Abstract
An enantioselective synthesis of platensimycin, a novel antibiotic natural product that inhibits bacterial β-ketoacyl-(acyl-carrier-protein) synthase (FabF), is described. Our synthetic strategy for the construction of the oxatetracyclic core involved an intramolecular Diels-Alder reaction. Our preliminary studies provided a complex tetracyclic product, by first undergoing an interesting 1,5-hydride shift followed by a Diels-Alder reaction. Further optimization of the diene’s electronic properties, by incorporation of a methoxy group, led to the oxatetracyclic core of platensimycin. The three required chiral centers, including two all-carbon quaternary chiral centers, were built in the intramolecular Diels-Alder step. The synthesis utilized natural (+)-carvone as the key chiral starting material, which determined the stereochemistry of the final product. The synthesis also featured an efficient Petasis olefination, a hydroboration sequence, a Gais’s asymmetric Horner-Wadsworth-Emmons reaction, and a mercury salt catalyzed enol ether isomerization.
Keywords: Total synthesis, Platensimycin, Antibacterial agent, Diels-Alder, FabF
Introduction
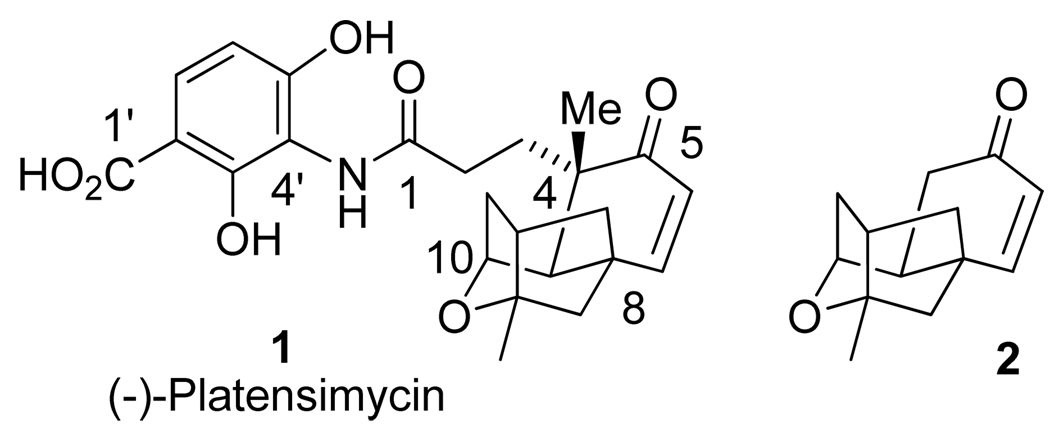
Antibiotic resistance is a serious medical problem worldwide. Currently available antibiotic drugs are becoming less and less effective due to the emergence of a range of lethal resistant strains.1 Therefore, the development of new antibiotics exhibiting novel mechanisms of action has become an urgent priority. Since the early 1960s, the discovery of novel antibiotics from natural products has been quite limited.2,3 Recent isolation of (-)-platensimycin (1, Figure 1), from a strain of Streptomyces platensis, by Merck has cast a new light on antibiotic research.4–6
Figure 1.
Structure of (−)-platensimycin 1 and ketone intermediate 2.
(-)-Platensimycin shows potent antibacterial activity against a broad range of Gram-positive organisms, in vivo efficacy, and no observed toxicity in mice.4 (-)-Platensimycin possesses a novel mechanism of action. It blocks bacterial fatty acid biosynthesis by targeting a key enzyme, bacterial β-ketoacyl-(acyl-carrier-protein) synthase (FabF), which catalyzes the crucial carbon-carbon bond formation in the chain-elongation step. Before the discovery of (-)-platensimycin, only weak antibiotics were known to target FabF.7 Platensimycin exhibited no cross-resistance to other key antibiotic-resistant strains, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus faecium. Unfortunately, (-)-platensimycin is not a good drug candidate due to its poor pharmacokinetic properties.8 However, it constitutes an important lead structure, which provided a starting template for structural modifications and drug development.
Due to its intriguing structure, especially the complex hydrophobic oxatetracyclic core, and its excellent biological activity, (-)-platensimycin has grasped the attention of many synthetic chemists around the world.9 The first total synthesis of platensimycin as a racemic mixture was achieved by Nicolaou and co-workers.10 Since then, a number of formal syntheses targeting the same ketone intermediate (2, Figure 1) in racemic or optically active forms have appeared in the literature.11–19 Syntheses and biological evaluation of several structural analogues have also been reported.20–23 More recently, Nicolaou and co-workers reported a detailed synthesis and structure-activity relationship study.24 We recently reported a highly stereoselective synthesis of the oxatetracyclic core of (-)-platensimycin by using an intramolecular Diels-Alder reaction as the key step.25 Based upon the results of our preliminary Diels-Alder reaction, we devised a route for the total synthesis of platensimycin. Herein, we report a detailed account of our studies leading to the total synthesis of (-)-platensimycin.
Results and Discussion
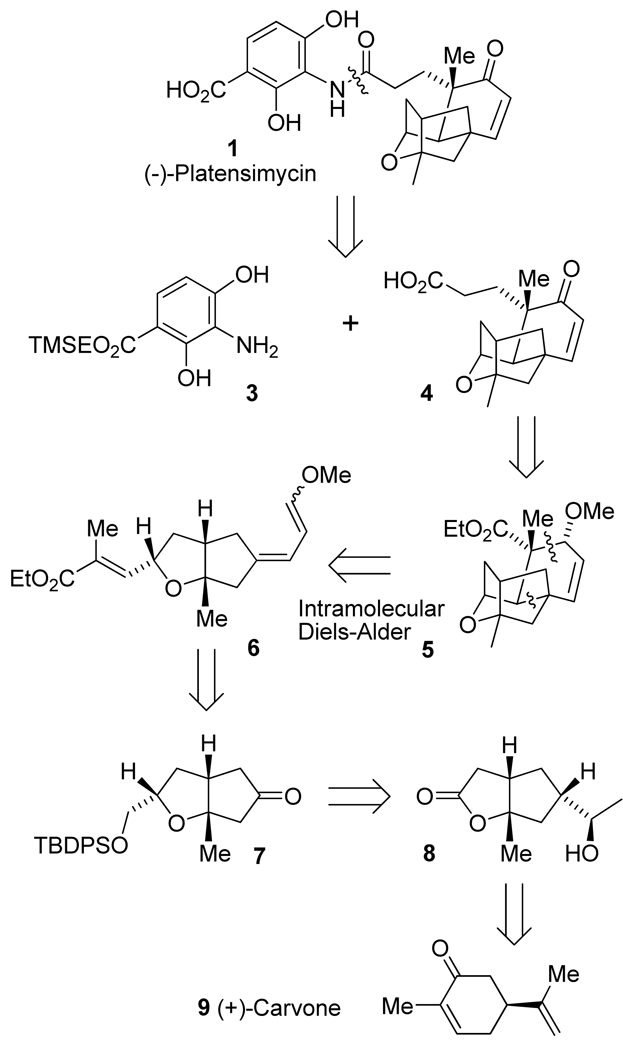
Our synthetic plan is outlined in Figure 2. Strategic disconnection of (-)-platensimycin at the amide bond provided a protected aniline precursor 3,26 and platensic acid 4.27 This TMSE-protected aniline derivative has been recently utilized in a platencin synthesis by Nicolaou and co-workers.26 Platensic acid 4 can be derived from ester 5 by elongation of the side-chain and subsequent oxidation to provide an enone moiety. The cage like (-)-platensimycin core 5 contains seven chiral centers. We planned to construct this core structure by using an intramolecular Diels-Alder reaction. The intramolecular Diels-Alder reaction is a powerful strategy for the construction of stereochemically defined polycyclic compounds in a single step operation.28 Thus, disconnection of ester 5 between the C4-C5 and C8-C9 single bonds provided the simpler bicyclic precursor triene 6. Both tri-substituted olefins in triene 6 could be obtained by a Wittig olefination reactions29,30 or by Horner-Wadsworth-Emmons reactions of the corresponding ketone and aldehyde.31,32 The precursor of triene 6 could be obtained from bicyclic ketone 7. The protected primary alcohol functionality in ketone 7 could be prepared by a hydroboration oxidation sequence from the corresponding enol ether derived from lactone 8. The olefination of lactone 8 can be achieved by Petasis olefination.33 The known lactone 8 can be synthesized from the commercially available natural product (+)-carvone 9.
Figure 2.
Retrosynthesis of (−)-Platensimycin (1).
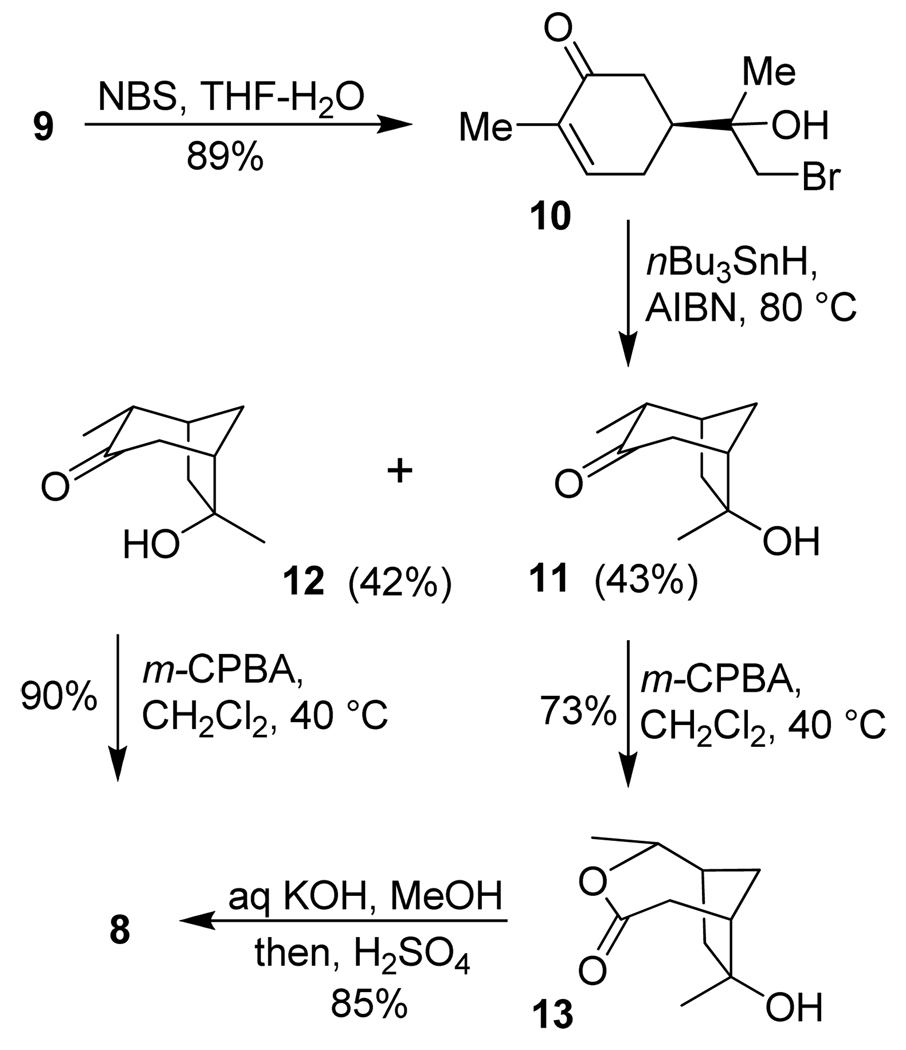
Our synthesis of the known lactone 8 is summarized in Scheme 1. (+)-Carvone 9 was converted to lactone 8 by a modified literature procedure.34–36 Initial treatment of 9 with Hg(OAc)2 in a mixture of THF and H2O followed by reduction with NaBH4 can provide ketones 11 and 12, as reported. However, this reaction generates stoichiometric amount of mercury as a by-product which is not convenient for large scale synthesis. We therefore devised a stepwise approach to these ketones.37 As shown, treatment of (+)-carvone with NBS and H2O provided bromide 10 in 89% yield.
Scheme 1.
Synthesis of lactone 8.
Radical cyclization of 10 in the presence of nBu3SnH and a catalytic amount of AIBN in refluxing benzene furnished ketones 11 and 12 in 85% combined yield as a 1:1 mixture. This mixture was separated by silica gel chromatography. Baeyer-Villiger oxidation of ketone 12 with m-CPBA in dichloromethane at reflux gave the corresponding seven membered lactone, which was opened by the adjacent tertiary alcohol to form the five membered lactone 8 in 90% yield. Ketone 11 was subjected to the same reaction which gave the seven membered lactone 13 in 73% yield. Saponification of lactone 13 followed by lactonization under acidic conditions provided lactone 8 in 85% yield with inversion of the quaternary chiral center.
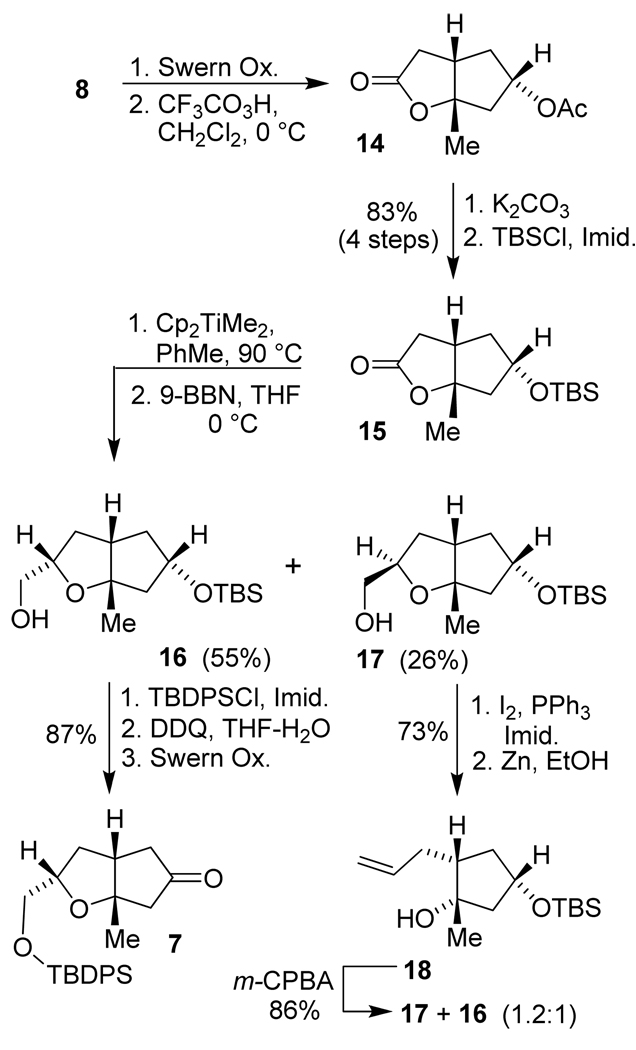
Lactone 8 was previously oxidized to ester 14 in a single operation using m-CPBA.34–36 However, this reaction proved to be extremely sluggish and the yield was low (~20%). Therefore, we explored a stepwise and more reliable route to this acetate which is shown in Scheme 2. The secondary alcohol in lactone 8 was first oxidized to the corresponding ketone using Swern oxidation.38
Scheme 2.
Synthesis of ketone 7.
It is noteworthy to mention that quenching the Swern oxidation with Et3N partially epimerized the ketone product. The use of sterically more hindered iPr2NEt rectified this problem. The resulting ketone was successfully converted to ester 14 by a Baeyer-Villiger oxidation with trifluoroperoxyacetic acid.39 Saponification of crude ester 14 gave the corresponding secondary alcohol, which was protected with TBSCl to provide the silyl ether 15 in 83% yield over 4 steps. Petasis olefination33 of lactone 15 with Cp2TiMe2 in toluene at 90 °C provided the corresponding enol ether. This product was subjected to hydroboration with 9-BBN followed by oxidation with H2O2 to provide the desired primary alcohol 16 and its diastereomer 17 as a 2:1 mixture in 81% yield.40 It is important to note that reaction times longer than 3 h led to diminished yield due to the isomerization of the double bond. While hydroboration by 9-BBN on the enol-ether is expected mainly from the less hindered concave face, the selectivity in the hydroboration step is surprisingly modest. The low selectivity may be due to developing non-bonded interaction between the ring junction methyl group and the bulky borane reagent’s approach on the enol ether from the concave face in the transition state. The diastereomeric alcohols 16 and 17 were separated by flash chromatography, and the major diastereomer 16 was protected with TBDPSCl to provide the corresponding bis-silyl ether in 98% yield. Selective cleavage of the secondary TBS ether with a catalytic amount of DDQ in a 9:1 mixture of THF/H2O furnished the secondary alcohol in 93% yield.41,42 Swern oxidation of this alcohol afforded ketone 7 in 96% yield.
Our many attempts to transform the primary alcohol 17 to the desired diastereomer 16 by oxidation of the primary alcohol and epimerization of the corresponding aldehyde or ester were not successful. However, we were able to partially convert 17 to 16 following the reaction sequence shown in Scheme 2. Alcohol 17 was first converted to an iodide by reaction with I2 and PPh3 in 91% yield. Reductive opening of the iodide with zinc dust in EtOH provided terminal olefin 18 in 85% yield. Epoxidation of 18 with m-CPBA generated the epoxide and subsequent epoxide opening by the adjacent alcohol afforded 16 and 17 as a mixture (1:1.2) in good yield. The mixture can be separated by silica gel chromatography.
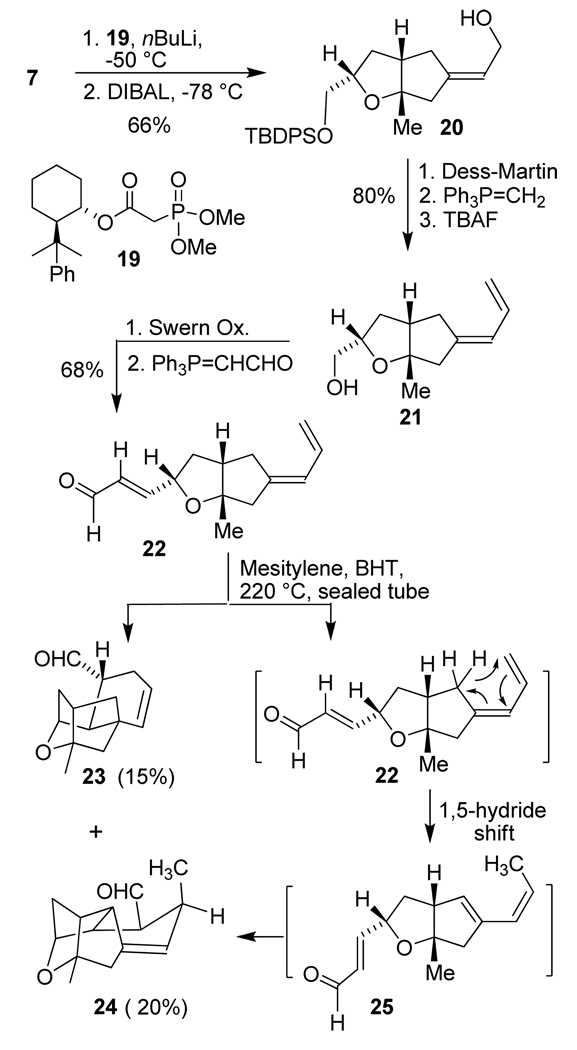
Our initial investigation of the crucial intramolecular Diels-Alder reaction is outlined in Scheme 3. The diene functionality in 22 was installed using a Horner-Wadsworth-Emmons reaction. As shown in Figure 2, the geometry of the trisubstituted olefin is expected to determine the orientation of the diene in the transition state for the Diels-Alder reaction. The approach of the flexible dienophile towards the diene will determine the configuration of the cage like oxatetracyclic structure. It appears that by controlling of the diene’s geometry, one can set the stereochemistry of the product. Since the ketone carbonyl possesses an α-methylene group on each side, the steric differentiation is marginal. The ring junction methyl group, however, may be utilized to improve selectivity. Our initial attempt of a selective olefination using a normal Horner-Wadsworth-Emmons olefination with LHMDS and triethyl phosphonoacetate provided only marginal selectivity for the E-olefin (3:2, by 1H-NMR analysis). Thus, we explored asymmetric olefination with chiral phosphonoacetate reagent 19, which was synthesized by transesterification of trimethyl phosphonoacetate with (+)-phenylnormenthol.43–45 The Horner-Wadsworth-Emmons reaction of 7 using chiral phosphonoacetate 19 at −50 °C furnished a mixture (4.5:1 by 1H NMR analysis) of the corresponding unsaturated E- and Z-esters in 94% yield. The E,Z mixture can be separated by flash chromatography on silica gel. DIBAL reduction of the E-unsaturated ester afforded allylic alcohol 20 in 86% yield and (+)-phenylnormenthol was recovered in 95% yield. Dess-Martin oxidation of the allylic alcohol afforded the aldehyde that was reacted with the corresponding Wittig reagent to provide the diene functionality. Removal of the TBDPS group with TBAF in THF furnished primary alcohol 21 in 80% yield over 3 steps. Swern oxidation of alcohol 21 gave the corresponding aldehyde, which was subjected to a Wittig reaction or a Horner-Wadsworth-Emmons olefination to provide substrates for the exploration of the key intramolecular Diels-Alder reaction.
Scheme 3.
Intramolecular Diels-Alder reaction of aldehyde 22.
We initially utilized an electron withdrawing ester group as the dienophile. The intramolecular Diels-Alder reaction of this substrate under thermal or Lewis acid catalyzed conditions did not provide any desired product. A Diels-Alder reaction without the α-methyl group on the dienophile was also unsuccessful, providing no desired cycloadduct. We then planned to examine an aldehyde functionality on the dienophile. Swern oxidation of alcohol 21 furnished the crude aldehyde which was treated with the corresponding Wittig reagent to provide unsaturated aldehyde 22 in 68% yield. A dilute solution (0.005M) of aldehyde 22 in mesitylene, in the presence of a catalytic amount of 2,6-di-tert-butyl-4-methyl phenol (BHT) as a radical inhibitor,46 was heated in a sealed tube at 220 °C for 2 h. The intramolecular Diels-Alder product 23 was isolated as a single isomer in 15% yield together with by-product 24 isolated in 20% yield. An examination of the 1H-NMR spectrum of compound 24, revealed that this compound resulted from an intramolecular Diels-Alder reaction of substrate 25. Conceivably, this thermodynamically more stable substrate was formed from 22 via a 1,5-hydride shift under the elevated reaction temperature.
Our further attempts to carry out the Diels-Alder reaction at lower temperatures in the presence of various Lewis acids were unsuccessful. We therefore focused our attention to improve the yield of desired cycloadduct 23. in this context, we planned to incorporate an alkoxy group on the diene and carry out the Diels-Alder reaction with an α,β-unsaturated ester as the dienophile. Since the diene is more substituted, a 1,5-hydride shift will be less likely to occur. Furthermore, the presence of an alkoxy group will presumably improve reactivity of the diene and the intramolecular Diels-Alder reaction may be accelerated.
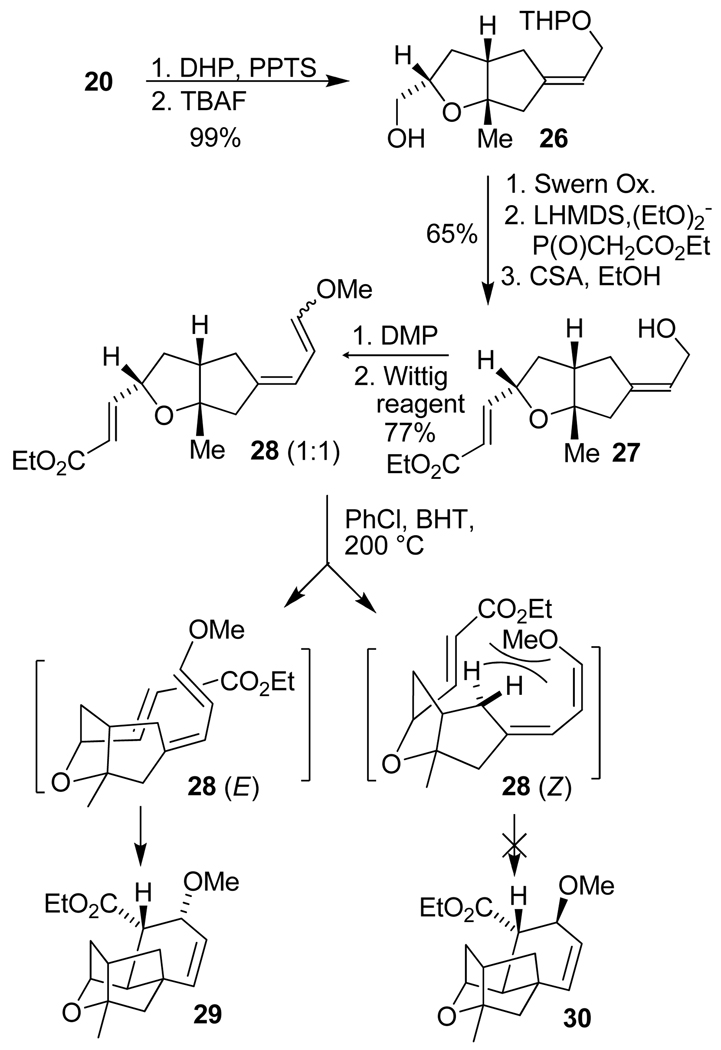
The synthesis of this new Diels-Alder substrate and the resulting intramolecular Diels-Alder reaction is outlined in Scheme 4. Protection of alcohol 20 as a THP ether followed by removal of TBDPS with TBAF gave alcohol 26 in nearly quantitative yield over 2 steps. Swern oxidation of alcohol 26 provided the corresponding aldehyde which was subjected to a Horner-Wadsworth-Emmons olefination with triethyl phosphonoacetate to give the dienophile moiety as a separable mixture (5:1) of E/Z-unsaturated esters. Removal of the THP group on the E-isomer with camphorsulfonic acid in EtOH furnished alcohol 27 in 65% overall yield. Alcohol 27 was converted to the Diels-Alder substrate 28 in two steps involving Dess-Martin oxidation of the allylic alcohol followed by Wittig olefination of the resulting aldehyde. Triene derivative 28 was obtained in 77% yield as an inseparable mixture (1:1) of E/Z enol ethers. This mixture of enol ethers was subjected to the intramolecular Diels-Alder reaction. In principle, both expected methoxy diastereomers can be transformed into the oxatetracyclic enone derivative. Thus, substrate 28 was heated in a sealed tube at 200 °C for 2 h. This has provided the Diels-Alder adduct 29 as a single isomer in 39% isolated yield and Z-enol ether (28-Z) was recovered in 38% isolated yield. The Z-isomer did not undergo Diels-Alder reaction presumably due to the developing non-bonding steric interactions between the terminal methoxy group and the methylene group on the bicyclic ring as shown in Scheme 4. The stereochemistry of the oxatetracyclic core 29 was initially established by extensive NMR experiments (1H, 13C, COSY, NOESY, HMQC, and HMBC). Subsequently, the X-ray crystal structure of 29 (see supporting information 1) conclusively established the stereochemical outcome.
Scheme 4.
Intramolecular Diels-Alder reaction of new substrate 28.
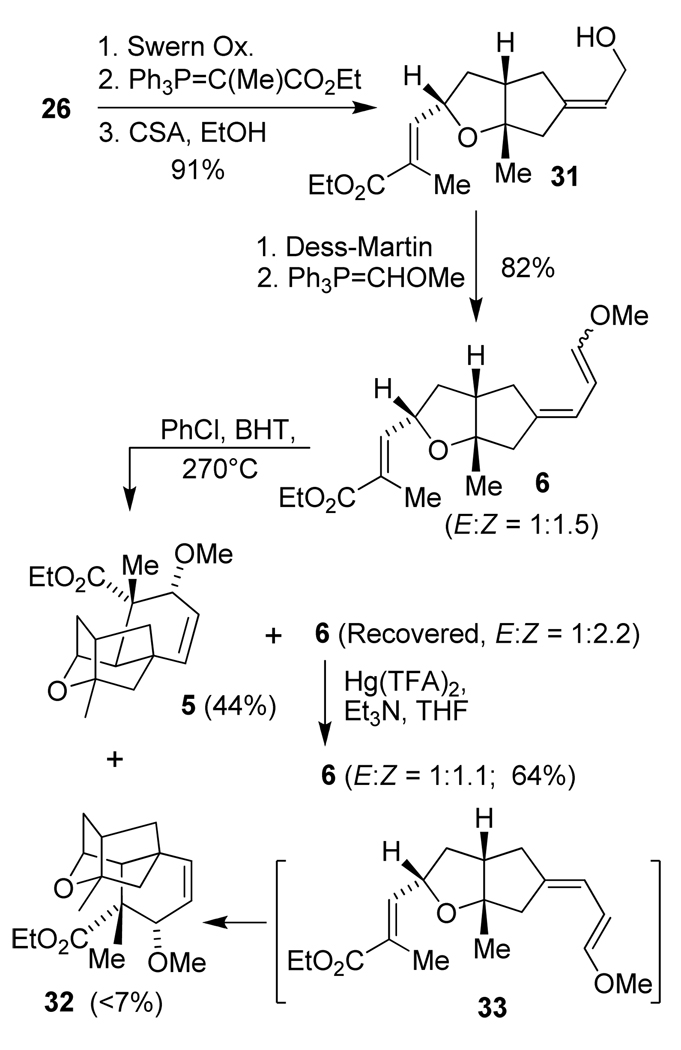
The successful intramolecular Diels-Alder reaction of enol ether 28 prompted us to incorporate the desired methyl group on the dienophile. The synthesis of the corresponding Diels-Alder substrate and its conversion to oxatetracyclic core 5 is shown in Scheme 5. Alcohol 31 containing an α-methyl ester was prepared in 91% yield over 3 steps following the similar route which yielded 27. It was converted to triene 6 in 82% yield as an inseparable mixture of E/Z enol ethers (2:3 ratio). This triene mixture was subjected to a thermal Diels-Alder reaction at 200 °C for 12 h. Unfortunately, the desired Diels-Alder adduct was not detected under these reaction conditions. This is possibly due to a steric interaction between the dienophile methyl group and the ring methylene in 6. This steric hindrance is much more severe when compared to that of the proton and the methylene group in compound 28. As a consequence, the activation energy of the reaction is higher and it may be necessary to increase the reaction temperature.47 The reaction temperature was raised to 270 ºC and heated at this temperature for 5 h. This provided the Diels-Alder adduct 5 in 36% isolated yield. The unreacted starting material 6 was isolated in 45% yield as a mixture of E/Z (1:2.2) enol ethers along with a small amount (<7%) of compound 32. The identity of compound 32 was established by an independent synthesis from triene 33 which was obtained as a minor isomer during the Horner-Wadsworth-Emmons olefination. Formation of 33 from 6 resulted after olefin isomerization. While we used BHT as a radical inhibitor in this reaction, it appears that the electron rich triene still underwent olefin isomerization to some degree.
Scheme 5.
Synthesis of core structure 5.
Mercury salts such as Hg(OAc)2 and Hg(TFA)2 have been widely used in transetherification and related annulation reactions.48 We explored the possibility of using a mercury salt to isomerize the major Z-enol ether present (E:Z = 4:9) in the recovered starting material 6. Recovered 6 was treated with a catalytic amount of Hg(TFA)2 in the presence of Et3N and stirred at 23 ºC for 4 h until the crude NMR showed nearly a 1:1 ratio of E/Z enol ethers. The yield was modest (< 64%) probably due to decomposition of the enol ether. The Diels-Alder reaction of this isomerized substrate 6 provided additional desired product 5 giving a combined yield of 44% for 5. Compound 32 was formed in less than 7% yield (by 1H-NMR analysis).
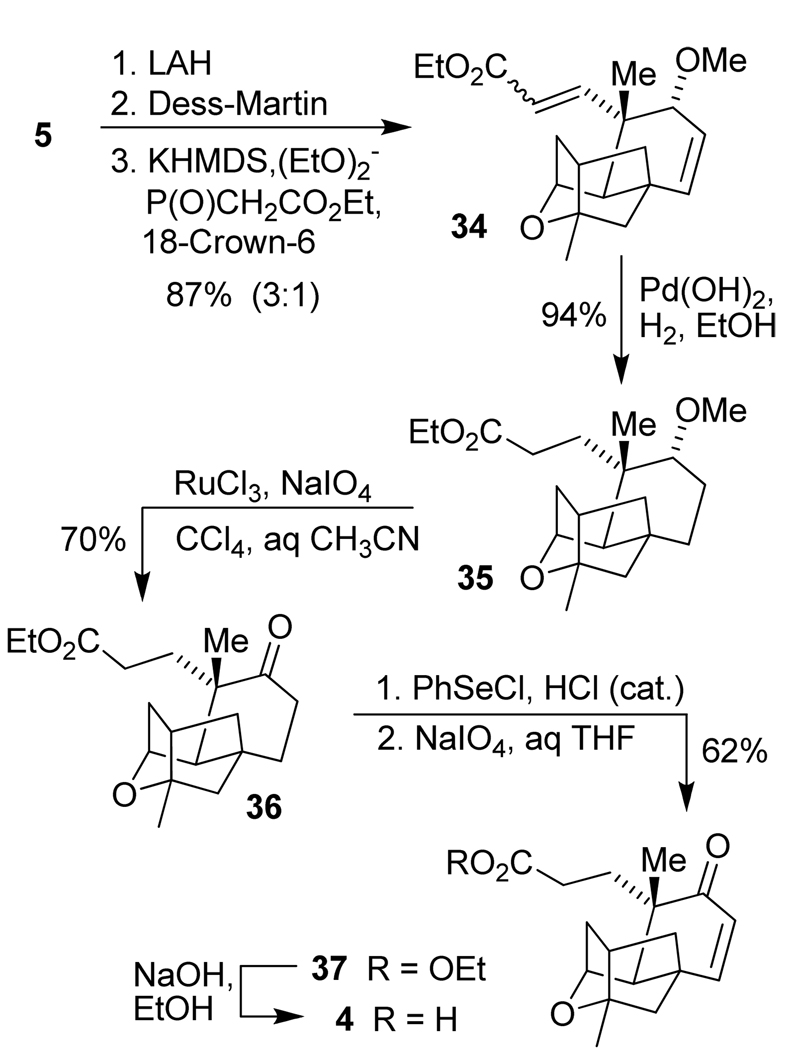
The synthesis of platensic acid 4 from compound 5 is outlined in Scheme 6. Reduction of the ester in 5 with LAH afforded the corresponding alcohol that was oxidized to the aldehyde intermediate with Dess-Martin periodinane. Horner-Wadsworth-Emmons olefination of this crude aldehyde was carried out in THF at reflux. Compound 34 was isolated in 87% yield over 3 steps as an inseparable mixture (3:1) of E/Z-unsaturated esters. Hydrogenation of 34 over Pearlman’s catalyst provided ester 35 which was subjected to a RuO4-based oxidation reaction.49 This successfully provided ketone 36 in 70% isolated yield. Ketone 36 was transformed to the corresponding TMS enol ether in 70% yield, however, oxidation of this enol ether with Pd(OAc)250 or IBX51 furnished enone 37 in very low yields (<20%). Ketone 36 was converted to the corresponding selenide with PhSeCl in EtOAc in the presence of 1 drop of concentrated HCl. Oxidation of this selenide derivative intermediate with NaIO4 gave enone 37 in 62% yield. Saponification of enone 37 with NaOH in EtOH provided platensic acid 4, which was used for the next step without further purification.
Scheme 6.
Synthesis of platensic acid 4.
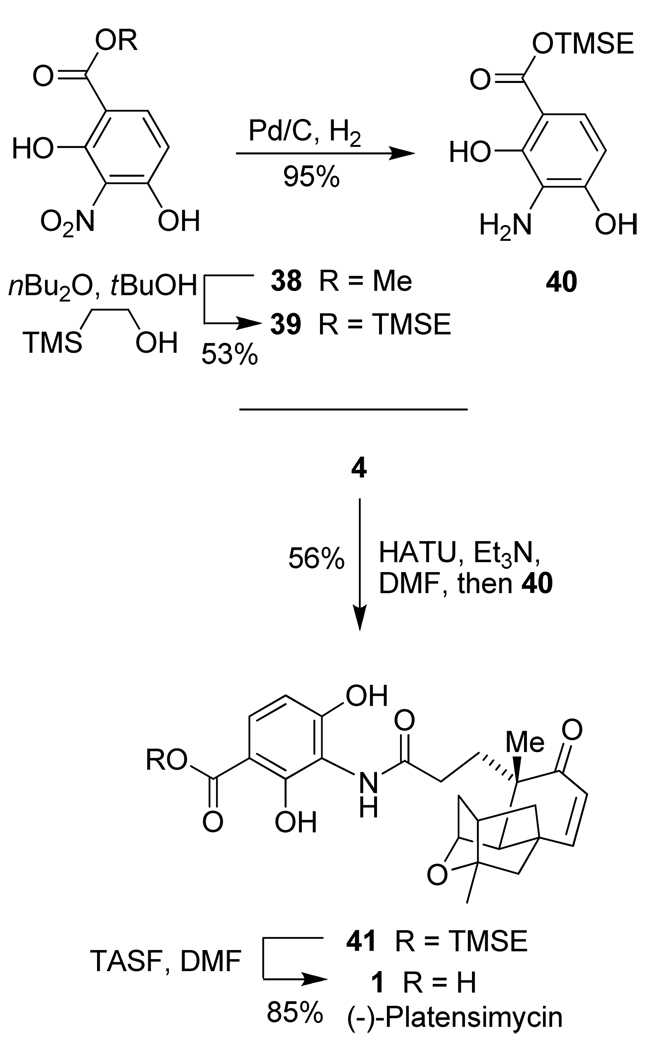
With platensic acid 4 in hand, we then focused on the synthesis of aniline derivative 40, utilized by Nicolaou and co-workers in their synthesis of platencin.26 Scheme 7 depicts the synthesis of TMSE-ester 40 and its subsequent conversion to platensimycin. The synthesis of trimethylsilylethyl ester 39 from methyl ester 38 was previously described by Giannis and co-workers.52 We modified the transesterification step by using only 2 equiv of 2-(trimethylsilyl)ethanol and an excess of bulky and cheap t-BuOH as the solvent.53 This reaction successfully provided the desired ester 39 in 53% yield. Hydrogenolysis of 39 over Pd-C furnished aniline fragment 40 in 95% yield. With both platensic acid 4 and aniline 40 in hand, the completion of platensimycin synthesis was carried out as follows. Platensic acid 4 was treated with Et3N and the peptide coupling reagent HATU in DMF for 10 min. A solution of aniline 40 in DMF was then added and the reaction mixture was stirred at 23 °C for 4 h. Amide 41 was isolated in 56% yield. Removal of the TMSE group with tris(dimethylamino)sulfonium difluorotrimethylsilicate ( TSAF) in DMF at 40 °C for 1 h furnished (-)-platensimycin 1 in 85% yield. Spectral data (1H- and 13C-NMR) and optical rotation ([α]D23 −49.2, c 0.24, MeOH) of the synthetic (-)-platensimycin are consistent with those reported for natural (-)-platensimycin ([α]D23 −51.1, c 0.13, MeOH).5
Scheme 7.
Synthesis of (−)-platensimycin 1.
Conclusion
In summary, we have achieved an enantioselective total synthesis of (-)-platensimycin. An intramolecular Diels-Alder reaction has been developed to construct the oxatetracyclic core of platensimycin. This Diels-Alder reaction has set three chiral centers including a quaternary carbon chiral center in a single operation. We have optimized the desired cycloaddition pathways by incorporation of a methoxy group on the diene, which prevented the undesired 1,5-hydride shift that led to a complex and undesired Diels-Alder adduct. We utilized (+)-carvone as the key starting material, which determined the stereochemistry of the final (-)-platensimycin. A modified protocol was developed for efficient preparation of bicyclic lactone 8 from (+)-carvone. Other key transformations include a Petasis olefination, a hydroboration sequence, a Gais’ asymmetric Horner-Wadsworth-Emmons reaction, and a mercury salt catalyzed enol ether isomerization reaction. Structural modifications of (-)-platensimycin are the subject of current investigation in our laboratories.
Experimental Section
Intramolecular Diels-Alder Products (5) and (32)
To a solution of 6 (627 mg, 2.05 mmol) in chlorobenzene (160 mL) was added BHT (46 mg, 0.21 mmol) at 23 °C. The reaction mixture was transferred into a sealed tube and heated to 270 °C for 5 h. The reaction was cooled to 23 °C concentrated in vacuo. Flash chromatography on silica gel (15% ethyl acetate in hexane with 1% Et3N) afforded 225 mg (0.74 mmol, 36%) of compound 5 and 282 mg (0.92 mmol, 45%) of a mixture of starting material 6 (E/Z = 4:9 by 1H-NMR analysis of crude mixture) and compound 32. To a solution of the above mixture (282 mg, 0.92 mmol) in THF (10 mL) at 23 °C was added Et3N (64 µL, 0.46 mmol) and Hg(TFA)2 (40 mg, 0.09 mmol). The reaction mixture was stirred at 23 °C for 4 h and then quenched with NaHCO3 (sat.). The aqueous layer was extracted with Et2O. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (15% ethyl acetate in hexanes with 1% Et3N) afforded 181 mg (0.6 mmol, 64%) of a mixture of E/Z (1:1.1) enol ethers 6 and compound 32 (~ 5:1). This mixture was dissolved in chlorobenzene (50 mL) and BHT (13 mg, 0.06 mmol) was added at 23 °C. The reaction mixture was put into a sealed tube and heated at 270 °C for 8 h. The reaction was cooled to 23 °C and the solution was concentrated in vacuo. Flash chromatography on silica gel (15% ethyl acetate in hexanes with 1% Et3N) afforded 40 mg (<7%) compound 32 and 52 mg (total 277 mg, 0.9 mmol, 44%) of compound 5. IR (neat) 1732, 1465, 1377; 1H NMR (500 MHz, CDCl3) δ 5.84 (dd, J = 10.0, 5.0 Hz, 1H), 5.55 (d, J = 10.0 Hz, 1H), 4.83 (brs, 1H), 4.18 (m, 2H), 3.45 (d, J = 5.5 Hz, 1H), 3.29 (s, 3H), 2.77 (brs, 1H), 2.25 (t, 1H), 2.00–1.94 (m, 1H), 1.88-1.82 (m, 2H), 1.79 (dd, J = 12.0, 4.0 Hz, 1H), 1.57-1.51 (m, 2H), 1.48 (d, J = 11.0 Hz, 1H), 1.41 (s, 3H), 1.30 (s, 3H), 1.28 (t, 3H); 13C NMR (125 MHz, CDCl3) δ 175.2, 138.6, 122.3, 87.1, 82.0, 78.8, 60.8, 57.7, 55.6, 48.5, 46.1, 44.9, 44.0, 42.8, 41.0, 23.6, 19.9, 14.7; HRMS (EI) [M]+ calcd for C18H26O4: 306.1831, found: 306.1830.
Unsaturated ester (34)
To a suspension of LiAlH4 (36 mg, 0.9 mmol) in Et2O (15 mL) at −40 °C was added a solution of 5 (277 mg, 0.9 mmol) in Et2O (5 mL). The reaction mixture was then stirred at 0 °C for 30 min. The reaction was quenched with a solution of potassium sodium tartrate and the mixture was vigorously stirred until the organic solution was clear. The aqueous layer was extracted with Et2O and the combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give the crude alcohol. To a stirred solution of the crude alcohol in CH2Cl2 (10 mL) was added NaHCO3 (400 mg) and Dess-Martin periodinane (500 mg, 1.17 mmol). The reaction mixture was stirred at 23 °C for 1 h. A solution of Na2S2O3 (2 g) in saturated NaHCO3 solution was added and the aqueous layer was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give the crude aldehyde. To a stirred solution of triethyl phosphonoacetate (1 mL, 4.98 mmol) and 18-crown-6 (2.4 g, 9 mmol) in THF (20 mL) at 0 °C was added KHMDS (0.5 M in toluene, 9 mL, 4.52 mmol) and a solution of the crude aldehyde in THF (5 mL). The reaction mixture was stirred at reflux for 12 h. The reaction was cooled to 23 °C and quenched with NH4Cl (sat.) and extracted with Et2O. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (20% ethyl acetate in hexane) afforded 261 mg (0.78 mmol, 87% over 3 steps) of 34 as a mixture of E/Z (3:1) unsaturated esters.
Saturated ester (35)
To a stirred solution of 34 (21 mg, 0.06 mmol) in EtOH (2 mL) was added Pd(OH)2/C (3.5 mg) in one portion. This reaction mixture was stirred under H2 at 23 °C for 12 h. The reaction mixture was filtered through celite and the filtrate was concentrated in vacuo. Flash chromatography on silica gel (8% ethyl acetate in chloroform) afforded 20 mg (0.78 mmol, 94%) of 35, 1H NMR (500 MHz, CDCl3) δ 4.32 (brs, 1H), 4.07 (q, 1H), 3.18 (s, 3H), 2.87 (d, J = 3.0 Hz, 1H), 2.24-2.15 (m, 3H), 1.98-1.88 (m, 3H), 1.88-1.80 (m, 2H), 1.80-1.66 (m, 2H), 1.66-1.60 (m, 1H), 1.58 (dd, J = 14.0, 4.0 Hz, 1H), 1.44 (dt, J = 3.5 Hz, 1H), 1.30 (s, 3H), 1.29 (d, J = 9.5 Hz, 1H), 1.23-1.18 (m, 1H), 1.20 (t, 3H), 0.98 (s, 3H), 0.91 (d, J = 10.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 174.4, 85.6, 82.0, 76.7, 60.1, 56.3, 55.4, 48.6, 45.3, 44.3, 43.3, 40.2, 38.0, 34.4, 28.4, 23.2, 22.5, 20.0, 14.1; HRMS (ESI) [M+Na]+ calcd for C20H32O4Na: 359.2198, found: 359.2201.
Ketone (36)
To a stirred solution of compound 35 (202 mg, 0.60 mmol) in CCl4 (8 mL), CH3CN (8 mL), and H2O (12 mL) was added NaIO4 (643 mg, 3 mmol) in one portion. The reaction mixture was stirred at 23 °C for 10 min before RuCl3 (15 mg, 0.06 mmol) was added. The reaction mixture was stirred at 23 °C for 48 h. The reaction was quenched with saturated NaHCO3 solution and extracted with EtOAc. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (40% ethyl ether in hexane) afforded 134 mg (0.42 mmol, 70%) of 36,[α]D23 −27.9 (c 0.38, CHCl3); IR (neat) 2360, 1730, 1173 cm−1; 1H NMR (500 MHz, CDCl3) δ 4.30 (brs, 1H), 4.08 (dq, J = 2.5 Hz, 1H), 2.54 (dt, J = 6.0 Hz, 1H), 2.32-2.22 (m, 4H), 2.04-1.92 (m, 5H), 1.82 (dt, J = 5.0 Hz, 2H), 1.77-1.69 (m, 1H), 1.62 (dd, J = 11.0, 3.5 Hz, 1H), 1.58 (dq, J = 14.0 Hz, 1H), 1.46-1.40 (m, 1H), 1.43 (d, J = 11.0 Hz, 1H), 1.37 (s, 3H), 1.22 (t, 3H), 1.18 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 214.5, 173.4, 86.1, 77.2, 60.3, 55.0, 50.0, 48.9, 44.6, 44.3, 42.5, 40.5, 36.1, 33.8, 32.8, 29.6, 23.4, 22.9, 14.1; HRMS (ESI) [M+Na]+ calcd for C19H28O4Na: 343.1888, found: 343.1887.
Enone (37)
To a stirred solution of 36 (16 mg, 0.05 mmol) in EtOAc (1 mL) was added PhSeCl (12 mg, 0.06) and 1 drop HCl (conc.). The reaction mixture was stirred at 23 °C for 5 h before saturated NaHCO3 solution was added. The aqueous layer was extracted with EtOAc and the combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by passing it through a short column of silica gel. To a stirred solution of the selenide intermediate in a mixture of THF (1 mL) and H2O (1.5 mL) was added NaIO4 (45 mg, 0.21 mmol). The reaction mixture was stirred at 23 °C for 12 h. THF was removed and the aqueous layer was extracted with EtOAc. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (25% ethyl ether in hexanes) afforded 10 mg (0.03 mmol, 62%) of 37,[α]D23 −28.1 (c 0.42, CHCl3); IR (neat) 2965, 1731, 1672, 1181 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.46 (d, J = 10.0 Hz, 1H), 5.88 (d, J = 10.0 Hz, 1H), 4.39 (brs, 1H), 4.10 (m, 2H), 2.40 (t, 1H), 2.35 (brs, 1H), 2.28-2.16 (m, 2H), 2.11-2.04 (m, 1H), 2.04-1.98 (m, 2H), 1.85 (dd, J = 11.0, 3.5 Hz, 1H), 1.78-1.69 (m, 2H), 1.60 (d, J = 11.0 Hz, 1H), 1.44 (s, 3H), 1.23 (t, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 203.1, 173.2, 153.3, 127.2, 86.9, 76.4, 60.3, 54.8, 46.2, 45.9, 44.6, 43.1, 40.5, 30.6, 29.2, 24.4, 22.9, 14.1; HRMS (ESI) [M+Na]+ calcd for C19H26O4Na: 341.1729, found: 341.1727.
(-)-Platensimycin TMSE ester (41)
To a stirred solution of enone 37 (15 mg, 0.05 mmol) in EtOH (1 mL) was added NaOH (1.0 M, 1 mL, 1.0 mmol). The reaction mixture was stirred at 23 °C for 30 min and then 1M HCl was added until pH ≈ 2. The mixture was extracted with CHCl3 and the combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude platensic acid 4 was used for the next step without further purification. 1H-NMR (500 MHz, CDCl3) δ 6.46 (d, J = 10.0 Hz, 1H), 5.88 (d, J = 10.0 Hz, 1H), 4.42 (brs, 1H), 4.08 (dq, J = 2.5 Hz, 1H), 2.40 (t, 1H), 2.35-2.31 (m, 2H), 2.30-2.21 (m, 2H), 2.12-2.05 (m, 1H), 2.01 (dd, J = 12.0, 4.0 Hz, 1H), 1.86 (dd, J = 11.0, 4.0 Hz, 1H), 1.79-1.69 (m, 2H), 1.60 (d, J = 11.0 Hz, 1H), 1.44 (s, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 203.2, 177.8, 153.5, 127.1, 87.1, 76.4, 54.7, 46.2, 45.8, 44.6, 43.0, 40.4, 30.4,28.8, 24.3, 22.8.
To a solution of the crude platensic acid 4 in DMF (0.2 mL) was added HATU (56 mg, 0.14 mmol) and Et3N (34 µL, 0.24 mmol). The reaction mixture was stirred at 23 °C for 10 min before a solution of 2-(trimethylsilyl)ethyl-3-amino-2,4-dihydroxybenzoate 40 (39 mg, 0.14 mmol) in DMF (0.2 mL) was added via cannula. The reaction mixture was stirred at 23 °C for 4 h then brine was added and the mixture was extracted with chloroform. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (15% acetone in hexanes) afforded 14.4 mg (0.03 mmol, 56%) of ester 41,[□]D23 −29.4 (c 0.51, CHCl3); IR (neat) 2360, 2338, 1697; 1H NMR (500 MHz, CDCl3) δ 11.80 (s, 1H), 11.03 (s, 1H), 8.09 (s, 1H), 7.55 (d, J = 9.0 Hz, 1H), 6.50 (d, J = 9.5 Hz, 2H), 5.92 (d, J = 10.0 Hz, 1H), 4.42 (m, 3H), 2.52 (ddd, J = 14.5, 11.5, 6.0 Hz, 1H), 2.45-2.31 (m, 3H), 2.14-2.07 (m, 1H), 2.06 (dd, J = 12.0, 3.5 Hz, 1H), 2.10 (d, J = 11.5 Hz, 1H), 1.90 (dd, J = 12.0, 6.0 Hz, 1H), 1.89 (dd, J = 11.5, 3.5 Hz, 1H), 1.78 (dd, J = 11.5, 6.5 Hz, 1H), 1.63 (d, J = 11.0 Hz, 1H), 1.45 (s, 3H), 1.27 (s, 3H), 1.13 (m, 2H), 0.08 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 203.5, 173.5, 170.4, 154.6, 153.8, 127.2, 127.0, 114.2, 111.0, 104.3, 86.9, 76.3, 63.6, 54.8, 46.6, 46.1, 46.0, 44.5, 43.0, 40.5, 32.0, 31.5, 24.1, 22.9, 17.3, −1.6; HRMS (ESI) [M+Na]+ calcd for C29H39NO7SiNa: 564.2394, found: 564.2396.
(-)-Platensimycin (1)
To a solution of compound 41 (10 mg, 0.02 mmol) in DMF (0.2 mL) was added TASF (10 mg, 0.04 mmol). The reaction mixture was stirred at 40 °C for 1 h. The reaction was cooled to 23 °C then brine was added and the mixture was extracted with CHCl3. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (60% acetone in hexanes followed by acetone, hexanes, acetic acid 60:40:1) afforded 7 mg (0.016 mmol, 85%) of (-)-platensimycin 1, [α]D23 −49.2 (c 0.24, MeOH); 1H NMR (500 MHz, C5D5N) δ 10.48 (s, 1H), 8.09 (d, J = 8.5 Hz, 1H), 6.85 (d, J = 9.0 Hz, 1H), 6.34 (d, J = 10.0 Hz, 1H), 5.92 (d, J = 10.0 Hz, 1H), 4.46 (s, 1H), 2.86-2.77 (m, 1H), 2.77-2.62 (m, 2H), 2.42 (s, 1H), 2.17 (t, 1H), 2.06-1.97 (m, 1H), 1.92-1.84 (m, 1H), 1.79 (d, J = 11.5 Hz, 1H), 1.70 (d, J = 10.5 Hz, 1H), 1.58-1.51 (m, 1H), 1.46 (d, J = 11.0 Hz, 1H), 1.37 (s, 3H), 1.12 (s, 3H); 13C NMR (125 MHz, C5D5N) δ 203.2, 174.7, 174.4, 158.3,158.0, 154.0, 129.4, 127.2, 115.3, 110.0, 107.1, 86.8, 76.4, 55.0, 46.7, 46.6, 46.1, 45.0, 43.0, 40.8, 32.1, 31.8, 24.4, 23.2; HRMS (ESI) [M+Na]+ calcd for C24H27NO7Na: 464.1685, found: 464.1686.
Supplementary Material
Acknowledgment
Financial support of this work was provided in part by the National Institutes of Health and Purdue University. The authors thank Dr. Phillip E. Fanwick (Purdue University) for assistance with the X-ray crystal structure analysis.
Footnotes
Supporting Information Available: Full experimental details and characterization of selected compounds; copies of 1H and 13C NMR spectra of selected compounds; comparison of 1H and 13C NMR spectra of synthetic and natural (−)-platensimycin. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Walsh C. Antibiotics: actions, origins, resistance. Washington, D.C.: ASM press; 2003. [Google Scholar]
- 2.Singh SB, Barrett JF. Biochem. Pharmacol. 2006;71:1006–1015. doi: 10.1016/j.bcp.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Butler MS, Buss AD. Biochem. Pharmacol. 2006;71:919–929. doi: 10.1016/j.bcp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang CW, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 5.Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang CW, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. J. Am. Chem. Soc. 2006;128:11916–11920. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- 6.Habich D, von Nussbaum F. ChemMedChem. 2006;1:951–954. doi: 10.1002/cmdc.200600145. [DOI] [PubMed] [Google Scholar]
- 7.Young K, Jayasuriya H, Ondeyka JG, Herath K, Zhang CW, Kodali S, Galgoci A, Painter R, Brown-Driver V, Yamamoto R, Silver LL, Zheng YC, Ventura JI, Sigmund J, Ha S, Basilio A, Vicente F, Tormo JR, Pelaez F, Youngman P, Cully D, Barrett JF, Schmatz D, Singh SB, Wang JAntimicrob. Agents Chemother. 2006;50:519–526. doi: 10.1128/AAC.50.2.519-526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herath KB, Attygalle AB, Singh SB. J. Am. Chem. Soc. 2007;129:15422–15423. doi: 10.1021/ja0758943. [DOI] [PubMed] [Google Scholar]
- 9.Tiefenbacher K, Mulzer J. Angew. Chem.,Int.Ed. 2008;47:2548–2555. doi: 10.1002/anie.200705303. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaou KC, Li A, Edmonds DJ. Angew. Chem. Int. Ed. 2006;45:7086–7090. doi: 10.1002/anie.200603892. [DOI] [PubMed] [Google Scholar]
- 11.Zou YF, Chen CH, Taylor CD, Foxman BM, Snider BB. Org. Lett. 2007;9:1825–1828. doi: 10.1021/ol070563g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew. Chem. Int. Ed. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou KC, Tang YF, Wang JH. Chem. Commun. 2007:1922–1923. doi: 10.1039/b704589a. [DOI] [PubMed] [Google Scholar]
- 14.Li PF, Payette JN, Yamamoto HJ. Am. Chem. Soc. 2007;129:9534–9535. doi: 10.1021/ja073547n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalic G, Corey EJ. Org. Lett. 2007;9:4921–4923. doi: 10.1021/ol702323s. [DOI] [PubMed] [Google Scholar]
- 16.Tiefenbacher K, Mulzer J. Angew. Chem. Int. Ed. 2007;46:8074–8075. doi: 10.1002/anie.200702852. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Jang KP, Choi SY, Chung YK, Lee E. Angew. Chem. Int. Ed. 2008;47:4009–4011. doi: 10.1002/anie.200800568. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo J-i, Takeuchi K, Ishibashi H. Org. Lett. 2008;10:4049–4052. doi: 10.1021/ol801584r. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou KC, Pappo D, Tsang KY, Gibe R, Chen DYK. Angew. Chem. Int. Ed. 2008;47:944–946. doi: 10.1002/anie.200705080. [DOI] [PubMed] [Google Scholar]
- 20.Nicolaou KC, Lister T, Denton RM, Montero A, Edmonds DJ. Angew. Chem. Int. Ed. 2007;46:4712–4714. doi: 10.1002/anie.200701548. [DOI] [PubMed] [Google Scholar]
- 21.Singh SB, Herath KB, Wang J, Tsou N, Ball RG. Tetrahedron Lett. 2007;48:5429–5433. [Google Scholar]
- 22.Nicolaou KC, Tang YF, Wang JH, Stepan AF, Li A, Montero A. J. Am. Chem. Soc. 2007;129:14850–14851. doi: 10.1021/ja076126e. [DOI] [PubMed] [Google Scholar]
- 23.Yeung Y-Y, Corey E. J. Org. Lett. 2008;10:3877–3878. doi: 10.1021/ol801400a. [DOI] [PubMed] [Google Scholar]
- 24.Nicolaou KC, Stepan AF, Lister T, Li A, Montero A, Tria GS, Turner CI, Tang Y, Wang J, Denton RM, Edmonds DJ. J. Am. Chem. Soc. 2008;130:13110–13119. doi: 10.1021/ja8044376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh AK, Xi K. Org. Lett. 2007;9:4013–4016. doi: 10.1021/ol701783z. [DOI] [PubMed] [Google Scholar]
- 26.Nicolaou KC, Tria GS, Edmonds D. J. Angew. Chem. Int. Ed. 2008;47:1780–1783. doi: 10.1002/anie.200800066. [DOI] [PubMed] [Google Scholar]
- 27.Herath KB, Zhang C, Jayasuriya H, Ondeyka JG, Zink DL, Burgess B, Wang J, Singh SB. Org.Lett. 2008;10:1699–1702. doi: 10.1021/ol800251v. [DOI] [PubMed] [Google Scholar]
- 28.Fallis AG. Can. J. Chem. 1984;62:183–234. [Google Scholar]
- 29.Wittig G, Schollkopf U. Chem. Ber. 1954;87:1318–1330. [Google Scholar]
- 30.Wittig G, Haag W. Chem. Ber. 1955;88:1654–1666. [Google Scholar]
- 31.Horner L, Hoffmann H, Wippel HG. Chem. Ber. 1958;91:61–63. [Google Scholar]
- 32.Wadsworth W, Emmons WD. J. Am. Chem. Soc. 1961;83:1733–1738. [Google Scholar]
- 33.Petasis NA, Bzowej EI. J. Am.Chem. Soc. 1990;112:6392–6394. [Google Scholar]
- 34.Weinges K, Reichert H. Synlett. 1991:785–786. [Google Scholar]
- 35.Weinges K, Reichert H, Huberpatz U, Irngartinger H. Liebigs Ann. Chem. 1993:403–411. [Google Scholar]
- 36.Weinges K, Reichert H, Braun R. Chem. Ber. 1994;127:549–550. [Google Scholar]
- 37.Srikrishna A, Hemamalini P. J. Org. Chem. 1990;55:4883–4887. [Google Scholar]
- 38.Omura K, Swern D. Tetrahedron. 1978;34:1651–1660. [Google Scholar]
- 39.Cooper MS, Heaney H, Newbold AJ, Sanderson WR. Synlett. 1990:533–535. [Google Scholar]
- 40.Lambert WT, Hanson GH, Benayoud F, Burke SD. J. Org. Chem. 2005;70:9382–9398. doi: 10.1021/jo051479m. [DOI] [PubMed] [Google Scholar]
- 41.Crouch RD. Tetrahedron. 2004;60:5833–5871. [Google Scholar]
- 42.Tanemura K, Suzuki T, Horaguchi T. J. Chem. Soc., Perkin Trans. 1992:2997–2998. [Google Scholar]
- 43.Comins DL, Salvador JM. J. Org. Chem. 1993;58:4656–4661. [Google Scholar]
- 44.Hatakeyama S, Satoh K, Sakurai K, Takano S. Tetrahedron Lett. 1987;28:2713–2716. [Google Scholar]
- 45.Gais HJ, Schmiedl G, Ossenkamp RKL. Liebigs Ann./Recl. 1997:2419–2431. [Google Scholar]
- 46.Coppinger GM. J. Am.Chem. Soc. 1964;86:4385–4388. [Google Scholar]
- 47.We did not examine Lewis acid catalysts for substrate 6, because of our attempts with Lewis acid catalyzed reaction with aldehyde 22 and some other designed substrates were unsuccessful. Also, we were concerned about the stability of the enol ether in the diene moiety in the presence of Lewis acids.
- 48.Watanabe WH, Conlon LE. J. Am. Chem. Soc. 1957;79:2828–2833. [Google Scholar]
- 49.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J. Org. Chem. 1981;46:3936–3938. [Google Scholar]
- 50.Ito Y, Hirao T, Saegusa T. J. Org. Chem. 1978;43:1011–1013. [Google Scholar]
- 51.Nicolaou KC, Gray DLF, Montagnon T, Harrison ST. Angew. Chem. Int. Ed. 2002;41:996–1000. doi: 10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Heretsch P, Giannis A. Synthesis. 2007:2614–2616. [Google Scholar]
- 53.Baumhof P, Mazitschek R, Giannis A. Angew. Chem. Int. Ed. 2001;40:3672–3674. doi: 10.1002/1521-3773(20011001)40:19<3672::aid-anie3672>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.