Abstract
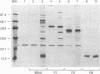
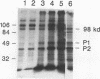
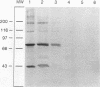
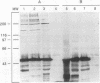
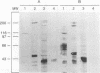
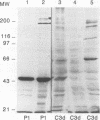
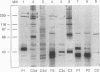
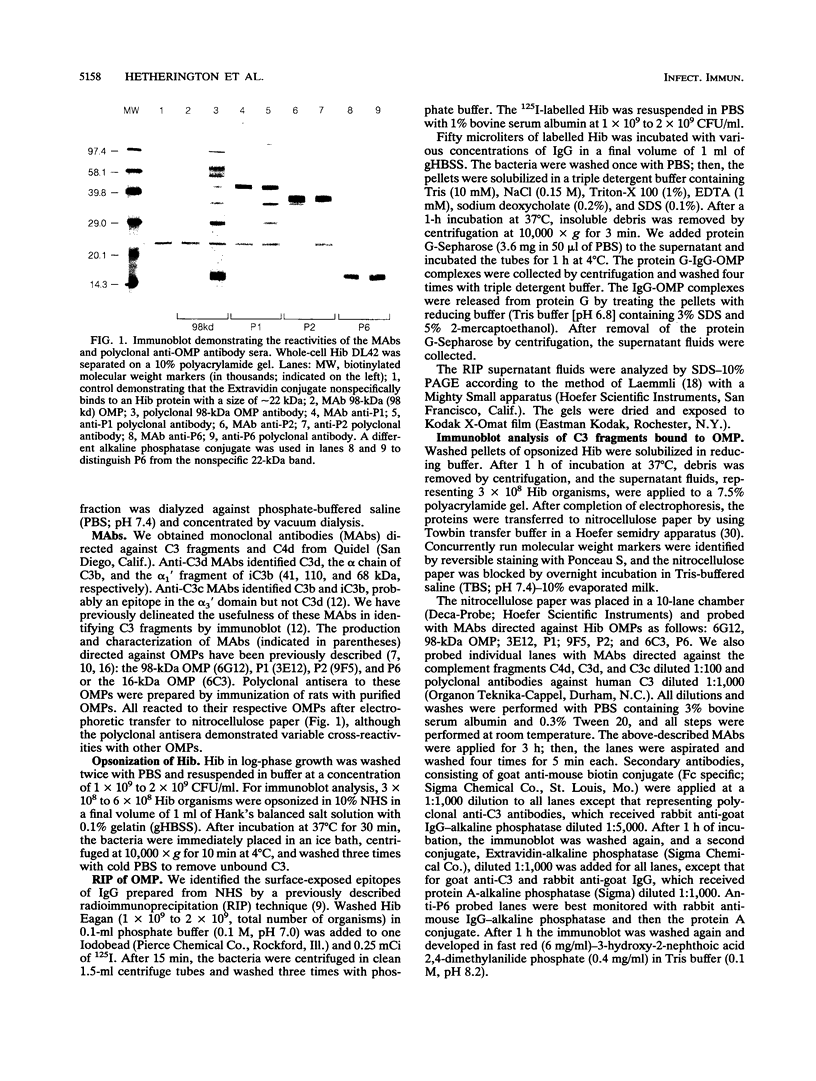
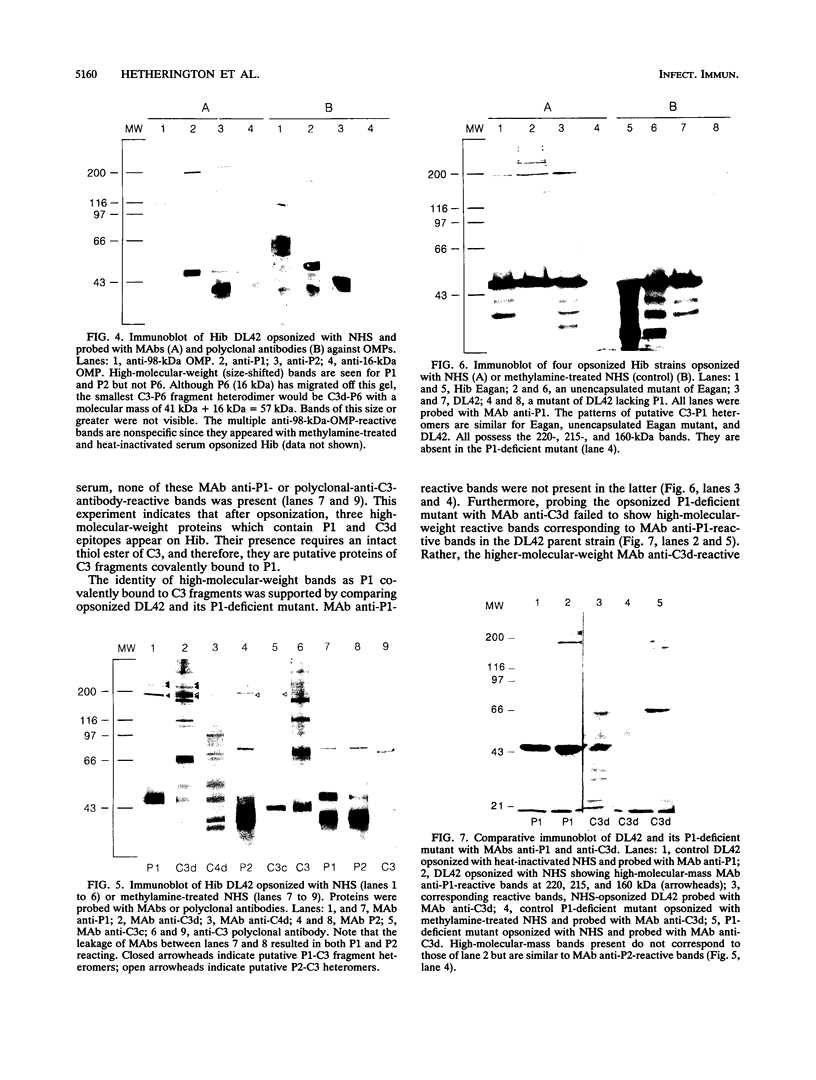
Complement component 3 (C3) binding to Haemophilus influenzae type b (Hib) is an important step in host defense against invasive disease, but the details of this process remain poorly understood. We have shown that the P1 and P2 outer membrane proteins (OMPs) serve as binding sites for C3 on serum-opsonized Hib. Whole-cell lysates of opsonized Hib were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resolved proteins were transferred to nitrocellulose. Immunoblot analysis with monoclonal antibodies (MAbs) to the 49-kDa P1 and 39-kDa P2 OMPs demonstrated high-molecular-weight bands that were not present when the bacteria were opsonized with heat-inactivated or methylamine-treated serum. Immunoblot analysis with MAbs to the 98- or 16-kDa (P6) OMPs did not reveal additional bands. An unencapsulated Hib mutant still lacked C3 bound to the 98-kDa or P6 OMP, indicating that the absence of C3 binding to these proteins was not the result of epitope masking by the capsule. Studies with MAbs to C3 fragments confirmed that the anti-P1- and anti-P2-reactive bands were C3 fragments bound to these OMPs. The molecular weights of proteins reactive to anti-OMP and anti-C3 antibodies indicated that multiple C3 fragments may be bound to P1 or that C3 may be bound to P2 multimers. Finally, the presence of other anti-C3-reactive proteins indicated that several other proteins serve as C3 targets during the opsonization of Hib.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinger-Kawahara C., Horwitz M. A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990 Oct 1;172(4):1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson F. J., Jr, Winkelstein J. A., Moxon E. R. Participation of complement in the nonimmune host defense against experimental Haemophilus influenzae type b septicemia and meningitis. Infect Immun. 1976 Oct;14(4):882–887. doi: 10.1128/iai.14.4.882-887.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg-Lagergård T. Target antigens for bactericidal and opsonizing antibodies to Haemophilus influenzae. Acta Pathol Microbiol Immunol Scand C. 1982 Aug;90(4):209–216. doi: 10.1111/j.1699-0463.1982.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Asmar B. I., Thirumoorthi M. C. Systemic Haemophilus influenzae disease: an overview. J Pediatr. 1979 Mar;94(3):355–364. doi: 10.1016/s0022-3476(79)80571-5. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R. Haemophilus influenzae non-type b infections in children. Am J Dis Child. 1987 Oct;141(10):1063–1065. doi: 10.1001/archpedi.141.10.1063. [DOI] [PubMed] [Google Scholar]
- Gonzales F. R., Leachman S., Norgard M. V., Radolf J. D., McCracken G. H., Jr, Evans C., Hansen E. J. Cloning and expression in Escherichia coli of the gene encoding the heat-modifiable major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2993–3000. doi: 10.1128/iai.55.12.2993-3000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Rockwell R. Experimental Haemophilus influenzae type b meningitis: immunological investigation of the infant rat model. Infect Immun. 1978 Jun;20(3):705–713. doi: 10.1128/iai.20.3.705-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Pelzel S. E., Orth K., Moomaw C. R., Radolf J. D., Slaughter C. A. Structural and antigenic conservation of the P2 porin protein among strains of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3270–3275. doi: 10.1128/iai.57.11.3270-3275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Cope L. D., Hansen E. J. Expression of the heat-modifiable major outer membrane protein of Haemophilus influenzae type b is unrelated to virulence. Infect Immun. 1989 Jun;57(6):1639–1646. doi: 10.1128/iai.57.6.1639-1646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henwick S., Hetherington S. V., Hostetter M. K. Specificity of three anti-complement factor 3 monoclonal antibodies. J Immunol Methods. 1992 Aug 30;153(1-2):173–184. doi: 10.1016/0022-1759(92)90320-s. [DOI] [PubMed] [Google Scholar]
- Hetherington S. V. Antibody to the outer membrane proteins is the dominant opsonic antibody in normal human serum against H. influenzae type b. Immunology. 1989 May;67(1):87–91. [PMC free article] [PubMed] [Google Scholar]
- Hetherington S. V., Patrick C. C. Complement component 3 binding to Haemophilus influenzae type b in the presence of anticapsular and anti-outer membrane antibodies. Infect Immun. 1992 Jan;60(1):19–24. doi: 10.1128/iai.60.1.19-24.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter M. K., Krueger R. A., Schmeling D. J. The biochemistry of opsonization: central role of the reactive thiolester of the third component of complement. J Infect Dis. 1984 Nov;150(5):653–661. doi: 10.1093/infdis/150.5.653. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingman K. L., Jansen E. M., Murphy T. F. Nearest neighbor analysis of outer membrane proteins of nontypeable Haemophilus influenzae. Infect Immun. 1988 Dec;56(12):3058–3063. doi: 10.1128/iai.56.12.3058-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988 Oct;56(10):2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Rice P. A., Nelson M. B., Dudas K. C., Apicella M. A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Invest. 1986 Oct;78(4):1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel G. J., Katz S., Edelson P. J. Complement-mediated early clearance of Haemophilus influenzae type b from blood is independent of serum lytic activity. J Infect Dis. 1988 Jan;157(1):85–90. doi: 10.1093/infdis/157.1.85. [DOI] [PubMed] [Google Scholar]
- Ross S. C., Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984 Sep;63(5):243–273. [PubMed] [Google Scholar]
- Schreiber J. R., Basker C. J., Siber G. R. Effect of complement depletion on anticapsular-antibody-mediated immunity to experimental infection with Haemophilus influenzae type b. Infect Immun. 1987 Nov;55(11):2830–2833. doi: 10.1128/iai.55.11.2830-2833.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Smith A. L., Anderson P., Smith D. H. The paradox of Hemophilus infuenzae type B bacteremia in the presence of serum bactericidal activity. J Clin Invest. 1976 Oct;58(4):1019–1029. doi: 10.1172/JCI108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Thomas W. R., Callow M. G., Dilworth R. J., Audesho A. A. Expression in Escherichia coli of a high-molecular-weight protective surface antigen found in nontypeable and type b Haemophilus influenzae. Infect Immun. 1990 Jun;58(6):1909–1913. doi: 10.1128/iai.58.6.1909-1913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]