Abstract
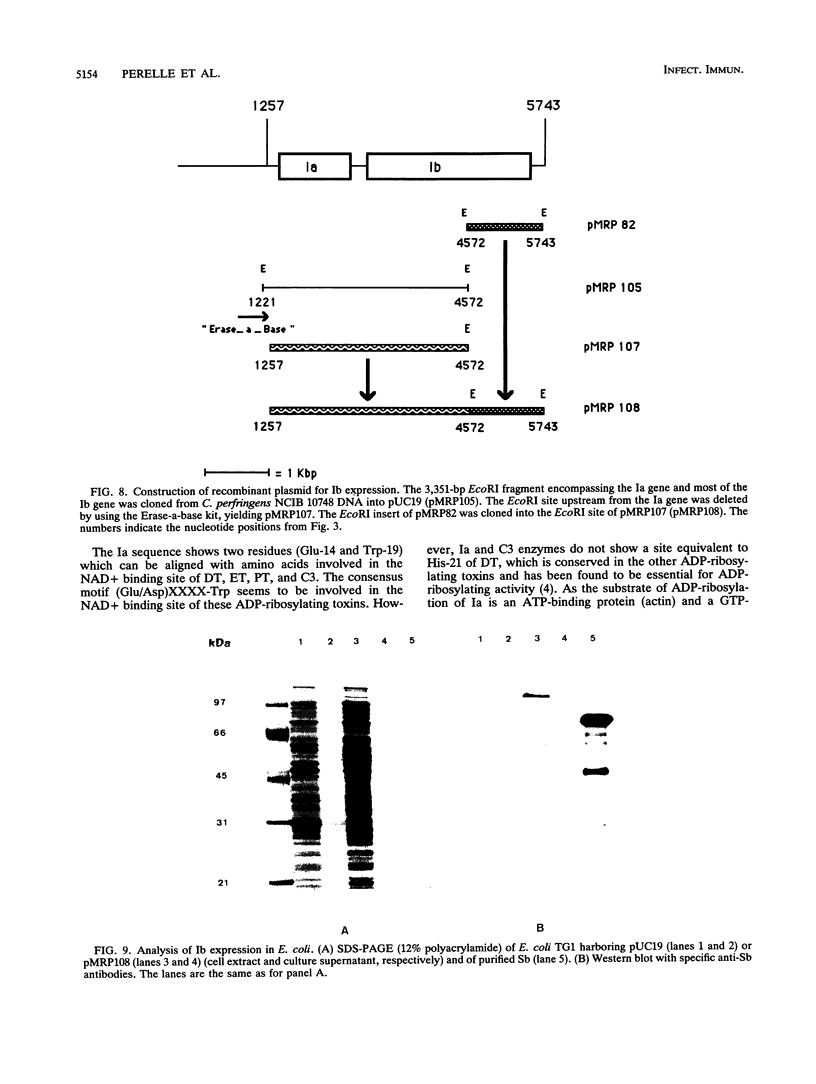
The iota toxin which is produced by Clostridium perfringens type E, is a binary toxin consisting of two independent polypeptides: Ia, which is an ADP-ribosyltransferase, and Ib, which is involved in the binding and internalization of the toxin into the cell. Two degenerate oligonucleotide probes deduced from partial amino acid sequence of each component of C. spiroforme toxin, which is closely related to the iota toxin, were used to clone three overlapping DNA fragments containing the iota-toxin genes from C. perfringens type E plasmid DNA. Two genes, in the same orientation, coding for Ia (387 amino acids) and Ib (875 amino acids) and separated by 243 noncoding nucleotides were identified. A predicted signal peptide was found for each component, and the secreted Ib displays two domains, the propeptide (172 amino acids) and the mature protein (664 amino acids). The Ia gene has been expressed in Escherichia coli and C. perfringens, under the control of its own promoter. The recombinant polypeptide obtained was recognized by Ia antibodies and ADP-ribosylated actin. The expression of the Ib gene was obtained in E. coli harboring a recombinant plasmid encompassing the putative promoter upstream of the Ia gene and the Ia and Ib genes. Two residues which have been found to be involved in the NAD+ binding site of diphtheria and pseudomonas toxins are conserved in the predicted Ia sequence (Glu-14 and Trp-19). The predicted amino acid Ib sequence shows 33.9% identity with and 54.4% similarity to the protective antigen of the anthrax toxin complex. In particular, the central region of Ib, which contains a predicted transmembrane segment (Leu-292 to Ser-308), presents 45% identity with the corresponding protective antigen sequence which is involved in the translocation of the toxin across the cell membrane.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chavan A. J., Nemoto Y., Narumiya S., Kozaki S., Haley B. E. NAD+ binding site of Clostridium botulinum C3 ADP-ribosyltransferase. Identification of peptide in the adenine ring binding domain using 2-azido NAD. J Biol Chem. 1992 Jul 25;267(21):14866–14870. [PubMed] [Google Scholar]
- Choe S., Bennett M. J., Fujii G., Curmi P. M., Kantardjieff K. A., Collier R. J., Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992 May 21;357(6375):216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- Considine R. V., Simpson L. L. Cellular and molecular actions of binary toxins possessing ADP-ribosyltransferase activity. Toxicon. 1991;29(8):913–936. doi: 10.1016/0041-0101(91)90076-4. [DOI] [PubMed] [Google Scholar]
- Domenighini M., Montecucco C., Ripka W. C., Rappuoli R. Computer modelling of the NAD binding site of ADP-ribosylating toxins: active-site structure and mechanism of NAD binding. Mol Microbiol. 1991 Jan;5(1):23–31. doi: 10.1111/j.1365-2958.1991.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Guyonnet F., Plommet M. Hémolysine gamma de staphylococcus aureus: purification et propriétés. Ann Inst Pasteur (Paris) 1970 Jan;118(1):19–33. [PubMed] [Google Scholar]
- Hatheway C. L. Toxigenic clostridia. Clin Microbiol Rev. 1990 Jan;3(1):66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. E., Clarke I. N., Kelly D. C., Titball R. W. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect Immun. 1992 Jan;60(1):102–110. doi: 10.1128/iai.60.1.102-110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Sugai M., Murooka Y., Paik S. Y., Hong Y. M., Ohgai H., Suginaka H. Molecular cloning and sequencing of the epidermal cell differentiation inhibitor gene from Staphylococcus aureus. Biochem Biophys Res Commun. 1991 Jan 31;174(2):459–464. doi: 10.1016/0006-291x(91)91438-i. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klimpel K. R., Molloy S. S., Thomas G., Leppla S. H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Hirayama T., Kato I., Matsuda F. Crystallization and properties of staphylococcal leukocidin. Biochim Biophys Acta. 1980 Nov 17;633(1):33–44. doi: 10.1016/0304-4165(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Boquet P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem Biophys Res Commun. 1988 May 16;152(3):1361–1368. doi: 10.1016/s0006-291x(88)80435-2. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Hauser D., Boquet P., Eklund M. W., Gill D. M. Characterization of the C3 gene of Clostridium botulinum types C and D and its expression in Escherichia coli. Infect Immun. 1991 Oct;59(10):3673–3679. doi: 10.1128/iai.59.10.3673-3679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M. R., Milward F. W., Bancillon B., Boquet P. Purification of the Clostridium spiroforme binary toxin and activity of the toxin on HEp-2 cells. Infect Immun. 1989 Aug;57(8):2462–2469. doi: 10.1128/iai.57.8.2462-2469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M. R., Rubin E. J., Gill D. M., Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect Immun. 1988 Sep;56(9):2299–2306. doi: 10.1128/iai.56.9.2299-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Stiles B. G., Zepeda H. H., Wilkins T. D. Molecular basis for the pathological actions of Clostridium perfringens iota toxin. Infect Immun. 1987 Jan;55(1):118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan J., Warner T. A., Scott P. T., Bannam T. L., Berryman D. I., Rood J. I. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid. 1992 May;27(3):207–219. doi: 10.1016/0147-619x(92)90023-4. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992 Dec;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon. 1986;24(8):767–773. doi: 10.1016/0041-0101(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect Immun. 1986 Dec;54(3):683–688. doi: 10.1128/iai.54.3.683-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Schering B., Bärmann M., Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987 Dec 10;225(1-2):48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989 Dec;3(12):1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Young M., Minton N. P., Staudenbauer W. L. Recent advances in the genetics of the clostridia. FEMS Microbiol Rev. 1989 Dec;5(4):301–325. doi: 10.1111/j.1574-6968.1989.tb03402.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]