Abstract
Haloduracin, a recently discovered two-peptide lantibiotic composed of the post-translationally modified peptides Halα and Halβ, is shown to have high potency against a range of Gram-positive bacteria and to inhibit spore outgrowth of Bacillus anthracis. The two peptides display optimal activity in a 1:1 stoichiometry and have efficacy similar to that of the commercially used lantibiotic nisin. However, haloduracin is more stable at pH 7 than nisin. Despite significant structural differences between the two peptides of haloduracin and those of the two-peptide lantibiotic lacticin 3147, these two systems show similarities in their mode of action. Like Ltnα, Halα binds to a target on the surface of Gram-positive bacteria, and like Ltnβ, the addition of Halβ results in pore formation and potassium efflux. Using Halα mutants, its B- and C-thioether rings are shown to be important but not required for bioactivity. A similar observation was made with mutants of Glu22, a residue that is highly conserved among several lipid II-binding lantibiotics such as mersacidin.
The continuing appearance of multidrug-resistant bacteria poses a significant threat to human health and presents an urgent need for the development of new antimicrobial drugs. Lantibiotics, a diverse and promising class of antimicrobial peptides produced by a variety of bacteria, display a broad spectrum of activity (1,2). Over 50 members have been identified to date, with several displaying potent antimicrobial activity at nanomolar levels against nosocomial pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and oxacillin-resistant Gram-positives (2,3). Importantly, development of significant levels of resistance has not been observed for nisin, the best-studied lantibiotic that has been used worldwide against food-born pathogens for more than four decades (4).
Lantibiotics are ribosomally synthesized as inactive precursors that undergo extensive enzyme-mediated post-translational modifications to their biologically active forms (3). The inactive precursor peptides (LanA) consist of an unmodified N-terminal leader sequence and a C-terminal structural region that is modified to the mature lantibiotic. The molecular details of the function of the leader sequence remain unclear (5); however, in addressed cases it is required for complete maturation of the structural region and for secretion of the bioactive product (3). Modifications observed in all lantibiotics involve the dehydration of select serine and threonine residues to produce dehydroalanine (Dha) and dehydrobutyrine (Dhb) residues, respectively. Following dehydration, an intramolecular Michael-type addition of cysteine thiols onto the unsaturated amino acids generates lanthionine (Lan) and methyllanthionine (MeLan) thioether rings (1).
Two-peptide lantibiotics function through the activities of two peptides whereby each peptide contributes specific roles in antimicrobial activity. In these systems, two prepeptides are ribosomally synthesized as inactive precursors (LanA1 and LanA2), which are both enzymatically modified to their mature, bioactive forms (Lanα and Lanβ). For all currently known two-peptide lantibiotics, the dehydration of hydroxyl-amino acids and subsequent cyclization to form (methyl)lanthionine residues results from the actions of a bifunctional modification enzyme termed LanM. In most two-peptide systems, the sequence homology between the LanA1 and LanA2 prepeptides is low; therefore two different bifunctional enzymes (LanM1 and LanM2) each act specifically on only one of the prepeptides (6,7). The modified LanA1 and LanA2 prepeptides are further processed by a LanT protein, which removes the leader peptides and secretes the final products. The mature peptides of several two-peptide systems share structural and sequence homology with known single-peptide lantibiotics. The mature α-peptides resemble the globular lantibiotic mersacidin with several fused thioether rings, whereas the mature β-peptides are typically elongated and more flexible (8,9) (1).
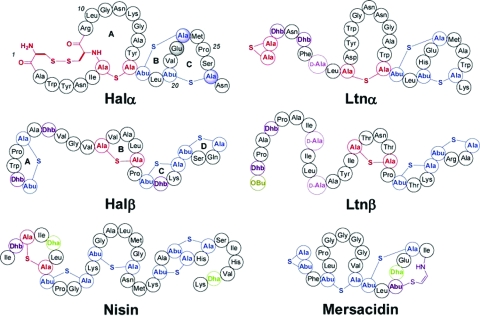
Figure 1.
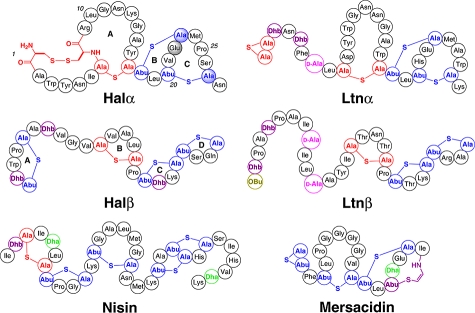
Structures of haloduracin, lacticin 3147, nisin, and mersacidin. Shaded circles of Halα indicate residues that were mutated in this study (Glu22, Cys23, and Cys27). Ala-S-Ala represents lanthionine, Abu-S-Ala represents methyllanthionine, and OBu represents 2-oxobutyryl.
Both nisin (10) and mersacidin (11) target the membrane-bound cell wall precursor lipid II, resulting in the inhibition of peptidoglycan biosynthesis (12) and, for nisin but not mersacidin, pore formation (13). Binding to lipid II inhibits peptidoglycan biosynthesis by physical sequestration, preventing the actions of transglycosylase and transpeptidase enzymes that polymerize lipid II and cross-link the glycan chains of the nascent cell wall, respectively (13). In a second mode of action, lipid II is utilized as a docking molecule for the generation of defined and stable pores that result in membrane damage and depolarization and ultimately cell death (14). An additional activity for several lantibiotics including nisin is the ability to prevent spore outgrowth of Gram-positive bacteria (15,16). A recent study suggested that inhibition of outgrowth is directly related to the ability of the lantibiotic to induce damage to the membrane of germinating spores, thereby preventing the establishment of oxidative metabolism and a membrane potential (17).
Haloduracin, a two-peptide lantibiotic recently discovered by genome mining, is produced by the Gram-positive alkaliphilic bacterium Bacillus halodurans C-125 (6,18). The lantibiotic consists of two post-translationally modified peptides, Halα and Halβ. Here we describe studies investigating the mode of action of haloduracin and compare it to nisin, the prototypical lantibiotic.
Results and Discussion
Haloduracin Peptides Function Optimally at a 1:1 Ratio
Prior to assessing the specific activity of haloduracin against a panel of bacterial strains, the ratio of Halα and Halβ that yields maximum bioactivity was established. For this purpose, the ability of Halα and Halβ to inhibit cell growth at varying concentrations and ratios was examined. As indicated by the resultant isobologram (2), haloduracin is active at nanomolar levels against the indicator strain Lactococcus lactis HP, and Halα and Halβ display optimal activity at a 1:1 ratio. This ratio was determined as the lowest concentration of individual Halα or Halβ peptide that when combined caused essentially complete growth inhibition (defined as >90%). The optimal synergy of Halα and Halβ at a 1:1 ratio is shared with other two-peptide lantibiotic systems such as staphylococcin C55, plantaricin W, and lacticin 3147 (19−21).
Figure 2.
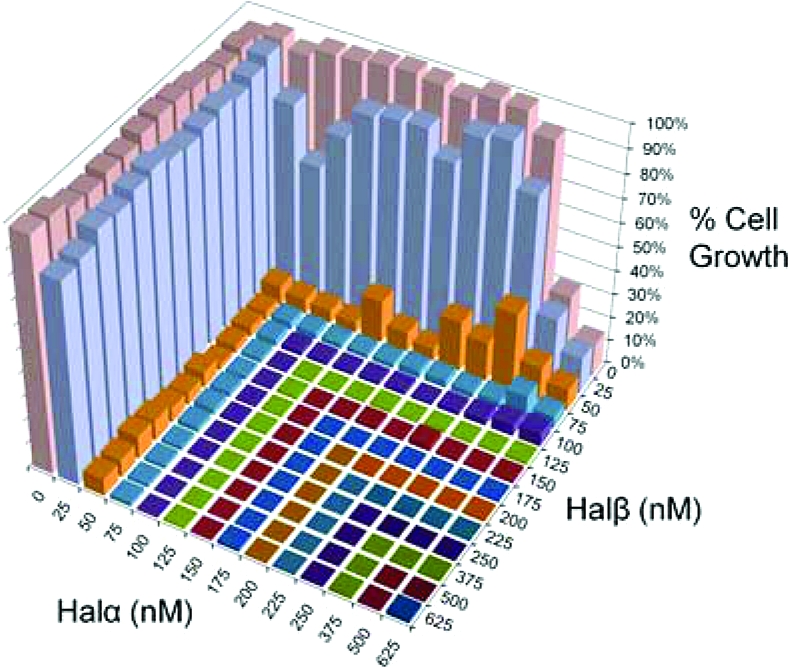
Isobologram indicating the concentrations and ratio of Halα and Halβ required to inhibit the growth of the indicator strain Lactococcus lactis HP.
Although the haloduracin peptides are most active in combination, at much higher concentrations both Halα and Halβ displayed independent activity against the highly susceptible L. lactis HP strain. Using liquid media growth assays, Halα and Halβ had independent IC50 values of 1031 ± 13 and 3146 ± 81 nM, respectively, corresponding to a 50- to 100-fold decrease in efficacy as compared to when the peptides were used together (1). Though further experiments will be necessary to fully identify how these peptides are able to possess bioactivity in the absence of their counterpart, we speculate on the basis of the structural similarity of Halα with mersacidin (1) that Halα likely binds to lipid II, thereby preventing peptidoglycan biosynthesis. Halβ may exert its independent activity through a mechanism similar to that of nisin in the absence of lipid II, in which the cationic Halβ peptide binds to anionic lipids of the cell membrane and the aggregation of Halβ monomers causes the transient formation of pore-like structures in the bacterial cell membrane (13).
Table 1. Comparison of haloduracin and nisin activities.
| Specific activity of haloduracin and nisin assessed by liquid growth inhibition assay | |||||
|---|---|---|---|---|---|
| Haloduracin |
Nisin |
||||
| Strain | Source | IC50 (nM) | MIC (nM) | IC50 (nM) | MIC (nM) |
| Lactococcus lactis HP | ATCCa 11602 | 29 ± 21 | 73.4 | 14 ± 5 | 32.2 |
| Lactococcus lactis 481 | CNRZb 481 | 63 ± 24 | 195 | 68 ± 6 | 127 |
| Lactococcus lactis 11454 | ATCC 11454 | 308 ± 13 | 625 | 1,759 ± 400 | 2,800 |
| Vancomycin-resistant Enterococcus faecium | C33105c | 265 ± 15 | 781 | 4,953 ± 187 | 12,500 |
| Bacillus anthracis Sterne 7702 | Gut et al.d | 363 ± 54 | 677 | 311 ± 216 | 417 |
| Bacillus subtilis | ATCC 6633 | 262 ± 54 | 469 | 414 ± 165 | 820 |
| methicillin-resistant Staphylococcus aureus | C5c | 2,616 ± 396 | 4,690 | 1,459 ± 7 | 1,560 |
| Staphylococcus aureus | ATCC 12600 | 1,144 ± 290 | 1,560 | 2,205 ± 523 | 4,690 |
| Staphylococcus epidermidis 15X | Ekkelenkamp et al.e | 273 ± 23 | 313 | 368 ± 152 | 508 |
| Micrococcus luteus | ATCC 4698 | 520 ± 17 | 1,250 | 266 ± 92 | 410 |
| Streptococcus mutans | ATCC 25175 | 1,837 ± 417 | 2,500 | 5,394 ± 732 | 12,500 |
| Activity assessed by agar diffusion growth inhibition assay | |||
|---|---|---|---|
| Strain | Source | Haloduracin | Nisin |
| Lactococcus lactis 117 | CNRZ 117 | + | + |
| Bacillus subtilis DB104 | Kawamura et al.f | + | + |
| Bacillus subtilis LH45 | Liu et al.g | + | + |
| Bacillus cereus Z4222 | INRAh Z4222 | + | + |
| Bacillus cereus TZ415 | INRA TZ415 | + | + |
| Enterobacter cloacae 10-19C | ATCC 29893 | − | − |
| Escherichia coli DH5α | UIUC-CMFi | − | − |
ATCC: American type Culture Collection.
CNRZ: National Centre for Zootechnical Research.
Clinical isolates from Carle Foundation Hospital (Urbana, IL).
Reference (17).
Reference (43).
Reference (44).
Reference (45).
INRA: Institut National de la Recherche Agronomique.
UIUC-CMF: University of Illinois Urbana-Champaign Cell and Media Facility.
Comparison of the Antimicrobial Potency of Haloduracin and Nisin
Agar diffusion growth inhibition assays were performed against a panel of Gram-positive and Gram-negative bacteria to obtain qualitative information regarding their sensitivity to treatment with haloduracin and nisin. These preliminary studies provided a binary outcome of either cell growth inhibition or proliferation. Strains that showed susceptibility toward haloduracin and nisin were subjected to growth inhibition assays in liquid media from which quantitative IC50 values were determined. Haloduracin and nisin shared a similar spectrum of inhibitory activity against a wide range of Gram-positive organisms but lacked potency against the Gram-negative bacteria tested (1). The concentrations of haloduracin and nisin required to inhibit several strains revealed that the effectiveness of the antimicrobial peptides is target-strain-dependent. For example, L. lactis HP was quite sensitive to both lantibiotics with IC50 values in the low-nanomolar range (29 ± 21 and 14 ± 5 nM for haloduracin and nisin, respectively). Some strains were more sensitive to haloduracin than to nisin (vancomycin-resistant Enterococcus) and vice versa (Micrococcus luteus). However, in most cases, only a 2- or 3-fold difference was observed in the IC50 values of haloduracin and nisin against a particular strain, and overall, haloduracin and nisin activity was roughly equivalent against the panel of strains examined in this study.
Enhanced Stability of Haloduracin as Compared with Nisin
Nisin shows promising activities in vitro and in mouse models against several clinically relevant bacteria strains (22); however, its inherent chemical instability (23,24) and poor solubility at physiological pH have prevented therapeutic development. Nisin is produced by acidophilic Lactococcus lactis strains that grow and produce the compound in highest quantities in an acidic environment. Accordingly, nisin has been shown to exhibit highest solubility and stability at low pH (25). A number of degradation products of nisin have been reported (23,24,26). In a previous study, nisin stability was systematically monitored in low buffer concentrations over a pH range of 2−8. The results showed that nisin is most stable at pH 3, and its stability was drastically compromised at both lower and higher pH values. Moreover, at pH 7 and 8, essentially complete degradation was observed in 10 days (27).
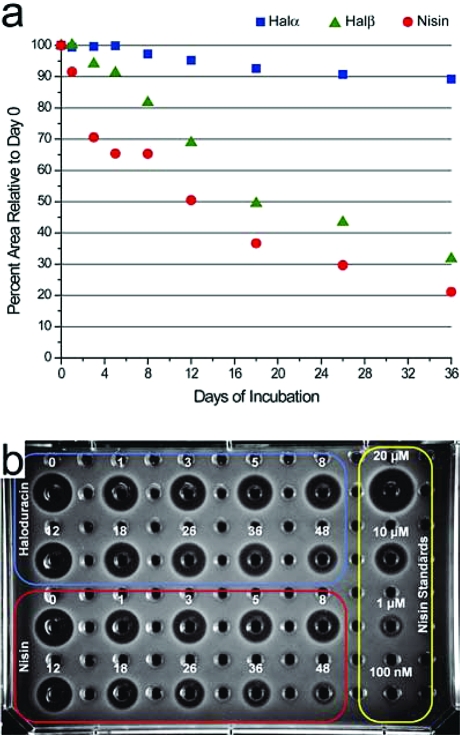
Haloduracin was discovered during a search for alkaliphilic lantibiotic-producing bacteria. Its producer, Bacillus halodurans C-125, flourishes in basic environments with a pH greater than 9.5. For this reason, we speculated that haloduracin may have improved chemical stability compared to nisin at neutral pH. We monitored the stability of Halα, Halβ, and nisin in a solution of low buffer concentration at pH 7.5 using HPLC and MALDI-TOF MS analysis (3, panel a). Halα displayed remarkable stability with >90% of intact peptide remaining after 36 days. The globular nature of this peptide, in addition to its low number of residues capable of undergoing oxidation, may contribute to its overall stability. Halβ showed moderate stability as compared with Halα, and the degradation of this linear peptide occurred gradually over the course of this assay. MS analysis of the additional peaks that were observed by HPLC shows that the products are mostly the result of oxidation (M + 16); breakdown of the oligopeptide chain was not observed. Nisin displayed the poorest stability of the three with many oxidation products observed at early time points followed by significant degradation of the peptide chain. The increased susceptibility toward oxidation of both Halβ and nisin may be due to their high number of thioether bridges. Agar diffusion assays were utilized to evaluate the antimicrobial activities of haloduracin and nisin over time, using the same samples that were analyzed by HPLC. As shown in 3, panel b, oxidation and degradation of nisin appears to have a much stronger deleterious effect on its bioactivity than oxidation of Halβ has on the antimicrobial activity of haloduracin. The observed activity is not due to solo activity of Halα, because Halα has much weaker activity in the absence of Halβ. It appears, therefore, that the structural requirements for Halβ activity are not critically dependent on unoxidized thioether cross-links.
Figure 3.
Haloduracin displays greater stability than nisin at physiological pH. (a) Assessment of relative stabilities of Halα, Halβ, and nisin at pH 7.5 using HPLC and MS analysis. (b) Bioassay against Lactococcus lactis HP using the same samples as analyzed by HPLC in panel a. The day of incubation is denoted above each well. Haloduracin: upper left (blue), nisin: lower left (red), nisin standards: right (yellow).
Halα and Halβ Act Sequentially To Affect Target Cells
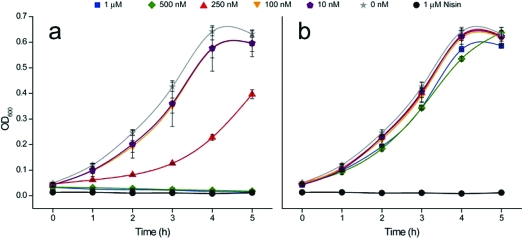
To address the potential roles of the individual peptides of haloduracin, we examined the order of binding events required to observe cell growth inhibitory activity. When target cells were incubated with Halα, followed by stringent washing to remove unbound peptide and subsequent incubation with Halβ, growth inhibition was observed (4, panel a). When the order of addition was reversed and cells were first exposed to Halβ, washed, and then treated with Halα, growth inhibition was not observed (4, panel b). When Halα and Halβ peptides were added at the same time, growth inhibition was observed (data not shown). The described outcome of sequential addition is similar to that observed for the two-peptide lantibiotic lacticin 3147 (21) and suggests a scenario in which Halα binds a target on the outer surface of the bacterial cell and the resulting complex is required for Halβ to exert its synergistic effect.
Figure 4.
Sequential activity of haloduracin peptides against L. lactis HP. Growth curves are shown for (a) Halα treatment, washing, and Halβ addition and (b) Halβ treatment, washing, and Halα addition. When errors bars are not visible, the error was smaller than the size of the symbol used.
Membrane Depolarization by Haloduracin
Given the known pore-forming activity of several lantibiotics, we examined the effect of haloduracin on membrane potential via flow cytometry with DiOC2, a membrane-potential-sensitive dye. Under physiological conditions, bacteria with intact cytoplasmic membranes maintain a membrane potential on the order of 100−200 mV (28). As shown in 5, haloduracin rapidly (within 10 min) dissipated the membrane potential of B. subtilis cells in a concentration-dependent manner and at levels comparable to or lower than nisin. The extensive dissipation of membrane potential likely reflects damage of the cell membrane.
Figure 5.
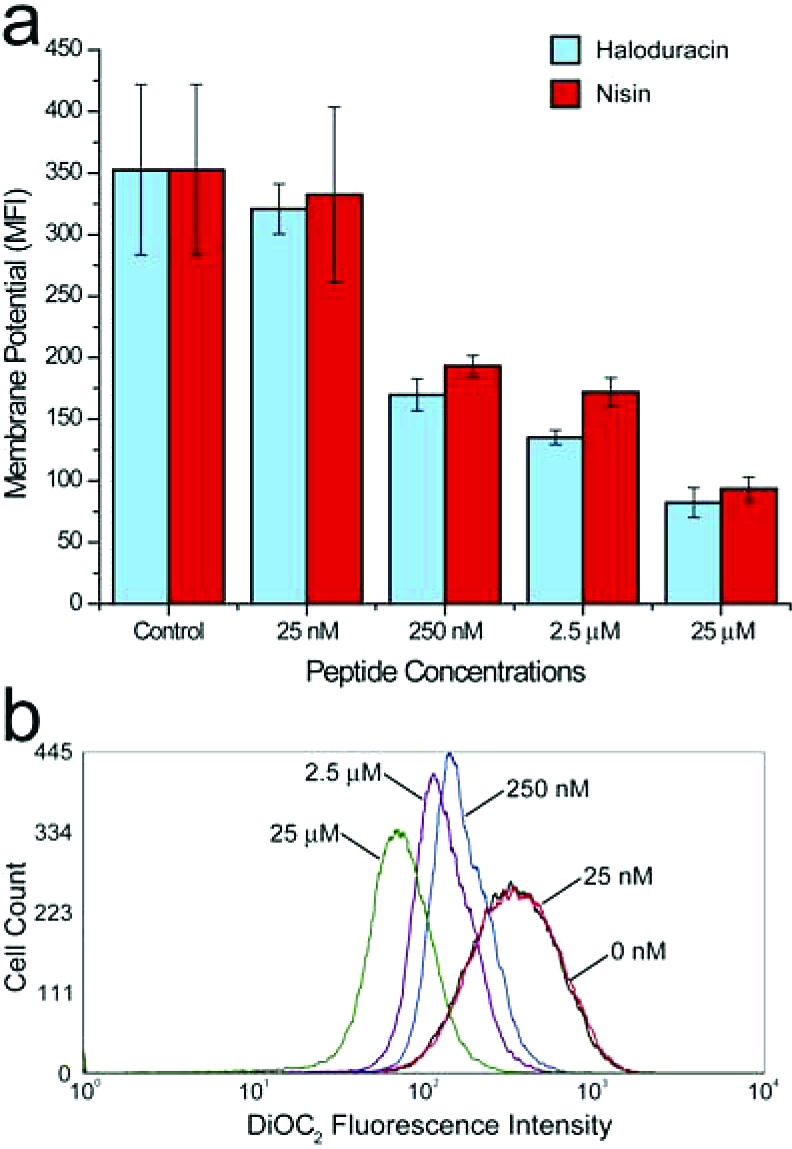
Haloduracin and nisin display comparable membrane depolarization activities. a) Mean fluorescence intensity (MFI) for haloduracin and nisin over a range of concentrations. b) Representative histogram of cell count versus DiOC2 fluorescence intensity for haloduracin. Populations shown are for 0 nM (control, black), 25 nM (red), 250 nM (blue), 2.5 μM (violet), and 25 μM haloduracin (green).
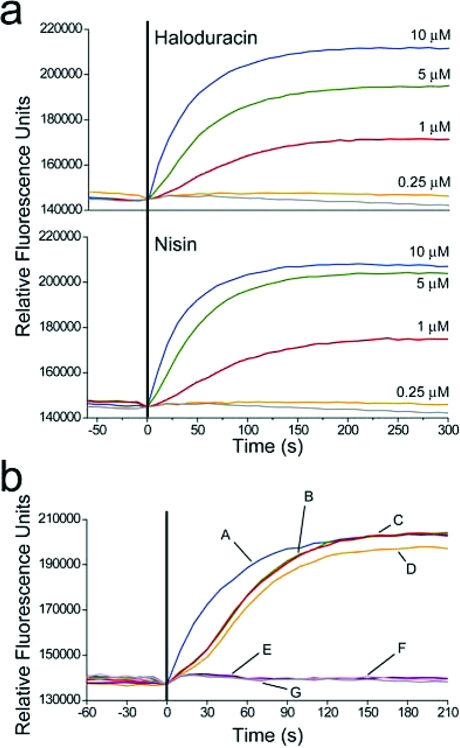
Pore Formation by Haloduracin and Efflux of Potassium Ions
The effects of haloduracin on membrane potential prompted the investigation of potassium efflux using a cell impermeable, potassium-sensitive dye (PBFI). Treatment of a M. luteus cell suspension with Halα and Halβ triggered rapid efflux of intracellular potassium (6, panel a). Nearly identical results were observed for other Gram-positive strains such as Bacillus subtilis ATCC 6633 (data not shown). Potassium ion release was detected after a short lag time followed by a rapid signal increase that reached a plateau within 4−5 min. The observed potassium release was dose-dependent, and efflux was not detected in untreated samples. When cells were treated with Halα or Halβ alone at low micromolar concentrations, no leakage of potassium was observed. These findings, in addition to the results obtained from the sequential binding study described above, prompted us to further investigate the individual roles of the haloduracin peptides in pore formation. When Halα and Halβ were added together or when cells were preincubated with Halβ for 5 min followed by the addition of Halα, a short lag was observed for 20−30 s followed by rapid K+ efflux. However, when cells were preincubated with Halα followed by the addition of Halβ, rapid onset of efflux was observed and the lag was essentially absent. The combined results of potassium efflux and sequential binding assays suggest that the biological role of Halα involves binding to its target, resulting in a complex that serves as a docking site for Halβ to bind and form pores. A similar mechanism has been proposed for lacticin 3147 (9), and it may be that this is a general mechanism for two-peptide lantibiotics for which the α-peptide has structural homology with mersacidin.
Figure 6.
Potassium ion release from Micrococcus luteus cells induced by haloduracin and nisin. (a) Reponses to 10 (blue), 5 (green), 1 (red), 0.25 (orange), and 0 μM (gray) haloduracin or nisin. (b) The effects on potassium release by preincubating either Halα or Halβ prior to addition of its counterpeptide: (A, blue) 5 μM Halα preincubation followed by 5 μM Halβ addition; (B, green) 5 μM Halβ preincubation followed by 5 μM Halα addition; (C, red) simultaneous addition of 5 μM Halα and 5 μM Halβ; (D, orange) addition of 5 μM nisin; (E, violet) addition of 5 μM Halα; (F, pink) addition of 5 μM Halβ; and (G, gray) a control without haloduracin. Vertical line (black) indicates time of antibiotic addition.
Halα Mutants
Antimicrobial activity in the nanomolar range often involves binding to a specific target molecule. On the basis of the comparable activity of haloduracin and nisin (a known lipid II-binding lantibiotic) against a range of bacteria, as well as the structural similarity of Halα with mersacidin, haloduracin most likely targets lipid II. The Halα peptide possesses a conserved CTLTXEC thioether bridge motif (B-ring), which is believed to be important for lipid II binding (6,29). This sequence is found in several class II lantibiotics including the single-peptide lantibiotics mersacidin and actagardine, as well as the Ltnα peptide from the two-peptide lantibiotic lacticin 3147 (30−32). Residing within this ring is a highly conserved glutamate residue, which has been shown for mersacidin to be essential for binding to lipid II (33).
Haloduracin biosynthesis was recently reconstituted in vitro(6), providing a method for the production of haloduracin mutants (29). The effects of individually disrupting the B- and C-thioether rings of Halα were probed by the mutation of Cys23 and Cys27 to alanines, respectively. Additionally, the conserved glutamate residing in the conserved CLTXEC ring (B-ring) was mutated to the isosteric glutamine (1). It has been shown previously that these mutations do not affect the formation of the other ring structures (29).
All Halα mutants retained the ability to inhibit L. lactis HP cell growth in liquid medium when incubated in the presence of wild-type Halβ. Quantitative IC50 values highlight the importance of the thioether rings of Halα in the antimicrobial activity of haloduracin with IC50 values of 335 ± 29 and 1180 ± 48 nM for wild-type Halβ combined with Halα C23A and C27A, respectively. The highly conserved glutamate residue residing in the B-ring of Halα also was important but not required for activity and displayed an IC50 of 964 ± 76 nM for Halα E22Q in combination with wild-type Halβ.
Maintaining biological activity at all despite the absence of conserved thioether rings present in Halα was unanticipated as these motifs are generally considered to be the basis of lantibiotic bioactivity and disruption of a ring has often resulted in abolished activity (1,34−39). The relatively modest reduction of bioactivity of the highly conserved B-ring mutant (10-fold) was therefore most unexpected. The retention of activity for the C27A and E22Q mutants was also unexpected as these mutants had no activity in agar diffusion assays (29). Similar increased activity of two-peptide lantibiotics in liquid media compared to solid agar media has been observed previously (40). Additional studies are necessary to investigate whether the reduced potency of Halα mutants in combination with wild-type Halβ is reflective of the ability of Halα mutants to bind lipid II, to effectively recruit Halβ for synergistic activity, or both.
Inhibition of Spore Outgrowth
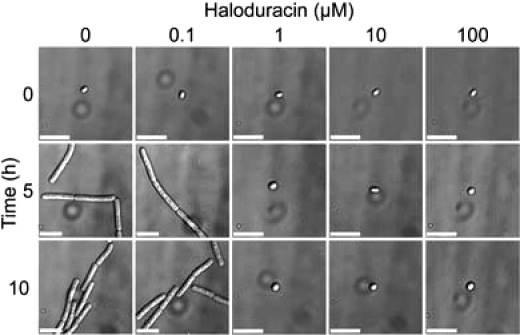
Nisin inhibits the outgrowth of spores from several pathogenic Gram-positive bacteria including Bacillus and Clostridium(15,41). To determine if haloduracin is capable of preventing spore outgrowth, Bacillis anthracis (Sterne 7702) was selected as a model. Spores were germinated in Brain Heart Infusion (BHI) medium in the presence of a range of haloduracin concentrations (7). For cultures containing low amounts of haloduracin (0.1 μM), differential interference contrast (DIC) microscopy revealed chains of vegetative bacilli after 5 and 10 h. However, no bacilli were present within cultures supplemented with 1, 10, or 100 μM haloduracin. This dose-dependent response shows that haloduracin was equally effective as nisin in the inhibition of B. anthracis spore outgrowth (17). Halα and Halβ also inhibited spore outgrowth when incubated independently, but only at 100 μM concentrations, whereas vegetative bacilli were observed in cultures containing 10 μM or less of either peptide (Supplementary Figure S5). Hence, the spore outgrowth inhibition depicted in 7 is the result of a synergistic mechanism of both peptides.
Figure 7.
Inhibition of Bacillus anthracis spore outgrowth by haloduracin. Scale bars equal 5.0 μm for all images.
Conclusion
This study illustrates the potent antimicrobial activity displayed in a synergistic manner by the two peptides of haloduracin. The activity of this two-peptide lantibiotic rivals that of nisin, which has been used worldwide as an effective preservative against food-borne pathogens. Haloduracin displayed stability higher than that of nisin at pH 7, which bodes well for potential use. Despite significant differences in the structures of both the α- and β-peptides of lacticin 3147 and haloduracin (1), these two compounds appear to operate by a similar mechanism that involves recognition by the α-peptides of a target at the cell surface, likely lipid II, and utilization of the β-peptides for membrane permeabilization. As such, these compounds combine the two activities of nisin into separate peptides (42).
Methods
Materials, Cultures, and Conditions
All chemicals and HPLC grade solvents were purchased from Sigma-Aldrich, unless otherwise stated. Tris and MOPS buffers were obtained from Fisher and α-cyano-4-hydroxy-cinnamic acid from Fluka. All media including Luria Bertoni (LB), Brain Heart Infusion (BHI), Todd Hewitt (TH), Mueller Hinton Broth (MHB), and M17 supplemented with 0.5% glucose (w/v) (GM17) were purchased from BD Biosciences. Factor Xa was purchased from New England Biolabs. Supplementary Table S1 describes the bacteria strains, source, and culture conditions for all bacteria used for this study. All Halα mutants were prepared as described previously (6,29).
Specific Activity Determination
Microtiter plates were used to determine the IC50 values (the concentration at which 50% growth inhibition is observed) of indicator strains. For assays with haloduracin, a 1:1 ratio of Halα:Halβ was used. Serial dilutions of Halα and Halβ peptides were prepared in sterile deionized water (SDW). Ninety-six-well and forty-eight-well microtiter plates (Corning Costar) were utilized for anaerobic and aerobic strains, respectively (see Materials, Cultures, and Conditions above). For 96-well plates, the total volume of culture in each well was 200 μL; the experimental wells contained 50 μL of diluted Halα and Halβ peptides at defined concentrations and 150 μL of a 1-in-10 dilution (approximately 1 × 108 CFU mL−1) of a culture of indicator strain diluted in fresh growth medium. In addition, each plate contained several blank (150 μL fresh growth medium and 50 μL SDW) and control wells (150 μL of untreated 1-in-10 diluted culture and 50 μL SDW). For 48-well plates, the total volume in each well was 300 μL; the experimental wells contained 75 μL of diluted Halα and Halβ peptides at defined concentrations and 225 μL of a 1-in-10 dilution (approximately 1 × 108 CFU mL−1) of a culture of indicator strain diluted in fresh growth medium. In addition, each plate contained several blank (225 μL fresh growth medium and 75 μL SDW) and control wells (225 μL of untreated 1-in-10 diluted culture and 75 μL SDW). The optical density at 600 nm (OD600) was recorded at hourly intervals from 0 to 5 h with an additional measurement at 18 h using a BioTek Synergy 2 plate reader. Plates were incubated according to optimal growth conditions (see Materials, Cultures, and Conditions above). The triplicate readings were averaged, and blanks (growth medium and SDW only) were subtracted from these readings. Growth curves were developed using control (culture and SDW only) readings to ensure sufficient OD changes for accurate inhibition assessment. Curve fits for IC50 determination were produced by fitting the data with Origin8 software using the dose−response curve with the equation y = A1 + (A2 − A1)/(1 + 10(Log x0 − x)p), with p = variable Hill slope. A 50% growth inhibition was determined as half the final OD600 ± 0.05. Nisin IC50 values were determined using identical procedures as described above.
Specific activities for individual peptides were determined essentially as described above, except that peptides were evaluated independently. Specific activity was assessed against the indicator strain L. lactis HP, and nisin standards were used as controls.
Membrane Potential Assays
Membrane potential was measured indirectly using the membrane potential-sensitive dye, 3,3′-diethyloxacarbocyanine iodide (DiOC2, Molecular Probes/Invitrogen). B. subtilis ATCC 6633 cultures (in triplicate) were grown to a density of 4 × 106 cells mL−1, and aliquots were transferred to tubes containing DiOC2 (final concentration of 300 nM). Cells were incubated with the dye for 20 min at 30 °C under aeration conditions prior to the addition of either haloduracin or nisin at 25 μM, 2.5 μM, 250 nM, 25 nM, or 0 nM. Following addition of peptides, cultures were incubated at 30 °C with aeration for an additional 10 min prior to analysis. The membrane potential was assessed by measuring the B. subtilis-associated DiOC2 fluorescence by flow cytometry (BD Biosciences LSR II flow cytometer) with excitation at 488 nm with an argon laser and measurement of emission through a band-pass filter at 530/30 nm. A minimum of 50,000 events were detected for each sample, and the data were analyzed using the FCS Express 3.00.0311 V Lite Stand-alone software. The data were plotted as the geometric mean of the fluorescence intensity (MFI).
Potassium Ion Release Assay
Potassium efflux was measured using the K+-sensitive fluorescent dye 1,3-benzenedicarboxylic acid, 4,4′-(1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-7,16-diylbis(5-methoxy-6,2-benzofurandiyl))bis (PBFI, Molecular Probes/Invitrogen). A culture of M. luteus was grown to an OD600 of 1.0 and washed five times with assay buffer (5 mM HEPES, 5 mM glucose, pH 7.2). Washed cells were resuspended in one-fifth the volume of assay buffer containing PBFI (final concentration of 10 μM) and aliquots were added to UV-transparent microtiter plates (Nunc). Sample fluorescence (excitation = 346 nm, emission = 505 nm) background data was collected (BioTek Synergy 2 plate reader, excitation filter 360/40, emission filter 508/20) until baseline signal was achieved, followed by the addition of haloduracin (Halα and Halβ in a 1:1 ratio) or nisin at 10 μM, 5 μM, 1 μM, 250 nM, or 0 nM. For preincubation studies of the haloduracin peptides, baseline signal was achieved, followed by the addition of either Halα or Halβ to wells at the concentrations indicated and incubation for 5 min prior to the addition of Halβ or Halα, respectively.
Inhibition of Bacillus anthracis Spore Outgrowth
B. anthracis spores at a concentration of 4.0 × 106 spores mL−1 were incubated in Brain Heart Infusion (BHI) medium supplemented with haloduracin (0.1, 1, 10, or 100 μM at a Halα:Halβ ratio of 1:1) or 0.1 M MOPS (pH 6.8) as a control. For assessment of Halα and Halβ used independently, spores were incubated in BHI medium supplemented with the peptide at 0.1, 1, 10, or 100 μM or with 0.1 M MOPS (pH 6.8) as a control. As a positive control, spores were treated with nisin under the same conditions and concentrations as used for Halα and Halβ. Cultures were incubated at 37 °C under aeration conditions and ambient CO2 (0.03% CO2). At 0, 5, and 10 h, samples were removed from the B. anthracis cultures and fixed by incubation in 3% formaldehyde (v/v) for 30 min at 37 °C. Samples were mounted on glass microscope slides in 20% glycerol (v/v). Differential interference contrast (DIC) microscopy images were collected with an Applied Precision assembled DeltaVision epifluorescence microscope equipped with an Olympus Plan Apo x100 oil objective with a numerical aperture of 1.42 and a working distance of 0.15 mm. Images were processed with SoftWoRX Explorer Suite.
Acknowledgments
We thank I. Gut for assistance with spore outgrowth and microscopy studies. This work was supported by the Howard Hughes Medical Institute.
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Chatterjee C.; Paul M.; Xie L.; van der Donk W. A. (2005) Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684. [DOI] [PubMed] [Google Scholar]
- Cotter P. D.; Hill C.; Ross R. P. (2005) Bacterial lantibiotics: strategies to improve therapeutic potential. Curr. Protein Pept. Sci. 6, 61–75. [DOI] [PubMed] [Google Scholar]
- Willey J. M.; van der Donk W. A. (2007) Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61, 477–501. [DOI] [PubMed] [Google Scholar]
- Enserink M. (1999) Promising antibiotic candidate identified. Science 286, 2245–2247. [DOI] [PubMed] [Google Scholar]
- Patton G. C.; Paul M.; Cooper L. E.; Chatterjee C.; van der Donk W. A. (2008) The importance of the leader sequence for directing lanthionine formation in lacticin 481. Biochemistry 47, 7342–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClerren A. L.; Cooper L. E.; Quan C.; Thomas P. M.; Kelleher N. L.; van der Donk W. A. (2006) Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. U.S.A. 103, 17243–17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe O.; Hill C.; Ross R. P. (2000) Each peptide of the two-component lantibiotic lacticin 3147 requires a separate modification enzyme for activity. Microbiology 146, 2147–2154. [DOI] [PubMed] [Google Scholar]
- Martin N. I.; Sprules T.; Carpenter M. R.; Cotter P. D.; Hill C.; Ross R. P.; Vederas J. C. (2004) Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 43, 3049–3056. [DOI] [PubMed] [Google Scholar]
- Wiedemann I.; Bottiger T.; Bonelli R. R.; Wiese A.; Hagge S. O.; Gutsmann T.; Seydel U.; Deegan L.; Hill C.; Ross P.; Sahl H. G. (2006) The mode of action of the lantibiotic lacticin 3147−a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61, 285–296. [DOI] [PubMed] [Google Scholar]
- Breukink E.; Wiedemann I.; van Kraaij C.; Kuipers O. P.; Sahl H.; de Kruijff B. (1999) Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286, 2361–2364. [DOI] [PubMed] [Google Scholar]
- Brötz H.; Bierbaum G.; Leopold K.; Reynolds P. E.; Sahl H. G. (1998) The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar K.; Walker S. (2002) Substrate analogues to study cell-wall biosynthesis and its inhibition. Curr. Opin. Chem. Biol. 6, 786–793. [DOI] [PubMed] [Google Scholar]
- Breukink E.; de Kruijff B. (2006) Lipid II as a target for antibiotics. Nat. Rev. Drug Discovery 5, 321–332. [DOI] [PubMed] [Google Scholar]
- Brötz H.; Josten M.; Wiedemann I.; Schneider U.; Götz F.; Bierbaum G.; Sahl H.-G. (1998) Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30, 317–327. [DOI] [PubMed] [Google Scholar]
- Campbell L. L. Jr.; Sniff E. E. (1959) Effect of subtilin and nisin on the spores of Bacillus coagulans. J. Bacteriol. 77, 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. C.; Dodd H. M.; Horn N.; Maclean K.; Lian L. Y.; Bycroft B. W.; Gasson M. J.; Roberts G. C. (1996) Structure-activity relationships in the peptide antibiotic nisin: role of dehydroalanine 5. Appl. Environ. Microbiol. 62, 2966–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut I. M.; Prouty A. M.; Ballard J. D.; van der Donk W. A.; Blanke S. R. (2008) Inhibition of Bacillus anthracis spore outgrowth by nisin. Antimicrob. Agents Chemother. 52, 4281–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton E. M.; Cotter P. D.; Hill C.; Ross R. P. (2007) Identification of a novel two-peptide lantibiotic, haloduracin, produced by the alkaliphile Bacillus halodurans C-125. FEMS Microbiol. Lett. 267, 64–71. [DOI] [PubMed] [Google Scholar]
- Navaratna M. A.; Sahl H. G.; Tagg J. R. (1998) Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 64, 4803–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holo H.; Jeknic Z.; Daeschel M.; Stevanovic S.; Nes I. F. (2001) Plantaricin W from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology 147, 643–651. [DOI] [PubMed] [Google Scholar]
- Morgan S. M.; O’Connor P, M.; Cotter P. D.; Ross R. P.; Hill C. (2005) Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob. Agents Chemother. 49, 2606–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. P.; Wei J.; Greenberg K.; Novick R. (1998) Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 42, 277–278. [PubMed] [Google Scholar]
- Chan W. C.; Bycroft B. W.; Lian L. Y.; Roberts G. C. K. (1989) Isolation and characterization of two degradation products derived from the peptide antibiotic nisin. FEBS Lett. 252, 29–36. [Google Scholar]
- Rollema H. S.; Metzger J. W.; Both P.; Kuipers O. P.; Siezen R. J. (1996) Structure and biological activity of chemically modified nisin A species. Eur. J. Biochem. 241, 716–722. [DOI] [PubMed] [Google Scholar]
- Hurst A. (1981) Nisin. Adv. Appl. Microbiol. 27, 85–123. [Google Scholar]
- Lian L. Y.; Chan W. C.; Morley S. D.; Roberts G. C.; Bycroft B. W.; Jackson D. (1992) Solution structures of nisin A and its two major degradation products determined by NMR. Biochem. J. 283, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H. S.; Kuipers O. P.; Both P.; de Vos W. M.; Siezen R. J. (1995) Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 61, 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo D. J.; Perlmutter N. G.; Hunt R. H.; Shapiro H. M. (2000) Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob. Agents Chemother. 44, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L. E.; McClerren A. L.; Chary A.; van der Donk W. A. (2008) Structure-activity relationship studies of the two-component lantibiotic haloduracin. Chem. Biol. 15, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.; Chatterjee S.; Lad S. J.; Phansalkar M. S.; Rupp R. H.; Ganguli B. N.; Fehlhaber H. W.; Kogler H. (1992) Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization. J. Antibiot. 45, 832–838. [DOI] [PubMed] [Google Scholar]
- Zimmermann N.; Metzger J. W.; Jung G. (1995) The tetracyclic lantibiotic actagardine. 1H-NMR and 13C-NMR assignments and revised primary structure. Eur. J. Biochem. 228, 786–797. [PubMed] [Google Scholar]
- Ryan M. P.; Jack R. W.; Josten M.; Sahl H. G.; Jung G.; Ross R. P.; Hill C. (1999) Extensive post-translational modification, including serine to d-alanine conversion, in the two-component lantibiotic, lacticin 3147. J. Biol. Chem. 274, 37544–37550. [DOI] [PubMed] [Google Scholar]
- Szekat C.; Jack R. W.; Skutlarek D.; Farber H.; Bierbaum G. (2003) Construction of an expression system for site-directed mutagenesis of the lantibiotic mersacidin. Appl. Environ. Microbiol. 69, 3777–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee C.; Patton G. C.; Cooper L.; Paul M.; van der Donk W. A. (2006) Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Kuipers O. P.; Bierbaum G.; Ottenwälder B.; Dodd H. M.; Horn N.; Metzger J.; Kupke T.; Gnau V.; Bongers R.; van den Bogaard P.; Kosters H.; Rollema H. S.; de Vos W. M.; Siezen R. J.; Jung G.; Götz F.; Sahl H. G.; Gasson M. J. (1996) Protein engineering of lantibiotics. Antonie van Leeuwenhoek 69, 161–169. [DOI] [PubMed] [Google Scholar]
- Chen P.; Novak J.; Kirk M.; Barnes S.; Qi F.; Caufield P. W. (1998) Structure-activity study of the lantibiotic mutacin II from Streptococcus mutans T8 by a gene replacement strategy. Appl. Environ. Microbiol. 64, 2335–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbaum G.; Szekat C.; Josten M.; Heidrich C.; Kempter C.; Jung G.; Sahl H. G. (1996) Engineering of a novel thioether bridge and role of modified residues in the lantibiotic Pep5. Appl. Environ. Microbiol. 62, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenwälder B.; Kupke T.; Brecht S.; Gnau V.; Metzger J.; Jung G.; Götz F. (1995) Isolation and characterization of genetically engineered gallidermin and epidermin analogs. Appl. Environ. Microbiol. 61, 3894–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kraaij C.; Breukink E.; Rollema H. S.; Bongers R. S.; Kosters H. A.; de Kruijff B.; Kuipers O. P. (2000) Engineering a disulfide bond and free thiols in the lantibiotic nisin Z. Eur. J. Biochem. 267, 901–909. [DOI] [PubMed] [Google Scholar]
- Galvin M.; Hill C.; Ross R. P. (1999) Lacticin3147 displays activity in buffer against Gram-positive bacterial pathogens which appear insensitive in standard plate assays. Lett. Appl. Microbiol 28, 355–358. [DOI] [PubMed] [Google Scholar]
- Mazzotta A. S.; Crandall A. D.; Montville T. J. (1997) Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 63, 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukink E. (2006) A lesson in efficient killing from two-component lantibiotics. Mol. Microbiol. 61, 271–273. [DOI] [PubMed] [Google Scholar]
- Ekkelenkamp M. B.; Hanssen M.; Danny Hsu S. T.; de Jong A.; Milatovic D.; Verhoef J.; van Nuland N. A. (2005) Isolation and structural characterization of epilancin 15X, a novel lantibiotic from a clinical strain of Staphylococcus epidermidis. FEBS Lett. 579, 1917–1922. [DOI] [PubMed] [Google Scholar]
- Kawamura F.; Doi R. H. (1984) Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 160, 442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Hansen J. N. (1991) Conversion of Bacillus subtilis 168 to a subtilin producer by competence transformation. J. Bacteriol. 173, 7387–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.