Abstract
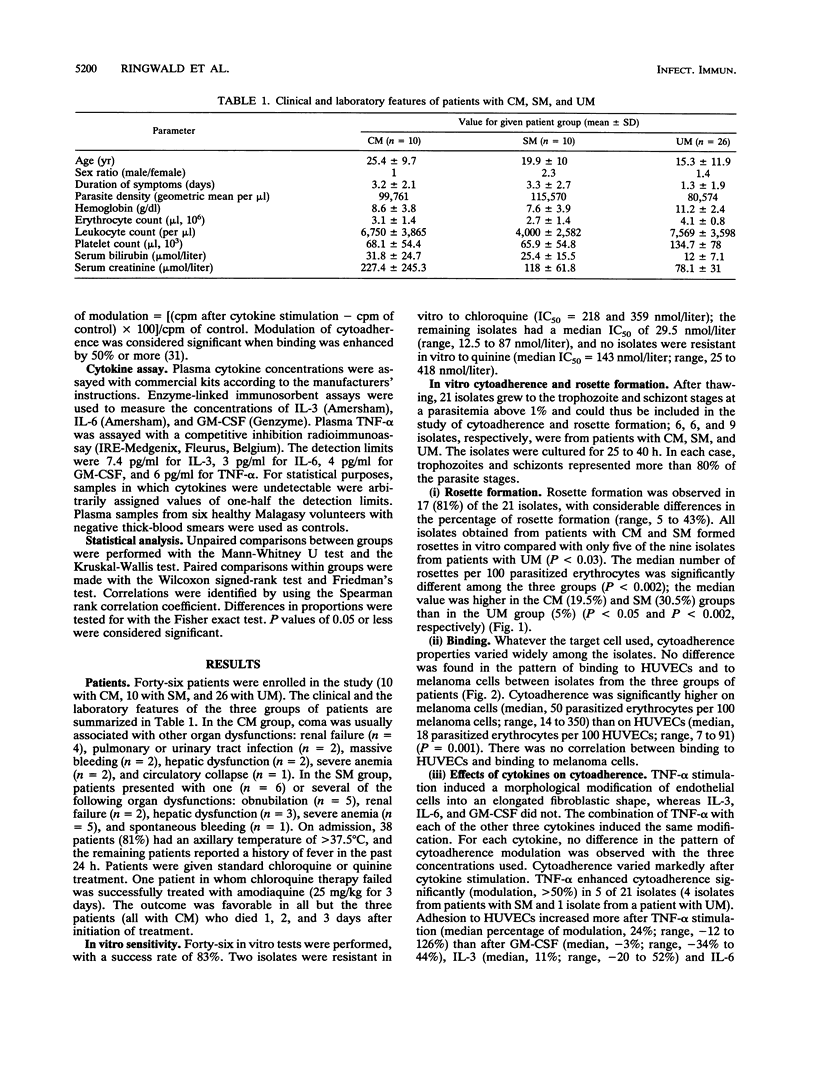
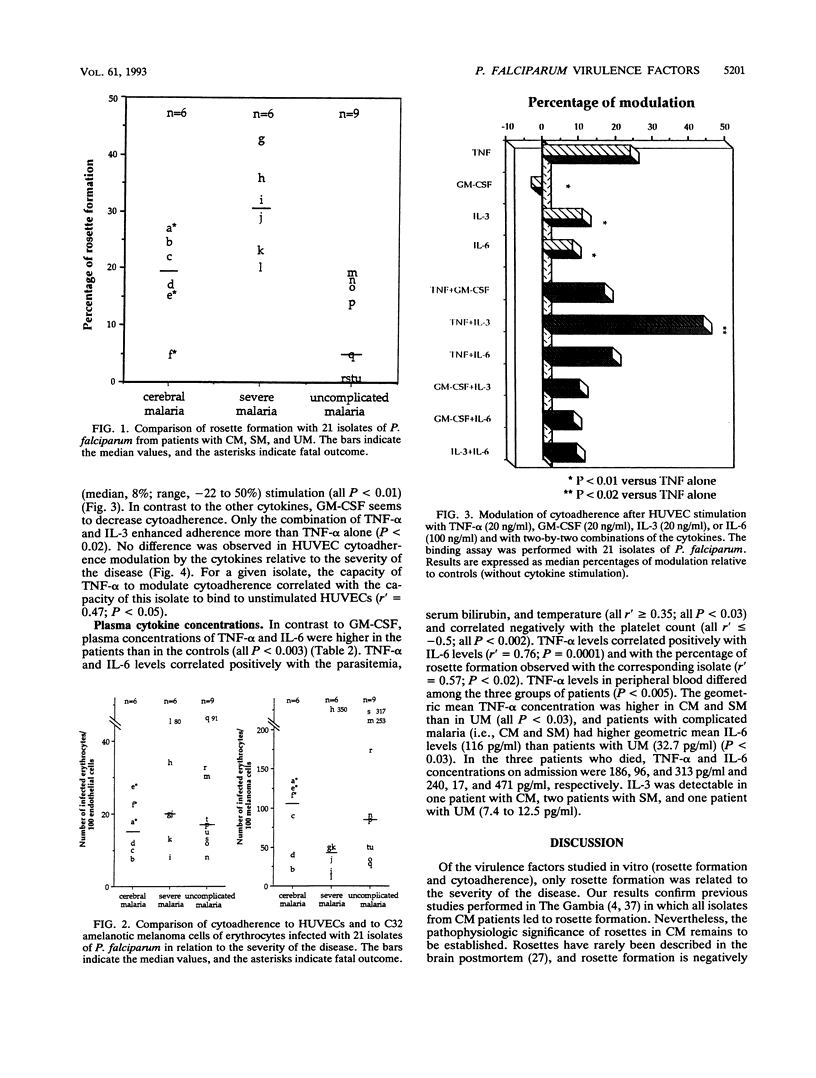
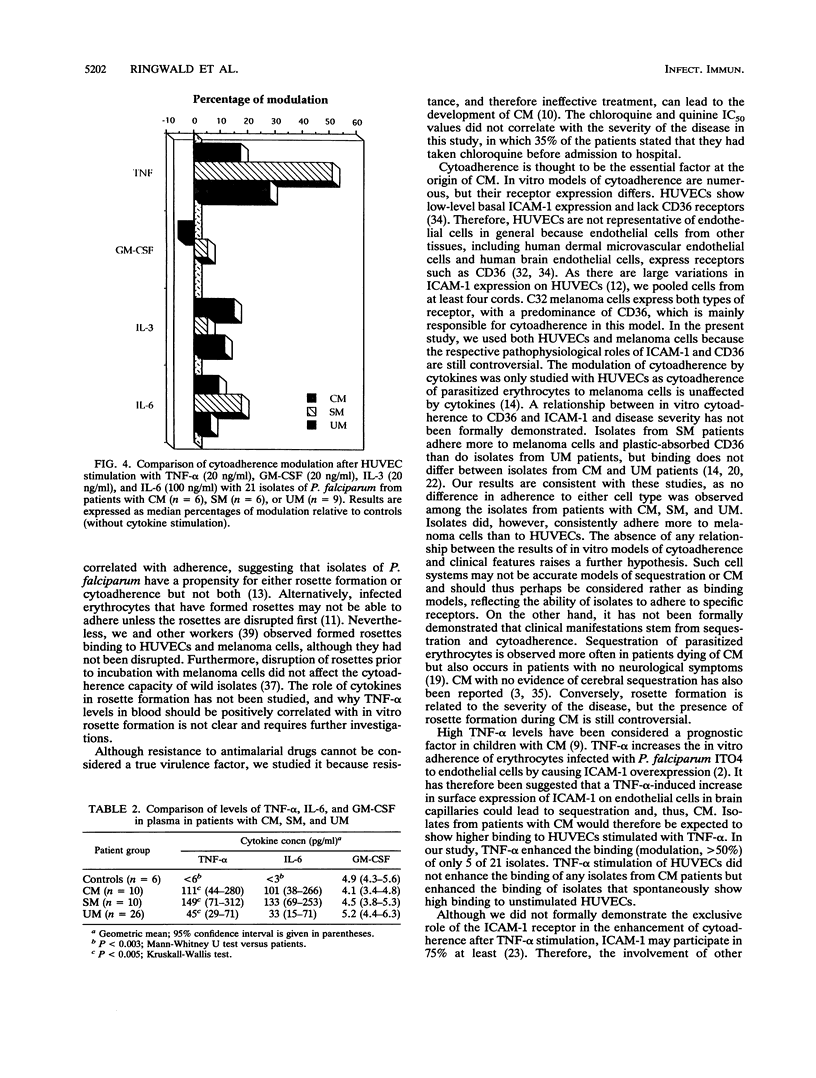
To determine virulence factors of isolates of Plasmodium falciparum and the potential role of cytokines in cerebral malaria, 46 Malagasy patients presenting with cerebral (n = 10), severe (n = 10), and uncomplicated (n = 26) malaria were enrolled in a study. The capacity of 21 of 46 P. falciparum isolates to form rosettes in vitro and to adhere to human umbilical vein endothelial cells (HUVECs) that express intercellular adhesion molecule-1 receptors and to C32 amelanotic melanoma cells that express mainly CD36 receptors was investigated together with the effects of tumor necrosis factor alpha (TNF-alpha), granulocyte macrophage-colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-6 alone and in two-by-two combinations on the cytoadherence of infected erythrocytes to HUVECs. Plasma levels of these cytokines were also measured in the patients at admission. The percentage of rosette formation was higher for the isolates from patients with cerebral (n = 6; 19.5%) and severe (n = 6; 30.5%) malaria than for those from patients with uncomplicated malaria (n = 9; 5%) (P < 0.002). The cytoadherence properties of the isolates did not differ among the three groups whatever the target cell used, but adherence to melanoma cells was systematically higher than that to HUVECs. Adhesion to HUVECs was increased more after TNF-alpha stimulation than after GM-CSF, IL-3, or IL-6 stimulation (P < 0.01). Only the combination of TNF-alpha and IL-3 enhanced cytoadherence more than TNF-alpha used alone (P < 0.02). No difference in the modulation of cytoadherence by cytokines was found in relation to the severity of the disease. TNF-alpha and IL-6 levels in peripheral blood were higher in the patients with cerebral and severe malaria than in the patients with uncomplicated malaria (P < 0.005). Most of the patients' sera contained little or no IL-3 or GM-CSF. Our results challenge the role of intercellular adhesion molecule-1 as the principal receptor mediating the cytoadherence of P. falciparum-infected erythrocytes and contrast with data obtained in the murine model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Asch A. S., Nachman R. L., Yamaya M., Aikawa M., Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989 Sep;84(3):765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig V., Boonpucknavig S., Udomsangpetch R., Nitiyanant P. An immunofluorescence study of cerebral malaria. A correlation with histopathology. Arch Pathol Lab Med. 1990 Oct;114(10):1028–1034. [PubMed] [Google Scholar]
- Carlson J., Helmby H., Hill A. V., Brewster D., Greenwood B. M., Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990 Dec 15;336(8729):1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- Dunn S. M., Hillam A. J., Stomski F., Jin B. Q., Lucas C. M., Boyd A. W., Krissansen G. W., Burns G. F. Leukocyte adhesion molecules involved in inflammation. Transplant Proc. 1989 Feb;21(1 Pt 1):31–34. [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Kindler V., Piguet P. F., Lambert P. H., Vassalli P. Prevention of experimental cerebral malaria by anticytokine antibodies. Interleukin 3 and granulocyte macrophage colony-stimulating factor are intermediates in increased tumor necrosis factor production and macrophage accumulation. J Exp Med. 1988 Oct 1;168(4):1499–1504. doi: 10.1084/jem.168.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Greenwood B., Marsh K., Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991 Oct;7(10):277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- Handunnetti S. M., Hasler T. H., Howard R. J. Plasmodium falciparum-infected erythrocytes do not adhere well to C32 melanoma cells or CD36 unless rosettes with uninfected erythrocytes are first disrupted. Infect Immun. 1992 Mar;60(3):928–932. doi: 10.1128/iai.60.3.928-932.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromatsu Y., Sato M., Yamada K., Nonaka K. Inhibitory effects of nicotinamide on recombinant human interferon-gamma-induced intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigen expression on cultured human endothelial cells. Immunol Lett. 1992 Jan;31(1):35–39. doi: 10.1016/0165-2478(92)90007-b. [DOI] [PubMed] [Google Scholar]
- Ho M., Davis T. M., Silamut K., Bunnag D., White N. J. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. 1991 Jun;59(6):2135–2139. doi: 10.1128/iai.59.6.2135-2139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Singh B., Looareesuwan S., Davis T. M., Bunnag D., White N. J. Clinical correlates of in vitro Plasmodium falciparum cytoadherence. Infect Immun. 1991 Mar;59(3):873–878. doi: 10.1128/iai.59.3.873-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Le Bras J., Andrieu B., Hatin I., Savel J., Coulaud J. P. Plasmodium falciparum: interprétation du semi-microtest de chimiosensibilité in vitro par incorporation de 3H-hypoxanthine. Pathol Biol (Paris) 1984 May;32(5):463–466. [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Marsh V. M., Brown J., Whittle H. C., Greenwood B. M. Plasmodium falciparum: the behavior of clinical isolates in an in vitro model of infected red blood cell sequestration. Exp Parasitol. 1988 Apr;65(2):202–208. doi: 10.1016/0014-4894(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Molyneux M. E., Taylor T. E., Wirima J. J., Grau G. E. Tumour necrosis factor, interleukin-6, and malaria. Lancet. 1991 May 4;337(8749):1098–1098. doi: 10.1016/0140-6736(91)91745-g. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Ho M., Tandon N. N., Van Seventer G. A., Shaw S., White N. J., Jamieson G. A., Chulay J. D., Webster H. K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991 Jul;164(1):163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tegoshi T., Maeno Y., Benjamin C., Ho M., Kan K. E., Thway Y., Win K., Aikawa M., Lobb R. R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992 Oct 1;176(4):1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Riganti M., Pongponratn E., Tegoshi T., Looareesuwan S., Punpoowong B., Aikawa M. Human cerebral malaria in Thailand: a clinico-pathological correlation. Immunol Lett. 1990 Aug;25(1-3):199–205. doi: 10.1016/0165-2478(90)90115-7. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Shaffer N., Grau G. E., Hedberg K., Davachi F., Lyamba B., Hightower A. W., Breman J. G., Phuc N. D. Tumor necrosis factor and severe malaria. J Infect Dis. 1991 Jan;163(1):96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Baird M., Lo S. K., Yesner L. M. Sense and antisense cDNA transfection of CD36 (glycoprotein IV) in melanoma cells. Role of CD36 as a thrombospondin receptor. J Biol Chem. 1992 Aug 15;267(23):16607–16612. [PubMed] [Google Scholar]
- Singh B., Ho M., Looareesuwan S., Mathai E., Warrell D. A., Hommel M. Plasmodium falciparum: inhibition/reversal of cytoadherence of Thai isolates to melanoma cells by local immune sera. Clin Exp Immunol. 1988 Apr;72(1):145–150. [PMC free article] [PubMed] [Google Scholar]
- Smith H., Nelson J. A., Gahmberg C. G., Crandall I., Sherman I. W. Plasmodium falciparum: cytoadherence of malaria-infected erythrocytes to human brain capillary and umbilical vein endothelial cells--a comparative study of adhesive ligands. Exp Parasitol. 1992 Nov;75(3):269–280. doi: 10.1016/0014-4894(92)90212-s. [DOI] [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Li L. J., Sepp N. T., Caughman S. W., Lawley T. J. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992 Jul 15;149(2):698–705. [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Wick T. M., Lawley T. J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992 Jan 1;148(1):78–83. [PubMed] [Google Scholar]
- Toro G., Román G. Cerebral malaria. A disseminated vasculomyelinopathy. Arch Neurol. 1978 May;35(5):271–275. doi: 10.1001/archneur.1978.00500290017004. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Treutiger C. J., Hedlund I., Helmby H., Carlson J., Jepson A., Twumasi P., Kwiatkowski D., Greenwood B. M., Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992 May;46(5):503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Leech J., Aikawa M., Miller L. H. An in vitro assay for sequestration: binding of Plasmodium falciparum-infected erythrocytes to formalin-fixed endothelial cells and amelanotic melanoma cells. J Protozool. 1985 Feb;32(1):88–90. doi: 10.1111/j.1550-7408.1985.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Webster H. K., Pattanapanyasat K., Pitchayangkul S., Thaithong S. Cytoadherence characteristics of rosette-forming Plasmodium falciparum. Infect Immun. 1992 Nov;60(11):4483–4490. doi: 10.1128/iai.60.11.4483-4490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Carlson J., Udomsangpetch R., Perlmann P. Why do Plasmodium falciparumm-infected erythrocytes form spontaneous erythrocyte rosettes? Parasitol Today. 1989 Jun;5(6):183–185. doi: 10.1016/0169-4758(89)90141-5. [DOI] [PubMed] [Google Scholar]
- Wright P. S., Cross-Doersen D., McCann P. P., Bitonti A. J. Plasmodium falciparum: a rapid assay for cytoadherence of [3H]hypoxanthine-labeled infected erythrocytes to human melanoma cells. Exp Parasitol. 1990 Oct;71(3):346–349. doi: 10.1016/0014-4894(90)90040-j. [DOI] [PubMed] [Google Scholar]



