Abstract
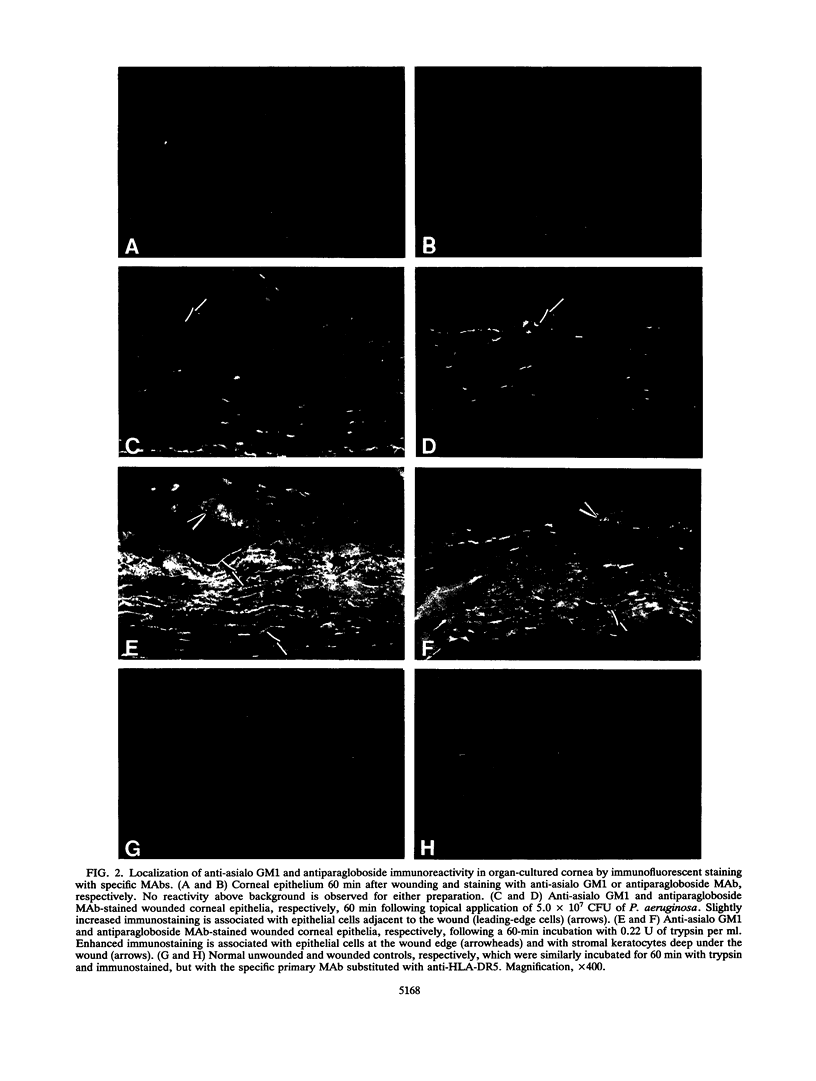
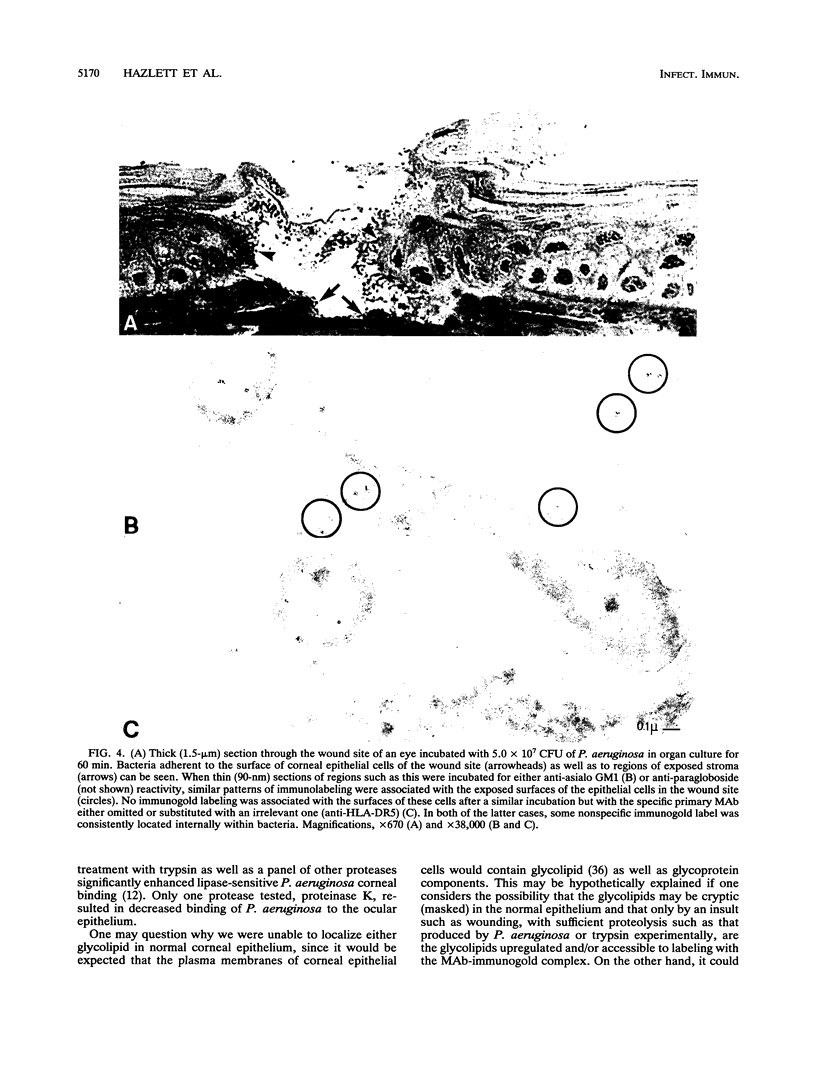
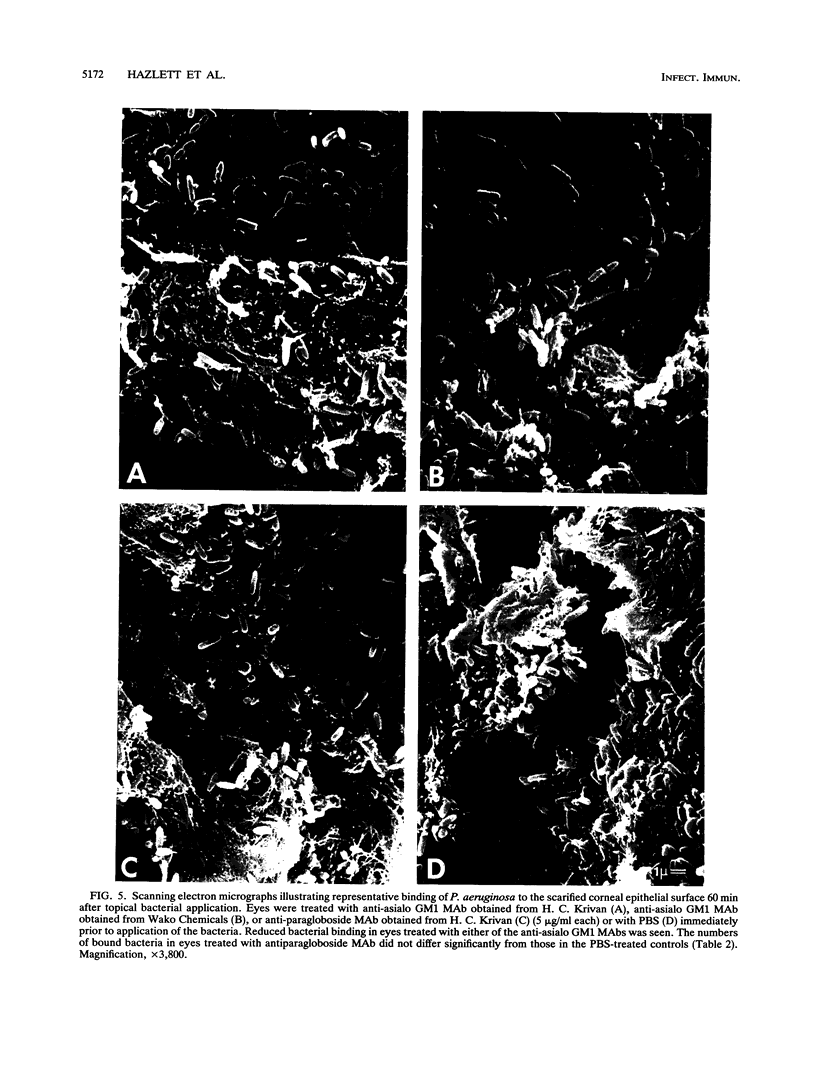
Anti-gangliotetraosylceramide (anti-asialo GM1) and antiparagloboside monoclonal antibodies (MAbs) were used in immunofluorescence, immunoelectron-microscopic, and in vitro binding inhibition assays to determine whether either of the glycolipids was detectable in the normal cornea, whether levels changed following corneal scarification and either trypsin treatment or incubation in vitro with Pseudomonas aeruginosa, and whether either of the MAbs could competitively inhibit P. aeruginosa binding to cornea. No immunostaining above background for either glycolipid was observed in frozen, unfixed sections or in lightly fixed, K4M-embedded antibody-gold-labeled thin sections of normal cornea. In frozen sections of organ-cultured scarified cornea, no increased immunostaining for anti-asialo GM1 or antiparagloboside reactivity was noted immediately or 60 min after corneal scarification. However, at 60 min after scarification and in vitro incubation of the eye with either trypsin or P. aeruginosa, enhanced immunostaining for both glycolipids was associated with cells within or immediately adjacent to the wound site. Trypsin increased immunoreactivity in the wound site more markedly compared with incubation with P. aeruginosa, but immunostaining was similarly localized with either treatment. No staining above background was seen in control sections. Similarly, with immunoelectron microscopy, increased immunogold-MAb staining for both glycolipids was seen on the plasma membranes of the wound-site cells of eyes incubated with either trypsin or P. aeruginosa compared with controls that were similarly immunostained but with the primary antibody either omitted or substituted with a nonspecific MAb. Competitive binding inhibition assays, in which the bacterial inoculum or the eye in organ culture was incubated with anti-asialo GM1 MAb prior to topical ocular application of the bacteria, showed significantly decreased P. aeruginosa adhesion compared with preparations similarly treated with phosphate-buffered saline or antiparagloboside MAb. These data provide evidence to support the hypothesis that asialo GM1, not paragloboside, serves as a receptor for P. aeruginosa binding to the scarified cornea of the adult mouse and spatially localizes both glycolipids in the wound site.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Minor V., Deal C., Shahrabadi M. S., Simpson D. A., Woods D. E. Pseudomonas aeruginosa exoenzyme S is an adhesion. Infect Immun. 1991 Sep;59(9):2859–2863. doi: 10.1128/iai.59.9.2859-2863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk R. S., Beisel K., Hazlett L. D. Genetic studies of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1981 Oct;34(1):1–5. doi: 10.1128/iai.34.1.1-5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk R. S., Leon M. A., Hazlett L. D. Genetic control of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1979 Dec;26(3):1221–1223. doi: 10.1128/iai.26.3.1221-1223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Paranchych W., Sastry P. A., Irvin R. T. Human buccal epithelial cell receptors of Pseudomonas aeruginosa: identification of glycoproteins with pilus binding activity. Can J Microbiol. 1989 Dec;35(12):1141–1145. doi: 10.1139/m89-189. [DOI] [PubMed] [Google Scholar]
- Enzan H., Hiroi M., Saibara T., Onishi S., Yamamoto Y., Yamamoto H., Hara H. Immunoelectron microscopic identification of asialo GM1-positive cells in adult rat liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(6):389–398. doi: 10.1007/BF02899571. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Girard M. T. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1779–1788. [PubMed] [Google Scholar]
- Galentine P. G., Cohen E. J., Laibson P. R., Adams C. P., Michaud R., Arentsen J. J. Corneal ulcers associated with contact lens wear. Arch Ophthalmol. 1984 Jun;102(6):891–894. doi: 10.1001/archopht.1984.01040030711025. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Masinick S., Berk R. S., Zheng Z. Proteinase K decreases Pseudomonas aeruginosa adhesion to wounded cornea. Exp Eye Res. 1992 Oct;55(4):579–587. doi: 10.1016/s0014-4835(05)80171-x. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Mathieu P. Glycoconjugates on corneal epithelial surface: effect of neuraminidase treatment. J Histochem Cytochem. 1989 Aug;37(8):1215–1224. doi: 10.1177/37.8.2754252. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Singh A., Berk R. S., Rudner X. L. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosa strains. Curr Eye Res. 1991 Apr;10(4):351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Strejc M., Berk R. S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1978–1985. [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Age-related susceptibility to Pseudomonas aeruginosa ocular infections in mice. Infect Immun. 1978 Apr;20(1):25–29. doi: 10.1128/iai.20.1.25-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Wells P., Spann B., Berk R. S. Penetration of the unwounded immature mouse cornea and conjunctiva by Pseudomonas: SEM-TEM analysis. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):694–697. [PubMed] [Google Scholar]
- Hazlett L., Barrett R., Klettner C., Berk R., Singh A. Pseudomonas aeruginosa: a probe to detail change with age in corneal receptor(s). Ophthalmic Res. 1991;23(3):141–146. doi: 10.1159/000267113. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jones D. B. Early diagnosis and therapy of bacterial corneal ulcers. Int Ophthalmol Clin. 1973 Winter;13(4):1–29. [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson P. R. Cornea and sclera. Arch Ophthalmol. 1972 Nov;88(5):553–574. doi: 10.1001/archopht.1972.01000030555018. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Panjwani N., Zaidi T. S., Gigstad J. E., Jungalwala F. B., Barza M., Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990 Jan;58(1):114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., McNiece M. T., Polack F. M. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann Ophthalmol. 1981 Apr;13(4):421–425. [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Yuen C. T., Stoll M. S., Feizi T. Differences in the binding specificities of Pseudomonas aeruginosa M35 and Escherichia coli C600 for lipid-linked oligosaccharides with lactose-related core regions. Infect Immun. 1992 Dec;60(12):5078–5084. doi: 10.1128/iai.60.12.5078-5084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner X. L., Zheng Z., Berk R. S., Irvin R. T., Hazlett L. D. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2185–2193. [PubMed] [Google Scholar]
- Schein O., Hibberd P., Kenyon K. R. Contact lens complications: incidental or epidemic? Am J Ophthalmol. 1986 Jul 15;102(1):116–117. doi: 10.1016/0002-9394(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Singh A., Hazlett L. D., Berk R. S. Characterization of Pseudomonas aeruginosa adherence to mouse corneas in organ culture. Infect Immun. 1990 May;58(5):1301–1307. doi: 10.1128/iai.58.5.1301-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Hazlett L., Berk R. S. Characterization of pseudomonal adherence to unwounded cornea. Invest Ophthalmol Vis Sci. 1991 Jun;32(7):2096–2104. [PubMed] [Google Scholar]
- Stern G. A., Weitzenkorn D., Valenti J. Adherence of Pseudomonas aeruginosa to the mouse cornea. Epithelial v stromal adherence. Arch Ophthalmol. 1982 Dec;100(12):1956–1958. doi: 10.1001/archopht.1982.01030040936014. [DOI] [PubMed] [Google Scholar]
- Thompson L. K., Horowitz P. M., Bentley K. L., Thomas D. D., Alderete J. F., Klebe R. J. Localization of the ganglioside-binding site of fibronectin. J Biol Chem. 1986 Apr 15;261(11):5209–5214. [PubMed] [Google Scholar]
- Woods D. E., To M., Sokol P. A. Pseudomonas aeruginosa exoenzyme S as a pathogenic determinant in respiratory infections. Antibiot Chemother (1971) 1989;42:27–35. doi: 10.1159/000417600. [DOI] [PubMed] [Google Scholar]
- Yue B. Y., Tao R. V., Lee B. C., Sugar J. Neutral glycosphingolipids isolated from porcine corneas. Curr Eye Res. 1990 Apr;9(4):337–342. doi: 10.3109/02713689008999621. [DOI] [PubMed] [Google Scholar]