the deletion does not produce a phenotype because the environment of definitive erythropoiesis favors the interaction of the LCR with the downstream β-globin gene. This competitive interaction of the LCR with the downstream adult β-globin gene compensates for the weak binding of the repressor complex when the ε silencer is deleted.
As mentioned earlier, in a previous study, a 125-bp deletion from the ε gene promoter containing sequences of the ε gene silencer was done in the context of a 155-kb β-YAC. Paradoxically, a severe decline in γ-globin mRNA was observed in the yolk sac in three transgenic mouse lines during embryonic erythropoiesis (23). Two of the three transgenic lines also exhibited a reduction of γ- and β-globin mRNA in the fetal erythropoiesis, and all three lines had reduction of β-globin gene expression in the erythroid cells residing in the adult spleen. However, characteristic of this study was striking variation in globin gene expression among lines (23). This variation suggests that these transgenic mice have been subjected to strong position effects. The low levels of γ and β gene expression most likely represents the result of integration of these transgenes at or near heterchromatic regions and suggest that the 155-kb β-YAC used in these studies, in contrast to the 213-kb β-YAC, is more prone to be subjected to position effects by the surrounding murine chromatin.
Acknowledgments
We thank Julie Stewart, Mary Stafford, Jennifer Hempelmann, Hadar H. Sheffer, and Andrew Stergachis for excellent technical support.
This work was supported by the National Institutes of Health grants DK 45365 and HL 20899.
References
- 1.Stamatoyannopoulos G, Grosveld F. Hemoglobin switching. In: Stamatoyannopoulos G, Majerus PW, Perlmutter RM, Varmus H, editors. Molecular Basis of Blood Diseases. 3rd ed. Philadephia: WB Saunders; 2001. pp. 135–182. [Google Scholar]
- 2.Tuan D, Solomon W, Li Q, London IM. The “beta-like-globin” gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985;82:6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 4.Forrester WC, Thompson C, Elder JT, Groudine M. A developmentally stable chromatin structure in the human β-globin gene cluster. Proc Natl Acad Sci U S A. 1986;83:1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrester WC, Novak U, Gelinas R, Groudine M. Molecular analysis of the human β-globin locus activation region. Proc Natl Acad Sci U S A. 1989;86:5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan TM, Behringer RR, Martin NC, Townes TM, Palmiter RD, Brinster RL. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human β-globin gene expression in transgenic mice. Genes Dev. 1989;3:314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- 7.Fraser P, Pruzina S, Antoniou M, Grosveld F. Each hypersensitive site of the human β-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 1993;7:106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
- 8.Navas PA, Peterson KR, Li Q, Skarpidi E, Rohde A, Shaw SE, Clegg CH, Asano H, Stamatoyannopoulos G. Developmental specificity of the interaction between the locus control region and embryonic or fetal globin genes in transgenic mice with an HS3 core deletion. Mol Cell Biol. 1998;18:4188–4196. doi: 10.1128/mcb.18.7.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magram J, Chada K, Costantini F. Developmental regulation of a cloned adult β-globin gene in transgenic mice. Nature. 1985;315:338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- 10.Townes TM, Lingrel JB, Chen HY, Brinster RL, Palmiter RD. Erythroid-specific expression of human β-globin genes in transgenic mice. Eur Mol Biol Org J. 1985;4:1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chada K, Magram J, Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986;319:685–689. doi: 10.1038/319685a0. [DOI] [PubMed] [Google Scholar]
- 12.Kollias G, Wrighton N, Hurst J, Grosveld F. Regulated expression of human Aγ-, β-, and hybrid γβ-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986;46:89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- 13.Raich N, Papayannopoulou T, Stamatoyannopoulos G, Enver T. Demonstration of a human ε-globin gene silencer with studies in transgenic mice. Blood. 1992;79:861–864. [PubMed] [Google Scholar]
- 14.Cao SX, Gutman PD, Dave HP, Schechter AN. Identification of a transcriptional silencer in the 5′-flanking region of the human ε-globin gene. Proc Natl Acad Sci U S A. 1989;86:5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutman PD, Cao SX, Dave HP, Mittelman M, Schechter AN. Binding of erythroid and non-erythroid nuclear proteins to the silencer of the human ε-globin-encoding gene. Gene. 1992;110:197–203. doi: 10.1016/0378-1119(92)90648-9. [DOI] [PubMed] [Google Scholar]
- 16.Trepicchio WL, Dyer MA, Baron MH. Developmental regulation of the human embryonic β-like globin gene is mediated by synergistic interactions among multiple tissue- and stage-specific elements. Mol Cell Biol. 1993;13:7457–7468. doi: 10.1128/mcb.13.12.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada-Kiyama Y, Peters B, Noguchi CT. The ε-globin gene silencer: characterization by in vitro transcription. J Biol Chem. 1992;267:11532–11538. [PubMed] [Google Scholar]
- 18.Shih DM, Wall RJ, Shapiro SG. A 5′ control region of the human ε-globin gene is sufficient for embryonic specificity in transgenic mice. J Biol Chem. 1993;268:3066–3071. [PubMed] [Google Scholar]
- 19.Raich N, Enver T, Nakamoto B, Josephson B, Papayannopoulou T, Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990;250:1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Blau CA, Clegg CH, Rohde A, Stamatoyannopoulos G. Multiple ε-promoter elements participate in the developmental control of ε-globin genes in transgenic mice. J Biol Chem. 1998;273:17361–17367. doi: 10.1074/jbc.273.28.17361. [DOI] [PubMed] [Google Scholar]
- 21.Filipe A, Li Q, Deveaux S, Godin I, Romeo PH, Stamatoyannopoulos G, Mignotte V. Regulation of embryonic/fetal globin genes by nuclear hormone receptors: a novel perspective on hemoglobin switching. Eur Mol Biol Org J. 1999;18:687–697. doi: 10.1093/emboj/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human ε-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14:2778–2794. doi: 10.1101/gad.822500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Bungert J, Engel JD. Mutation of gene-proximal regulatory elements disrupts human ε-, γ-, and β-globin expression in yeast artificial chromosome transgenic mice. Proc Natl Acad Sci U S A. 1997;94:169–174. doi: 10.1073/pnas.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navas PA, Peterson KR, Li Q, McArthur M, Stamatoyannopoulos G. The 5′HS4 core element of the human β-globin locus control region is required for high-level globin gene expression in definitive but not in primitive erythropoiesis. J Mol Biol. 2001;312:17–26. doi: 10.1006/jmbi.2001.4939. [DOI] [PubMed] [Google Scholar]
- 25.Peterson KR, Clegg CH, Huxley C, Josephson BM, Haugen HS, Furukawa T, Stamatoyannopoulos G. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human β-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci U S A. 1993;90:7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson KR, Li QL, Clegg CH, Furukawa T, Navas PA, Norton EJ, Kimbrough TG, Stamatoyannopoulos G. Use of yeast artificial chromosomes (YACs) in studies of mammalian development: production of β-globin locus YAC mice carrying human globin developmental mutants. Proc Natl Acad Sci U S A. 1995;92:5655–5659. doi: 10.1073/pnas.92.12.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson KR, Zitnik G, Huxley C, Lowrey CH, Gnirke A, Leppig KA, Papayannopoulou T, Stamatoyannopoulos G. Use of yeast artificial chromosomes (YACs) for studying control of gene expression: correct regulation of the genes of a human β-globin locus YAC following transfer to mouse erythroleukemia cell lines. Proc Natl Acad Sci U S A. 1993;90:11207–11211. doi: 10.1073/pnas.90.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raich N, Clegg CH, Grofti J, Romeo PH, Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human ε-globin gene. Eur Mol Biol Org J. 1995;14:801–809. doi: 10.1002/j.1460-2075.1995.tb07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Noguchi CT, Miller W, Hardison R, Schechter AN. Multiple regulatory elements in the 5′-flanking sequence of the human ε-globin gene. J Biol Chem. 1998;273:10202–10209. doi: 10.1074/jbc.273.17.10202. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Fang X, Han H, Stamatoyannopoulos G. The minimal promoter plays a major role in silencing of the galago γ-globin gene in adult erythropoiesis. Proc Natl Acad Sci U S A. 2004;101:8096–8101. doi: 10.1073/pnas.0402594101. [DOI] [PMC free article] [PubMed] [Google Scholar]