Abstract
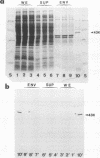
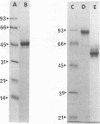
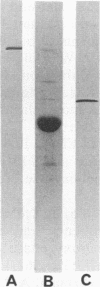
Flanking DNA regions of the fimbrilin gene (designated fimA), which encodes the major subunit protein of Porphyromonas (Bacteroides) gingivalis fimbriae, were cloned in several manners from the P. gingivalis chromosome into Escherichia coli by screening with probes derived from a 2.5-kb SacI DNA fragment previously cloned. A total of 10.4 kb of DNA fragments from the P. gingivalis genome was cloned in the pUC plasmid. Expression of the fimA gene and possible flanking genes in the fragments cloned was examined in a pUC plasmid vector system and in a bacteriophage T7 RNA polymerase-promoter expression vector system. The results show that in the pUC plasmid system, a 45-kDa protein, a product of fimA, was only poorly expressed as a precursor of the fimbrilin protein (FimA) and could be detected from cell extracts in Western blotting (immunoblotting) analysis as a sharp band but not in colony immunoblotting analysis. On the other hand, in the T7 RNA polymerase-promoter system, the product of fimA and products of the possible flanking genes responsible for fimbriation were overproduced as thick bands of the 45-kDa protein and as 63-, 50-, and 80-kDa proteins, respectively, in stained electrophoresis gels. All of the recombinant proteins were insoluble and seemed to be expressed as precursors with leader peptides. The 63-kDa, 45-k*Da (a truncated protein of the 50-kDa protein), and 80-kDa proteins were purified after solubilization with sodium dodecyl sulfate. N-terminal amino acid sequences of the 45-k*Da and 80-kDa proteins were analyzed up to the first 35 residues with a gas-phase sequencer. Monospecific antibodies directed to the recombinant proteins, i.e., the 63-kDa, 45-k*Da, and 80-kDa proteins, were raised in rabbits. By using the antibodies, localization of their matured proteins in P. gingivalis was investigated by Western blotting analysis. Immunoblotting analysis suggests that at least the 50- and 80-kDa proteins, encoded by genes downstream from the fimA gene, are minor components associated with fimbriae.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Goguen J. D., Sun D., Klemm P., Beachey E. H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J Bacteriol. 1987 Dec;169(12):5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Deal C. D., Kaplan S. Solubilization, isolation, and immunochemical characterization of the major outer membrane protein from Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6524–6529. [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltzel A., Gambill D., Jackson W. J., Totis P. A., Summers A. O. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J Bacteriol. 1987 Jul;169(7):3379–3384. doi: 10.1128/jb.169.7.3379-3384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita E., Amano A., Hanioka T., Tamagawa H., Shizukuishi S., Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect Immun. 1986 May;52(2):421–427. doi: 10.1128/iai.52.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Jacob-Dubuisson F., Dodson K., Kuehn M., Slonim L., Striker R., Hultgren S. J. Adhesin presentation in bacteria requires molecular chaperones and ushers. Infect Immun. 1992 Nov;60(11):4445–4451. doi: 10.1128/iai.60.11.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel K. W., Clark V. L. The RNA polymerases of Porphyromonas gingivalis and Fusobacterium nucleatum are unrelated to the RNA polymerase of Escherichia coli. J Dent Res. 1990 Sep;69(9):1567–1572. doi: 10.1177/00220345900690090601. [DOI] [PubMed] [Google Scholar]
- Kurkela S., Lehväslaiho H., Palva E. T., Teeri T. H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988 Dec 20;73(2):355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Morris C., Rosenberg A. H., Studier F. W. Utilization of bacteriophage T7 late promoters in recombinant plasmids during infection. J Mol Biol. 1981 Dec 15;153(3):527–544. doi: 10.1016/0022-2836(81)90406-x. [DOI] [PubMed] [Google Scholar]
- Mizunoe Y., Nakabeppu Y., Sekiguchi M., Kawabata S., Moriya T., Amako K. Cloning and sequence of the gene encoding the major structural component of mannose-resistant fimbriae of Serratia marcescens. J Bacteriol. 1988 Aug;170(8):3567–3574. doi: 10.1128/jb.170.8.3567-3574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Bouchard D., Deslauriers M., Lamonde L. Immunochemical identification and preliminary characterization of a nonfimbrial hemagglutinating adhesin of Bacteroides gingivalis. Infect Immun. 1989 Feb;57(2):566–573. doi: 10.1128/iai.57.2.566-573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. H., Trust T. J., Kay W. W. Fimbriation genes of Salmonella enteritidis. J Bacteriol. 1989 Sep;171(9):4648–4654. doi: 10.1128/jb.171.9.4648-4654.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies A., Nies D. H., Silver S. Cloning and expression of plasmid genes encoding resistances to chromate and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989 Sep;171(9):5065–5070. doi: 10.1128/jb.171.9.5065-5070.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F. Characterization of Porphyromonas (bacteroides) gingivalis hemagglutinin as a protease. Biochem Biophys Res Commun. 1991 Jul 15;178(1):336–342. doi: 10.1016/0006-291x(91)91819-x. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F., Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33(1):75–80. doi: 10.1111/j.1348-0421.1989.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Ogawa T., McGhee M. L., Moldoveanu Z., Hamada S., Mestecky J., McGhee J. R., Kiyono H. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989 Apr;76(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Shimauchi H., Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989 Nov;57(11):3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hoschützky H., Jann K., Van Die I., Hacker J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol. 1988 Sep;170(9):3983–3990. doi: 10.1128/jb.170.9.3983-3990.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Tenhunen J., Väisänen-Rhen V., Pere A., Båga M., Korhonen T. K. Fimbriation and P-antigen recognition of Escherichia coli strains harbouring mutated recombinant plasmids encoding fimbrial adhesins of the uropathogenic E. coli strain KS71. J Gen Microbiol. 1986 Jan;132(1):71–77. doi: 10.1099/00221287-132-1-71. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J Bacteriol. 1984 Sep;159(3):1034–1039. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Stephens R. L., Haygood M. G., Lidstrom M. E. Identification of putative methanol dehydrogenase (moxF) structural genes in methylotrophs and cloning of moxF genes from Methylococcus capsulatus bath and Methylomonas albus BG8. J Bacteriol. 1988 May;170(5):2063–2069. doi: 10.1128/jb.170.5.2063-2069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yoshimura F., Takahashi K., Tani H., Suzuki T. Detection of fimbriae and fimbrial antigens on the oral anaerobe Bacteroides gingivalis by negative staining and serological methods. J Gen Microbiol. 1988 Oct;134(10):2713–2720. doi: 10.1099/00221287-134-10-2713. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Yoshimura F., Kawanami M., Kato H. Detection of fimbrilin gene (fimA) in Porphyromonas (Bacteroides) gingivalis by Southern blot analysis. J Periodontal Res. 1992 Nov;27(6):599–603. doi: 10.1111/j.1600-0765.1992.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington O. R., Deslauriers M., Stevens D. P., Lyford L. K., Haque S., Yan Y., Flood P. M. Generation and purification of recombinant fimbrillin from Porphyromonas (Bacteroides) gingivalis 381. Infect Immun. 1993 Mar;61(3):1040–1047. doi: 10.1128/iai.61.3.1040-1047.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L., Goldrick D., Hong J. S. Identification of the products and nucleotide sequences of two regulatory genes involved in the exogenous induction of phosphoglycerate transport in Salmonella typhimurium. J Bacteriol. 1988 Sep;170(9):4299–4303. doi: 10.1128/jb.170.9.4299-4303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeung M. K., Cisar J. O. Cloning and nucleotide sequence of a gene for Actinomyces naeslundii WVU45 type 2 fimbriae. J Bacteriol. 1988 Sep;170(9):3803–3809. doi: 10.1128/jb.170.9.3803-3809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Sugano T., Kawanami M., Kato H., Suzuki T. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal diseases. Microbiol Immunol. 1987;31(9):935–941. doi: 10.1111/j.1348-0421.1987.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takasawa T., Yoneyama M., Yamaguchi T., Shiokawa H., Suzuki T. Fimbriae from the oral anaerobe Bacteroides gingivalis: physical, chemical, and immunological properties. J Bacteriol. 1985 Aug;163(2):730–734. doi: 10.1128/jb.163.2.730-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I., Zuidweg E., Hoekstra W., Bergmans H. The role of fimbriae of uropathogenic Escherichia coli as carriers of the adhesin involved in mannose-resistant hemagglutination. Microb Pathog. 1986 Feb;1(1):51–56. doi: 10.1016/0882-4010(86)90031-8. [DOI] [PubMed] [Google Scholar]