Abstract
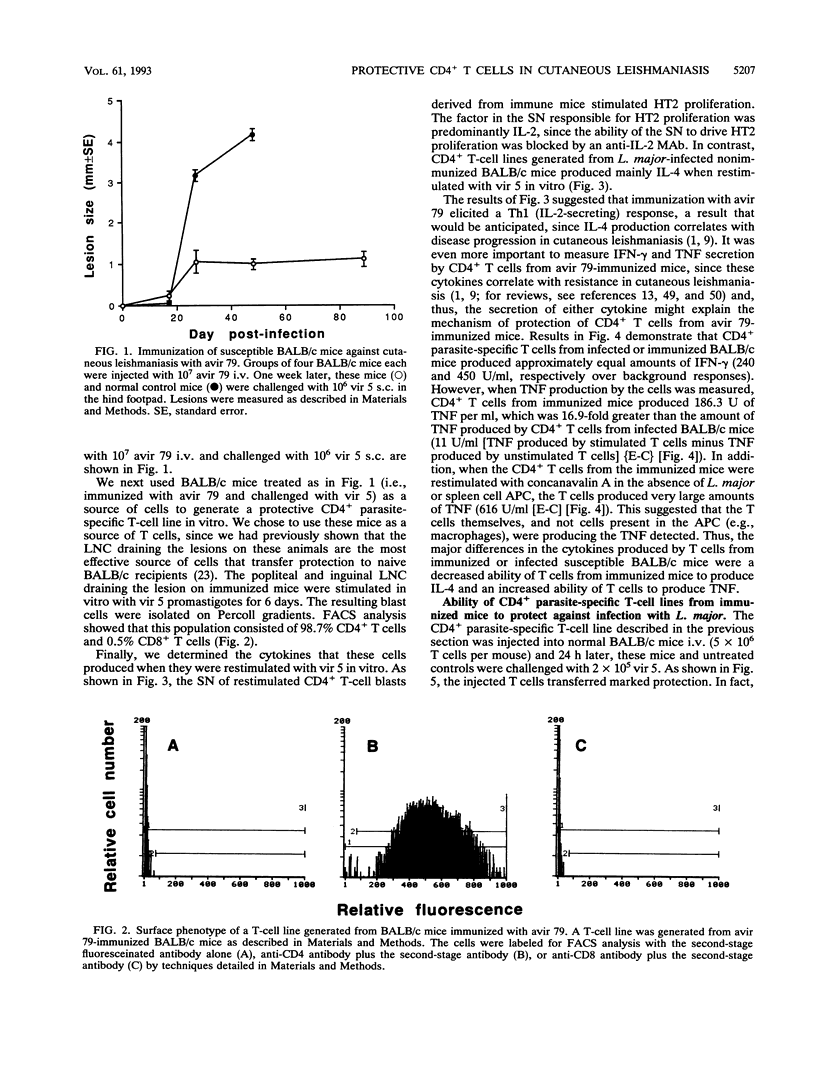
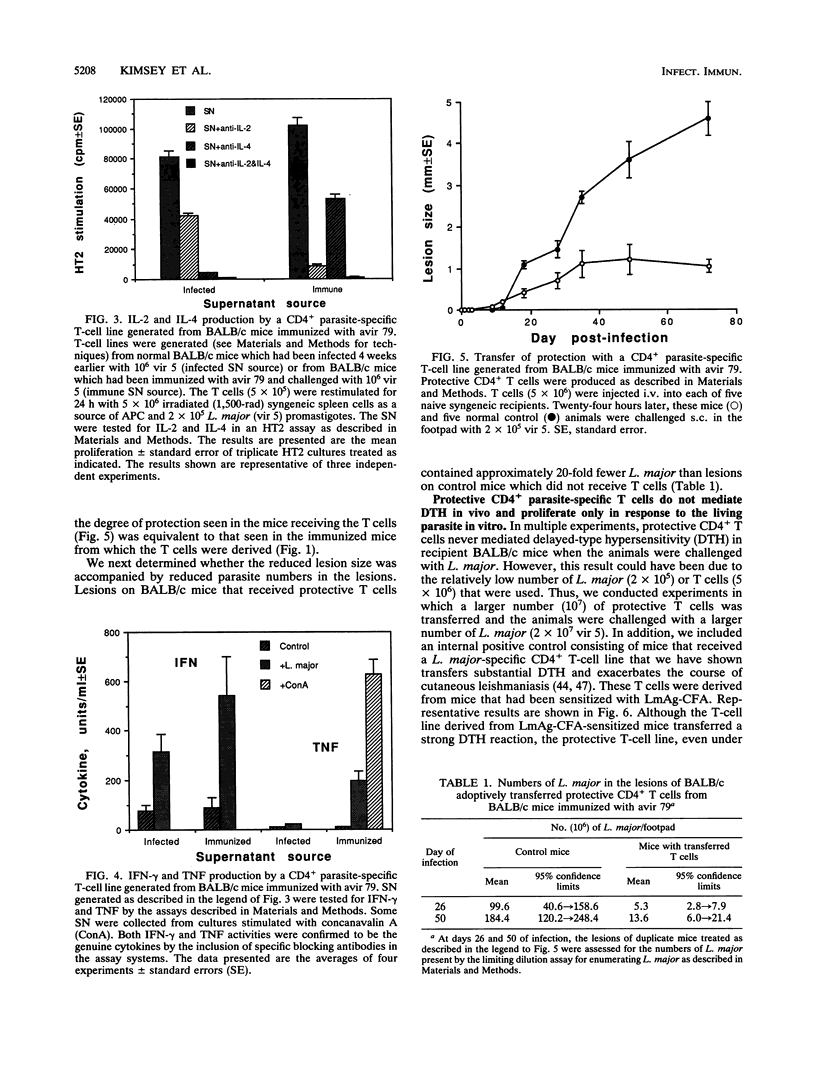
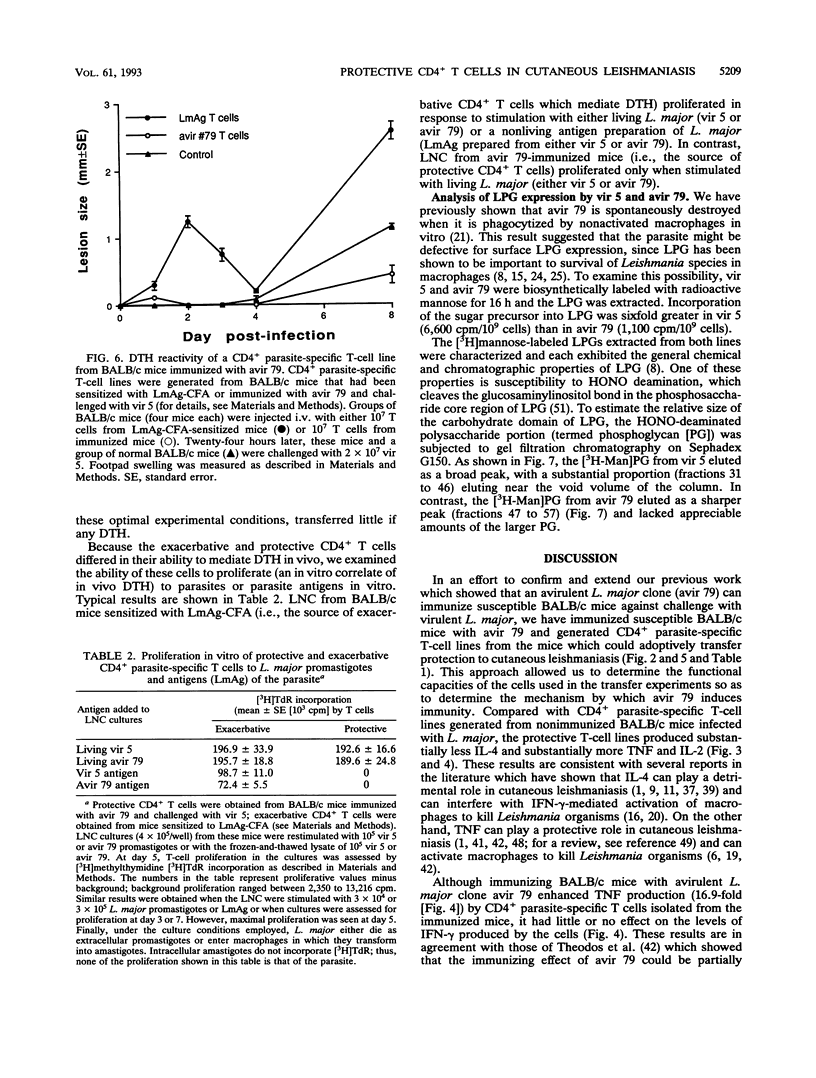
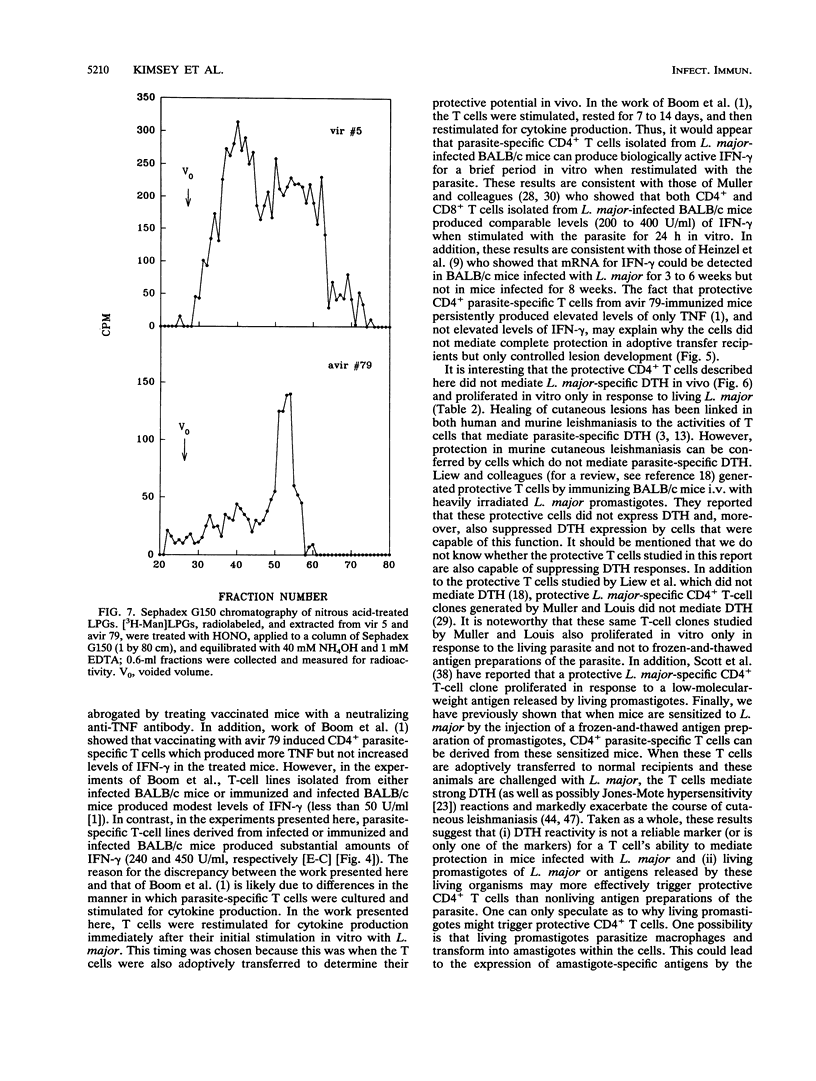
An avirulent clone of Leishmania major was used to immunize susceptible BALB/c mice against challenge with virulent L. major. By using the immunized animals as a source of cells, CD4+ parasite-specific T-cell lines could be generated in vitro which, when adoptively transferred to naive BALB/c recipients, conferred marked protection against challenge with virulent L. major. Compared with CD4+ parasite-specific T-cell lines generated from nonimmunized BALB/c mice infected with L. major, the protective T-cell lines generated from immunized mice produced substantially less interleukin-4 and substantially more tumor necrosis factor and interleukin-2. Interestingly, the protective CD4+ T cells did not mediate L. major-specific delayed-type hypersensitivity in vivo and proliferated in vitro only in response to living L. major and not to frozen-and-thawed antigen preparations of the parasite. Finally, the avirulent clone of L. major was found to express the major surface glycolipid of L. major, lipophosphoglycan, at a level that was sixfold less than expression of this molecule by virulent L. major. In addition, lipophosphoglycan of the avirulent parasite failed to mature into the larger, or metacyclic, form of the molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom W. H., Liebster L., Abbas A. K., Titus R. G. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect Immun. 1990 Dec;58(12):3863–3870. doi: 10.1128/iai.58.12.3863-3870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Gallagher G., Baillie A. J., Alexander J. The induction of protective immunity to Leishmania major in the BALB/c mouse by interleukin 4 treatment. Eur J Immunol. 1989 Apr;19(4):779–782. doi: 10.1002/eji.1830190432. [DOI] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rondón A. J. Diffuse cutaneous leishmaniasis: a disease due to an immunological defect of the host. Trans R Soc Trop Med Hyg. 1972;66(4):603–610. doi: 10.1016/0035-9203(72)90306-9. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Farrell J. P., Muller I., Louis J. A. A role for Lyt-2+ T cells in resistance to cutaneous leishmaniasis in immunized mice. J Immunol. 1989 Mar 15;142(6):2052–2056. [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Schnur L. F., Spithill T. W., Mitchell G. F. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986 Dec 1;137(11):3608–3613. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Awwad M., North R. J. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. J Exp Med. 1989 May 1;169(5):1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday B. J., Sadick M. D., Wang Z. E., Reiner S. L., Heinzel F. P., Parslow T. G., Locksley R. M. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991 Sep 1;147(5):1653–1658. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- Jarpe M. A., Hayes M. P., Russell J. K., Johnson H. M., Russell S. W. Causal association of interferon-gamma with tumor regression. J Interferon Res. 1989 Apr;9(2):239–244. doi: 10.1089/jir.1989.9.239. [DOI] [PubMed] [Google Scholar]
- King D. L., Turco S. J. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol Biochem Parasitol. 1988 Apr;28(3):285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Lezama-Davila C. M., Williams D. M., Gallagher G., Alexander J. Cytokine control of Leishmania infection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 1992 Jan;14(1):37–48. doi: 10.1111/j.1365-3024.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from L-arginine. Immunology. 1990 Dec;71(4):556–559. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Marchand M., Daoud S., Titus R. G., Louis J., Boon T. Variants with reduced virulence derived from Leishmania major after mutagen treatment. Parasite Immunol. 1987 Jan;9(1):81–92. doi: 10.1111/j.1365-3024.1987.tb00490.x. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McGurn M., Boon T., Louis J. A., Titus R. G. Leishmania major: nature of immunity induced by immunization with a mutagenized avirulent clone of the parasite in mice. Exp Parasitol. 1990 Jul;71(1):81–89. doi: 10.1016/0014-4894(90)90010-a. [DOI] [PubMed] [Google Scholar]
- McNeely T. B., Tolson D. L., Pearson T. W., Turco S. J. Characterization of Leishmania donovani variant clones using anti-lipophosphoglycan monoclonal antibodies. Glycobiology. 1990 Sep;1(1):63–69. doi: 10.1093/glycob/1.1.63. [DOI] [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol. 1990 Apr 1;144(7):2745–2750. [PubMed] [Google Scholar]
- Moll H., Röllinghoff M. Resistance to murine cutaneous leishmaniasis is mediated by TH1 cells, but disease-promoting CD4+ cells are different from TH2 cells. Eur J Immunol. 1990 Sep;20(9):2067–2074. doi: 10.1002/eji.1830200927. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Müller I., Louis J. A. Immunity to experimental infection with Leishmania major: generation of protective L3T4+ T cell clones recognizing antigen(s) associated with live parasites. Eur J Immunol. 1989 May;19(5):865–871. doi: 10.1002/eji.1830190513. [DOI] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Kropf P., Louis J., Milon G. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int Immunol. 1991 Jun;3(6):587–597. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Puentes S. M., Da Silva R. P., Sacks D. L., Hammer C. H., Joiner K. A. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol. 1990 Dec 15;145(12):4311–4316. [PubMed] [Google Scholar]
- Sacks D. L., Brodin T. N., Turco S. J. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):225–233. doi: 10.1016/0166-6851(90)90165-i. [DOI] [PubMed] [Google Scholar]
- Sacks D. L. Metacyclogenesis in Leishmania promastigotes. Exp Parasitol. 1989 Jul;69(1):100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypek J. P., Matzilevich M. M., Wyler D. J. Th2 lymphocyte clone can activate macrophage antileishmanial defense by a lymphokine-independent mechanism in vitro and can augment parasite attrition in vivo. Cell Immunol. 1991 Mar;133(1):178–186. doi: 10.1016/0008-8749(91)90189-i. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Wyler D. J. Antileishmanial defense in macrophages triggered by tumor necrosis factor expressed on CD4+ T lymphocyte plasma membrane. J Exp Med. 1991 Oct 1;174(4):755–759. doi: 10.1084/jem.174.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodos C. M., Povinelli L., Molina R., Sherry B., Titus R. G. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect Immun. 1991 Aug;59(8):2839–2842. doi: 10.1128/iai.59.8.2839-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Milon G., Marchal G., Vassalli P., Cerottini J. C., Louis J. A. Involvement of specific Lyt-2+ T cells in the immunological control of experimentally induced murine cutaneous leishmaniasis. Eur J Immunol. 1987 Oct;17(10):1429–1433. doi: 10.1002/eji.1830171007. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Müller I., Kimsey P., Cerny A., Behin R., Zinkernagel R. M., Louis J. A. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991 Mar;21(3):559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. The involvement of TNF, IL-1 and IL-6 in the immune response to protozoan parasites. Immunol Today. 1991 Mar;12(3):A13–A16. doi: 10.1016/S0167-5699(05)80005-2. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Theodos C. M., Kimsey P. B., Shankar A., Hall L., McGurn M., Povinelli L. Role of T cells in immunity to the intracellular pathogen, Leishmania major. Subcell Biochem. 1992;18:99–129. doi: 10.1007/978-1-4899-1651-8_4. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Orlandi P. A., Jr, Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989 Apr 25;264(12):6711–6715. [PubMed] [Google Scholar]



