Abstract
Background
The neuroanatomical basis of lexical retrieval has been studied intensively. The current review focuses on the special case of proper nouns.
Aims
This article reviews a program of research that has used both lesion-deficit and functional imaging (PET) approaches to investigate the neuroanatomical basis for lexical retrieval of proper nouns. In lesion-deficit studies, we found that damage to the left temporal polar (TP) region leads to reliable and specific impairments in naming famous persons (e.g., “George Clooney”) and famous landmarks (e.g., “Golden Gate Bridge”). In functional imaging studies, we found that when participants name famous persons and landmarks, they produce specific activation (increases in regional cerebral blood flow) in the left TP region.
Main Contribution
These findings converge with lesion and functional imaging data from other laboratories to support the idea that the left TP region is important for the retrieval of names for unique concrete entities, persons and landmarks being typical examples of such categories of entities.
Conclusions
We have interpreted these results within a theoretical framework that suggests that left TP contains convergence regions that operate as intermediaries between conceptual knowledge retrieval and lexical retrieval for classes of unique concrete entities.
Keywords: aphasia, anomia, proper nouns, visual naming, lesion, positron emission tomography
1. INTRODUCTION
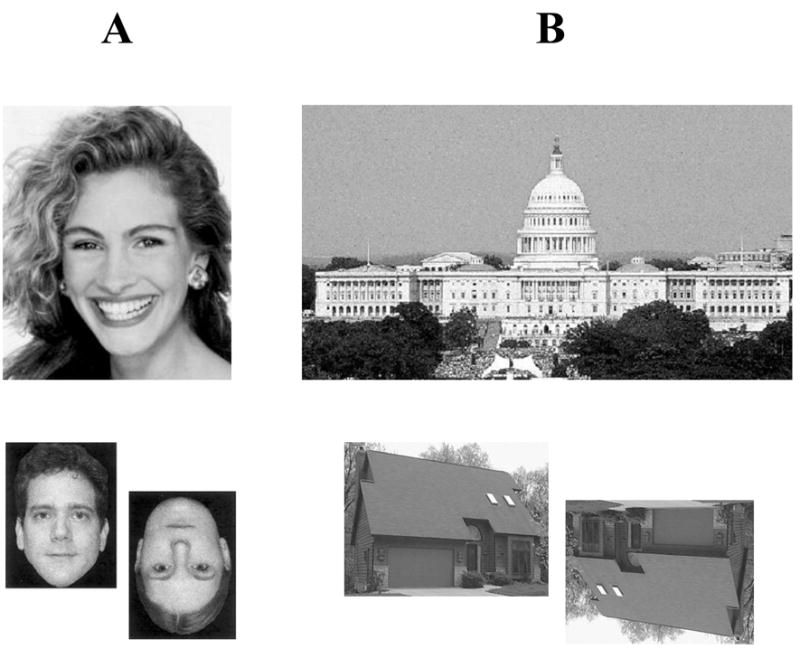
Consider what happens when you view famous persons or famous landmarks, such as those exemplified in Figure 1. Your brain engages a series of processes that lead to the retrieval of conceptual knowledge about the stimuli—i.e., information about the meaning of the stimuli, such as their attributes, properties, associations with other memories, and semantic facts that make up their definitions. Another important piece of information that will be retrieved is the name of the item, and normally, the name retrieval process unfolds virtually automatically in the wake of the conceptual knowledge retrieval process (in fact, the naming process is not even amenable to conscious suppression). These processes—from retrieving the meaning of an entity to retrieving its name—happen extremely quickly and (mostly) highly accurately. What our brains are accomplishing here is the identification of unique concrete entities, that is, the retrieval of specific and precise knowledge, including a proper name, for stimuli that are concrete (i.e., having tangible, definable physical properties) and unique (i.e., being one-of-a-kind, matchless). These entities have unique conceptual and lexical associations, and they have been termed “semantically unique items” (Gorno-Tempini & Price, 2001). The categories represented by examples in Figure 1, famous persons and famous landmarks, comprise such entities: the items are unique and denoted by a proper name.
Figure 1.
Examples of (A) famous persons and (B) famous landmarks.
It is important to clarify that there is a distinction between the processes that relate to knowing an entity and those that relate to naming an entity (processes typically referred to in the neuropsychological literature as “recognition” and “naming,” respectively). Normally, these processes are seamless, automatic, and indistinguishable. But they are not the same—they are not interchangeable, and they can be dissociated. Consider the fairly common experience in which one encounters a well known individual who is recognized immediately, but the name of the person cannot be recalled (even though it is well known). Aging, fatigue, distraction, and other such factors can adversely affect the ability to retrieve proper names. In patients with brain damage to critical structures, this phenomenon can be amplified many times over, and the patients may exhibit a striking inability to retrieve many, sometimes even most, of the proper names for unique entities whose meaning they can perfectly well recognize. In short, there is a clear separation between knowing and naming that can occur (but usually doesn’t) in normal healthy brains, and that can be dramatically exaggerated in damaged brains. This underscores the fact that the processes for knowing and naming are at least partially separate, and this is true not only at the cognitive/psychological level but also at the neuroanatomical level.
The current article focuses on the retrieval of proper names for unique concrete entities, and in particular, on how proper name retrieval is reliably related to a particular neuroanatomical sector, namely, the left temporal polar region (Brodmann area 38; see Figure 2). Proper naming can be disrupted by damage in many different brain areas (see Semenza, Mondini, & Zettin, 1995, for a review), as well as by degenerative diseases that cause more diffuse and widespread brain dysfunction (such as Alzheimer’s disease; e.g., Hodges, Salmon, & Butters, 1993), and as noted earlier, proper naming is also susceptible to adverse effects from commonplace, non-neurological factors such as fatigue and aging (e.g., Cohen & Burke, 1993). But what becomes more interesting from a neuroscience perspective is the fact that focal damage restricted to the left temporal pole can produce severe and circumscribed impairment in retrieving proper names. In addition, healthy persons who are engaged in a proper naming task show focal activation (increased blood flow) in the left temporal polar region. This evidence comes together to support a consistent and compelling account of a particular brain-behavior relationship, viz., that proper name retrieval is associated with the left temporal pole. This account is the storyline for the current article.
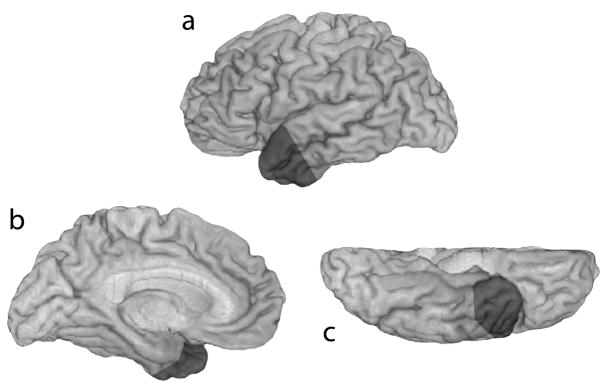
Figure 2.
The temporal polar (TP) region is marked in dark gray shading on lateral (a), mesial (b), and ventral (c) views of the left hemisphere.
The evidence is presented within the following outline. In Section 2, work from our laboratory regarding the retrieval of proper names for famous persons is presented. Lesion studies are presented first, followed by functional imaging studies. In Section 3, work regarding the retrieval of proper names for famous landmarks is presented; again, the lesion approach is presented first, followed by functional imaging. For both categories of stimuli, the lesion-deficit and functional imaging approaches provide complementary and convergent findings that hone in on the basic message regarding proper name retrieval and the left temporal pole. The fact that the message is similar for different categories of unique entities (persons and landmarks) suggests that this brain-behavior relationship has something to do with uniqueness and proper naming, as opposed to persons or landmarks per se, or word retrieval per se. In Section 4, a theoretical framework is briefly sketched out, and the findings are situated in the context of work from other laboratories that has converged on conclusions similar to those from our laboratory.
2. RETRIEVING NAMES FOR PERSONS
2.1. Lesion-Deficit Approach
A variety of case studies, and some preliminary observations of patients in our research program, had suggested that damage to the left temporal polar (TP) region could produce a circumscribed impairment in naming familiar persons (e.g., Damasio, Damasio, Tranel, & Brandt, 1990; Drane, Ojemann, Aylward, Ojemann, Johnson, Silbergeld, Miller, & Tranel, 2008; Glosser, Salvucci, & Chiaravalloti, 2003; Seidenberg, Griffith, Sabsevitz, Moran, Haltiner, Bell, Swanson, Hammeke, & Hermann, 2002; Tranel, Damasio, & Damasio, 1997a; Tsukiura, Fujii, Fukatsu, Otsuki, Okuda, Umetsu, Suzuki, Tabuchi, Yanagawa, Nagasaka, Kawashima, Fukuda, Takahashi, & Yamadori, 2002; Viskontas, McAndrews, & Moscovitch, 2002). Picking up on this idea, we conducted several systematic studies in which we investigated the reliability of this association in large groups of neurological patients (Damasio, Grabowski, Tranel, Hichwa, & Damasio, 1996; Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004; Tranel, 2006). In regard to proper name retrieval, the main hypothesis in these lesion studies was that impaired proper naming would be associated specifically with damage to the left TP region.
2.1. a. Initial study (Damasio et al., 1996)
Our initial study was conducted in the mid-1990s, when we took a large group of neurological patients with focal brain lesions and measured their naming abilities with a detailed series of standardized tests. The portion of this work described here focuses on the proper naming aspect of the study. (The patients have also been investigated extensively with tests of common naming, e.g., naming of nonunique concrete entities such as animals, fruits/vegetables, and tools/utensils. Since the current review is focused on proper naming, the common naming data will not be reported in detail. However, a comment about those data will be included in the Discussion of the findings, because this is critical to the consideration of how specific the proper naming deficit is in the patients.)
Participants
The participants included 127 neurological patients selected from our Patient Registry.1 All patients gave informed consent. The patients were selected to have lesions that would allow us to sample most sectors of the left and right hemispheres. The lesions were caused by either cerebrovascular disease (n = 106), herpes simplex encephalitis (n = 5), or temporal lobectomy (n = 16). The patients had to have strong indication of left-hemisphere language dominance, as determined from neurological and neuropsychological testing (and by Wada testing in the temporal lobectomy patients). 115 patients were right-handed, 6 were left-handed, and 6 were mixed-handed. The patients did not have general intellectual impairment (as determined by Wechsler Adult Intelligence Scale-III testing), and they did not have difficulty attending to or perceiving visual stimuli (as determined by detailed neuropsychological assessment). Some patients had been diagnosed acutely with aphasia, but in the chronic epoch their residual aphasia was not severe enough to prevent them from providing valid responses in the proper naming task (that is, the patients understood the task demands, and understood that they should attempt to produce a proper name for each stimulus, or indicate that they did not know the stimulus). All data, including standard neuropsychological measures, neuroanatomical data, and the proper naming performances, were obtained in the chronic phase, when patients were at least 3 months post lesion onset.2 We also studied 55 normal, healthy participants, who were matched to the neurological patients on age, education, and gender distribution.
Task and Procedure
The experimental task was to name pictures of famous persons. The stimuli were 133 pictures of famous persons (presented as faces). The pictures were shown one-by-one in free field, and for each, the participant was requested to state who the person was. If a vague or superordinate response was produced (e.g., “a blonde lady” or “an actress”), the participant was prompted as follows: “Be more specific; say exactly who you think that is.” Time limits were not imposed.
Scoring
For each of the 133 faces, if the participant produced the correct name, the naming response was scored as correct. If the participant did not produce a name, gave the wrong name, or gave a vague or superordinate response, the naming response was scored as incorrect. Items that were named correctly were scored as being correctly recognized. For items not named correctly, the information the participant had produced regarding specific characteristics of the item was used to judge whether the participant had produced an acceptable identification of the person. If no information was generated, or if the information generated was inaccurate, vague, or generic, the item was scored as a recognition failure. If a participant produced the wrong name, but still identified the stimulus accurately, the response was scored as a recognition success but a naming failure. The descriptive responses provided by participants (transcriptions) were reviewed by raters (laboratory personnel who were blind to the objectives of the study), and if raters could identify the famous person from the description the participant had provided, then the response was scored as a correct recognition; otherwise, it was scored as incorrect. For each participant, the naming score was calculated by dividing the number of items named correctly by the number recognized correctly (and converting to percent). It should be noted that this procedure does not penalize participants for failing to name items they do not recognize. This method is summarized in Figure 3. Following neuropsychological convention, naming scores that were 2 or more standard deviations below the mean of the normal comparison group were classified as “impaired.”
Figure 3.

Example of scoring procedure for “recognition” and “naming.”
Correct naming (and correct recognition): “Michael Jordan”
Correct recognition, incorrect naming: “He was a really great basketball player; won championships in Chicago; tried to play baseball.”
Incorrect recognition: “I’m not sure…. actor? I think he was in movies or on a television show.”
Neuroanatomical Analysis
Neuroanatomical analysis was based on magnetic resonance (MR) data (or CT data in a few patients), reconstructed in three dimensions. For each patient, the lesion was transposed and manually warped into a normal 3-D reconstructed brain. This method permits the determination of the maximal overlap of lesions relative to patients grouped by a particular neuropsychological defect (in this case, impaired person naming) (see Damasio et al., 2004, for details regarding this method).
Results
Of the group of 127 brain-damaged patients, there were 13 who had impaired person naming. These 13 patients had an average person naming score of 49.6% correct (SD = 23.1), significantly below the mean score of the remaining 114 patients with unimpaired person naming (M = 89.3% correct, SD = 4.9; t(125) = −6.17, p < .001). Looking at the lesion overlap in the 13 impaired patients, we found that the highest region of overlap was in the left TP region. The maximal overlap, which at its highest entailed lesions from all 13 affected patients, included the lateral, inferior, and mesial aspects of the TP, and included both cortex and subcortical white matter. These findings provided strong support for the hypothesis that left TP damage would cause impaired naming of famous persons.
Although a detailed consideration of the common naming abilities of the patients is outside the scope of this review, it is important to note that of the 13 patients with impaired proper naming, 7 had a defect restricted to the category of persons (and did not have defects in naming animals or tools/utensils). In follow-up analyses (see Figure 2 in Damasio et al., 1996), we found that there was a strong association between lesions restricted to the TP region, and naming defects restricted to the category of proper names. These additional findings point to the specificity of the temporal pole-proper naming relationship, a point that is taken up further in the Discussion below.
2.1. b. Follow-up study (Damasio et al., 2004)
A replication and extension of our initial study was conducted in a follow-up investigation several years later, using an expanded patient population and refined data analysis techniques (Damasio et al., 2004). Again, the main hypothesis pertinent to lesions and proper naming was that impaired naming of famous persons would be associated specifically with damage to the left TP region.
Participants
The participants included 139 neurological patients selected from our Patient Registry. The lesions were caused by either cerebrovascular disease (n = 113), herpes simplex encephalitis (n = 5), or temporal lobectomy (n = 21). The cohort of patients started out with an N of 169, but was narrowed down to 139 because we focused the analyses on patients who had been tested in five different categories of concrete entities, to facilitate between-category comparisons. The majority of these patients were initially reported in the Damasio et al. (1996) study described in section 2.1. a above, although several dozen new patients were included in the Damasio et al. (2004) follow-up study. Of the 139 patients, 126 were right-handed, 4 were left-handed, and 9 were mixed-handed; the patients otherwise conformed to the same specifications as in the initial study described above.
Task, Scoring, and Neuroanatomical Analysis
The proper naming task and scoring procedures were the same as in the initial study. The neuroanatomical analysis was also the same, with the following exceptions. Instead of simply calculating an overlap map for the patients with impaired naming, we calculated two overlap maps and then subtracted them. Specifically, an overlap map was calculated for the impaired patients, and an overlap map was calculated for the unimpaired patients. The unimpaired map was then subtracted from the impaired map, on a voxel-by-voxel basis. This subtraction yields a volume, which we call a lesion-difference map, which shows regions for which there is an excess of lesions in the impaired group relative to the unimpaired group. Using an “excess criterion” of five, we identified areas that were considered specifically related to impaired proper naming. In other words, such regions, at a given voxel, had at least 5 more impaired patients relative to unimpaired patients.3
Results
There were 39 patients in the overall sample with impaired person naming (scores ≥2 SDs below the normal mean). The lesion-difference map showed a marked concentration of impaired > unimpaired in the left TP region, where the excess of impaired patients was as high as 11. There was no other region in the left hemisphere, and no region anywhere in the right hemisphere, that showed a concentration of lesions associated with impaired naming of famous persons. This outcome replicates and extends our previous results, and again supports the conclusion that damage to left TP is reliably and specifically associated with impaired person naming.
2.1. c. Another replication (Tranel, 2006)
A recent study provided another replication of the two larger lesion studies reported above, but using a somewhat different approach. Specifically, in the more recent study (Tranel, 2006) the neuroanatomical status of the patients served as the independent variable, and the neuropsychological outcome (person naming score) served as the dependent variable. This is a reverse of the approach used in the two previous studies, where the neuropsychological status had served as the independent variable and the neuroanatomical status was the dependent variable. The hypothesis was that impaired person naming would be associated specifically with left TP damage.
Participants
The participants included 11 patients with left TP lesions and 10 patients with right TP lesions, selected from our Patient Registry, and 90 normal, healthy comparison participants. The patients conformed to the same general specifications as detailed above (Section 2.1. a), and the left TP, right TP, and comparison groups had comparable demographics. To be included in the TP groups, patients had to have lesions that encroached into area 38 in either the left or right hemisphere.
Task and Procedure
The task was the same as the one we have used previously to measure famous person naming; however, we developed a new Famous Faces Test that included 155 faces of actors, sports figures, and politicians. The procedures for administering and scoring the test were the same as those described earlier, and the dependent measure was each patient’s naming score, specifically, the number of persons named correctly divided by the number of persons recognized correctly (in percent). The three groups—left TP, right TP, and normal comparison—were contrasted on the naming score.
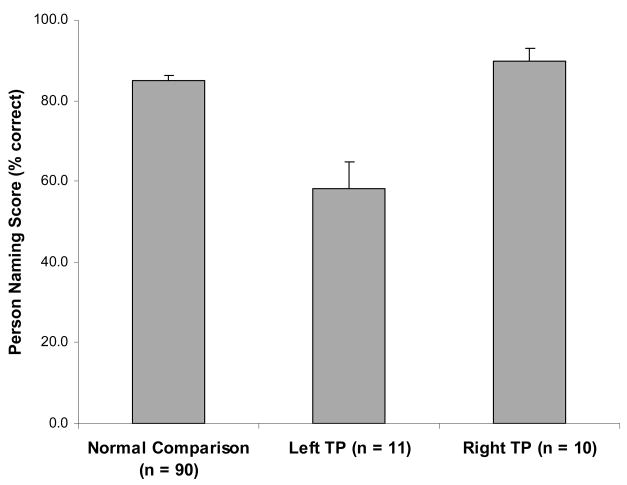
Results
The 11 left TP patients had a mean person naming score of 58.1 (SD = 22.5). By contrast, the 10 right TP patients had a mean naming score of 89.8 (SD = 9.7), and the 90 normal comparison participants had a mean naming score of 85.0 (SD = 11.1) (Figure 4). The score of the left TP group is 26.9 percentage points (and more than 2 SDs) below the mean of the normal comparison group, and significantly lower than the score of the right TP group (t(19) = 4.11, p < .001). These findings again show a reliable and specific association between left TP damage and impaired person naming.
Figure 4.
Person naming performances in normal participants and in patients with left TP or right TP brain damage, in mean percent correct (SEM).
Although it was not a focus of this study, the recognition performances of the patients were also calculated, and consistent with previous work (Tranel, Damasio, & Damasio, 1997b; Viskontas et al., 2002), we found that patients with right TP lesions tended to have mild to moderate deficits in famous person recognition, with scores 1 to 2 standard deviations below those of normal comparison participants. Such deficits were not evident for recognition of landmarks, however, as the right TP patients as a group had landmark recognition scores (M = 65.3 percent correct, SD = 8.3) that were at the same level as normal comparison participants (M = 62.0 percent correct, SD = 18.0). The differences between landmarks and persons, insofar as recognition is concerned, raise interesting questions about whether different neural systems may underpin recognition capacities for these various categories, and this remains an important unanswered question for future research.
2.2. Functional Imaging Approach
Convergent evidence for the relationship between left TP and person naming has come from functional imaging studies, both from our laboratory (Damasio et al., 1996, 2004; Grabowski, Damasio, Tranel, Ponto, Hichwa, & Damasio, 2001) and others (Gorno-Tempini & Price, 2001; Tsukiura et al., 2002; Tsukiura, Namiki, Fujii, & Iijima, 2003). The main studies we have conducted on this topic are summarized here. In this work, we have used positron emission tomography (PET), which affords certain advantages over fMRI in looking at the temporal polar region. The main hypothesis was that naming famous persons would produce specific activation in left TP.
2.2. a. Initial study (Damasio et al., 1996)
Participants
The participants were 9 healthy persons (7 women, 2 men4), with no history of neurological or psychiatric illness, all of whom were fully right-handed, native English speakers, and at least high school educated. They ranged in age from 22 to 49.
Task
The task involved naming famous faces. The faces were presented on a computer monitor, at a rate of one every 2.5 seconds, and participants were requested to name each face (the overall naming success rate was 83%). A baseline task was also administered, and this entailed presenting unfamiliar faces either right side up or upside down, and requiring the participant to judge the orientation and respond “up” or “down” (Figure 5). The naming task and the baseline task were performed twice by each participant.
Figure 5.
Examples of stimuli used in the PET studies from our laboratory: A. Famous face naming (target) task and face orientation judgment (baseline) task. B. Famous landmark naming (target) task and house orientation judgment (baseline) task.
Procedure and Data Analysis
PET data were acquired on a GE whole-body tomograph, after intravenous bolus administration of 50 mCi of [15O] water. Regional cerebral blood flow was estimated using standard methods (Herscovitch, Markham, & Raichle, 1983). MR and PET data were co-registered (Woods, Mazziotta, & Cherry, 1993), and the search volume (including left TP) was defined. (The search volume comprises the specific neural regions where we looked for rCBF changes.) PET data were analyzed with a pixelwise linear model (Friston, Holmes, Worsley, Poline, Frith, & Frackowiak, 1991), and the critical t value (p < 0.05) was set at 3.82. To determine the activation associated with person naming, the baseline task was subtracted from the person naming task.
Results
The contrast between the person naming task and the baseline task revealed two main areas of significant activation: (1) Left TP (−37, +3, −33; 1,022 mm3; t=4.57;); and (2) Right TP (+42, 0, −34; 1,090 mm3; t=+4.50). The left TP activation is squarely in the same region that was identified in the lesion studies as being important for person naming, and this result thus provides convergent evidence for the association between left TP and person naming. We interpreted the right TP activation as evidence of the recognition process that invariably accompanies the naming process in normal individuals (e.g., Sergent, Ohta, & MacDonald, 1992). (Convergent evidence for this interpretation comes from our previous lesion work, which, as noted in Section 2.1. c. above, has indicated that right TP lesions can lead to impairments in person recognition (Tranel et al., 1997b).)
2.2. b. Follow-up study (Grabowski et al., 2001)
Participants
The participants were 10 (5 men, 5 women) healthy individuals with an average age of 28 years, all native English speakers, fully right-handed, and at least high school educated.
Task
The person naming task was similar to the previous study. Participants were presented with pictures of famous faces, and asked to name each one. Faces were presented every 2.5 seconds, and a total of 15 faces were presented for each PET injection (two replications of the person naming task were performed). The baseline task (judging the orientation of unfamiliar faces) was the same as in the previous study, and it was also performed twice.
Procedure and Data Analysis
The PET procedure was the same as that used previously. Spoken naming responses were audiotaped, allowing us to determine response accuracy and latency. (As it turned out, person naming accuracy was M = 84.4% correct, and the median response latency was 1556 msec.) The search volume for the PET activations comprised the left and right temporal poles (the latter because we expected the recognition part of the process to activate the right TP region, as before). The threshold t value (p = 0.05) for the search volume was 3.09. For purposes of analysis, the activation associated with the baseline task was subtracted from the activation associated with the person naming task.
Results
The person naming minus baseline contrast revealed a significant area of activation in the left TP region, as predicted (−39, +15, −17; Tmax=3.56; t65=3.97), which we associate with the process of lexical retrieval for proper names. This contrast also yielded a significant area of activation in the right TP region, which, again, we interpret as evidence for the recognition component of the process (+45, +17, −13; Tmax=3.37). These results are similar to those obtained in our first PET study, and they provide further confirmation of the relationship between proper name retrieval and the left temporal pole.
2.2. c. Another follow-up study (Damasio et al., 2004)
In our summary article published in 2004 (Damasio et al.), we reanalyzed and summarized results from previous PET studies that had investigated person naming.
Participants
The participants were 16 (8 men, 8 women) healthy individuals with an average age of 28.9 years, drawn from the Damasio et al. (1996) and Grabowski et al. (2001) studies.
Task, Procedure, and Data Analysis
The person naming task, baseline task, PET procedures, and data analysis approach were the same as in the previous studies. In brief, participants were engaged in naming famous faces, and the activation associated with this task was contrasted with a baseline task (face orientation judgment) by subtracting the latter from the former. The average naming performance accuracy by the 16 participants was 82% correct, and the average response latency was 1332 msec.
Results
The person naming minus baseline contrast revealed a significant area of maximal activation in the left TP region (−40, +7, −24; t=4.10). This maximum falls within 10 mm from the maximum we found in the Damasio et al. (1996) study, and within 10.6 mm from the maximum we found in the Grabowski et al. (2001) study. These activation peaks are very close to one another, and they are all squarely within the left TP region. Together, the PET studies provide consistent confirmation of the lesion data in suggesting a relationship between the left TP region and person name retrieval.
3. RETRIEVING NAMES FOR LANDMARKS
An interesting and theoretically important question that emerged during our work on face processing and proper naming is whether the brain-behavior relationships that have been established for famous persons would extend to other categories of concrete entities. We have taken the theoretical position on this issue that the factors driving the placement of neural systems for proper name retrieval in the left temporal polar region pertain to the nature of the stimuli (unique, concrete, numerous) and the nature of the processing requirements (identification at a level of unique specificity), and this model predicts that any category in which such factors are operative will build on the neural systems in left TP for retrieval of the lexical items associated with the entities in the category.5 (Although it is beyond the scope of the current review, our framework likewise predicts that for categories in which these factors are not operative, the neural operators will be outside the left TP region, e.g., in other parts of the left inferotemporal region. This point was touched on in section 2.1. a, where we noted that lesions restricted to left TP tend to cause naming impairments that are restricted to proper nouns, and do not affect naming in other categories such as animals, tools/utensils, and fruits/vegetables, where a common noun naming response is appropriate.) A category that is much like persons in this regard is landmarks: like persons, landmarks are unique, and they are identified by a proper name. Lesion studies had shown that patients with impaired person naming frequently demonstrated a parallel defect in naming geographical items, including landmarks (e.g., Fery, Vincent, & Brédart, 1995; Lyons, Hanley, & Kay, 2002; Otsuka, Suzuki, Fuji, Miura, Endo, Kondo, & Yamadori, 2005). Hence, a natural next step in our program of work was to study the neuroanatomical correlates of retrieving names for landmarks. As in the case of persons, the focus of this work has been on the left TP region, and in parallel with the face-related work presented in Section 2, we have investigated the landmark category using both a lesion-deficit approach and a functional imaging approach.
3.1. Lesion-Deficit Approach
We have used a lesion-deficit approach to study the neuroanatomical basis of landmark naming (Tranel, 2006), and the results of this work are summarized below. The main hypothesis we tested in this study was that impaired naming of famous landmarks would be associated with lesions in the left TP region.
Participants
The participants were 74 neurological patients selected from our Patient Registry. The patients had focal, stable lesions to either the left (n = 40) or right (n = 34) hemisphere, caused by cerebrovascular disease (n = 45), temporal lobectomy (n = 13), surgical resection of arteriovenous malformation or benign tumor (n = 15), or herpes simplex encephalitis (n = 1). 69 patients were right-handed and 5 were left-handed. Otherwise, the patients had the same specifications as in the previous studies on person naming. As in previous studies, the data (neuropsychological, neuroanatomical, and experimental) were obtained in the chronic phase. All patients gave informed consent to participate in the study.
Experimental Task
The participants were administered the Landmark Recognition and Naming Test (hereafter, “Landmark Test”; Tranel, Enekwechi, & Manzel, 2005). The test contains 65 famous landmarks, depicted in color photographs free of identifying text. A trained experimenter administered the Landmark Test to each participant individually. The participant was shown each of the 65 pictures, one-at-a-time, and asked to identify them. No time limit was imposed, and responses were recorded verbatim. The procedure was designed so that data regarding both recognition and naming of the items could be collected. Naming was defined as production of a specific proper noun corresponding to the stimulus (e.g., “Devil’s Tower” for the rock monument in northeastern Wyoming). Recognition was defined as either accurate naming (which was accepted as evidence of accurate identification), or, if naming failed, provision of accurate information regarding specific characteristics of the stimulus, such as its location and physical features (e.g., “that big rock monument out West, that was in the movie ‘Close Encounters of the Third Kind’”). The procedure for distinguishing between recognition and naming is the same as that used for person identification, as described in Section 2 above.
Data Quantification
Each of the 65 items on the Landmark Test has a unique proper name. If the participant produced the correct name, then the naming response was scored as correct. If the participant did not produce a name, gave the wrong name, or gave a vague or superordinate response, the naming response was scored as incorrect. If the participant produced the wrong name, but still identified the stimulus accurately, the response was scored as a recognition success but a naming failure. Following the procedure we used for person identification, we first determined which items had been recognized accurately, and then the naming score was calculated by dividing the number of items named correctly by the number recognized correctly. This procedure does not penalize participants for failing to name items that they do not recognize.
Neuroanatomical Data
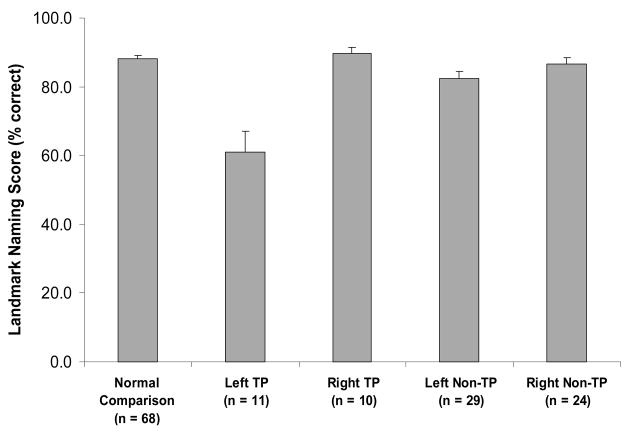
The neuroanatomical analysis was based on MR data (or CT data in a few cases). Each patient’s lesion was transposed and manually warped into a normal 3-D reconstructed brain. Patients were then grouped according to lesion site, and their naming performances on the Landmark Test were analyzed. Four groups of patients were created: (1) Left TP: 11 patients whose lesions involved the left temporal pole region; (2) Right TP: 10 patients whose lesions involved the right temporal pole region; (3) Left Non-TP: 29 patients who had left hemisphere lesions outside the temporal pole region; (4) Right Non-TP: 24 patients who had right hemisphere lesions outside the temporal pole region.
Results
The Landmark Naming scores of the 4 patient groups are presented in Figure 6 (along with data from the normal participants studied in Tranel et al., 2005). A one-way ANOVA yielded a significant result (F(3,70) = 12.94, p < .001; η2 = .36), and follow-up analyses indicated that the Left TP group was significantly different from all of the other groups (all p’s < .001), whereas the other groups did not differ significantly from one another. As Figure 6 shows, the Left TP group generated a Landmark Naming performance that was well below that of the other groups. The findings support the hypothesis that impaired naming of famous landmarks is associated with lesions in left TP.
Figure 6.
Landmark naming performances in normal participants and in patients with left TP, right TP, left non-TP, or right non-TP brain damage, in mean percent correct (SEM).
3.2. Functional Imaging Approach
We have also used a functional imaging approach (PET) to investigate the relationship between proper naming of landmarks and the left temporal polar region (Grabowski et al., 2001). The main hypothesis was that naming famous landmarks would activate left TP.
Participants
The participants were 10 (5 men, 5 women) healthy individuals with an average age of 28 years, all native English speakers, fully right-handed, and at least high school educated.
Task
The landmark naming task involved presenting pictures of famous landmarks and asking participants to name the landmarks. Pictures were presented every 2.5 seconds, and a total of 15 landmarks were presented for each PET injection (two replications of the landmark naming task were performed). A baseline task, in which participants judged the orientation of unfamiliar houses presented every 1.0 second either right side up (“up”) or upside down (“down”), was also performed twice (see Figure 5).
Procedure and Data Analysis
The PET procedure followed our standard approach (Grabowski et al., 2001). Spoken naming responses were audiotaped, allowing us to determine response accuracy and latency. (As it turned out, naming accuracy was M = 90.0% correct, and the median response latency was 1326 msec.) The search volume for the PET activations included the left temporal pole, and the threshold t value (p = 0.05) for the search volume was 3.09. For purposes of analysis, the activation associated with the baseline task was subtracted from the activation associated with the landmark naming task.
Results
The landmark naming minus baseline contrast revealed a significant area of activation in left TP region, as predicted (−33, +11, −24; Tmax=4.46; t65=6.57). This result provides further corroboration of the notion that the process of lexical retrieval for proper names is dependent on neural systems in left TP. We also conducted a direct comparison between person naming and landmark naming in this study, since the participants had performed both types of tasks and it was possible to perform a within-subject comparison between the two categories of unique concrete entities. In the left temporal pole, when person naming was compared directly with landmark naming, no significant activation was observed (Tmax = 1.34). Also, the task by category interaction in this contrast was nonsignificant (Tmax = 0.19). The observed difference in counts between the two naming tasks, in fact, was less than 1 percent (range −4.4 counts to +6.6 counts). These results indicate that person naming and landmark naming both engaged the left TP region, but neither category engaged left TP more than the other. This outcome supports the idea that left TP is engaged by lexical retrieval at unique level, regardless of conceptual category.
4. THEORETICAL FRAMEWORK
The findings from our research program are consistent with a growing body of evidence from other laboratories supporting the notion that left TP is important for retrieving proper names for unique concrete entities, whether the category be familiar faces or landmarks (for important related perspectives, see Barsalou, Simmons, Barbey, & Wilson, 2003; Gallese & Lakoff, 2005; Martin & Caramazza, 2003; Rogers, Garrard, McClelland, Lambon Ralph, Bozeat, Hodges, & Patterson, 2004). It is instructive to situate these findings in a theoretical context. We have proposed a framework in which systems in left TP termed “convergence regions” serve as third-party intermediaries that broker between conceptual knowledge retrieval and word retrieval for classes of unique concrete entities (Damasio et al., 2004). A key principle governing this arrangement is the requirement for unique naming—that is, the specialization in left TP is keyed to the demand for proper names. In our framework, the specialization of left TP is also driven by the need to associate a proper name with a stimulus that is part of a domain of items that tend to be numerous and, in many cases, visually similar, and that have to be disambiguated at specific level (one of a kind) in order for normal identification to take place. Similar to other conceptualizations of the operating principles of this region (e.g., Rogers et al., 2004), we envision that higher levels of specificity and complexity engage and require neural processing systems further “forward” in the temporal lobes, and the temporal poles (and perhaps interconnected prefrontal regions) constitute the rostral-most node of this processing stream.
We conceptualize the “convergence zone” structures in left TP as flexible, probability-driven system components, not rigid centers. Within normal neurobiological variability, the convergence zones involved in various classes of naming tasks are likely to be found in the same large-scale convergence region of the brain in most individuals, and specifically in left TP in the case of proper names. Large-scale convergence regions would be localized at the resolution of relatively large neural sectors, e.g., left TP, left posterior IT, left inferior frontal gyrus. But the micro-scale convergence zones, which would localize at a higher resolution on the order of a centimeter or even millimeters and would comprise subunits within large-scale convergence zones, would not be expected to be in equal sites across individuals. For example, depending on factors such as the exact nature of the stimulus, task demands, and the nature of a particular individual’s abilities, the same task might engage different convergence zones across relatively comparable individuals. Thus, we would predict that nearly equal lesions would not necessarily be associated with precisely the same deficits, and we would predict that the same tasks would not activate precisely the same neural coordinates in functional imaging studies. To recapitulate, on our view the process of anatomical selection of convergence zones both during learning and during subsequent operation is probability-driven, flexible, and individualized. The relationship between proper naming and left TP may not be mandatory, but it surely appears to be reliable.
According to our theoretical framework (Damasio et al., 2004), the intermediary systems in TP/IT are proposed to have bi-directional operation (cf. Marinković, 2004). That is, these systems function as two-way relays and support the process of triggering word form retrieval given conceptual knowledge, and the process of triggering conceptual knowledge retrieval given word forms. Data from functional imaging studies have shown that meanings of concrete entities that entail various types of perceptual knowledge rely on brain regions that are associated with encoding that knowledge, in the appropriate sensory cortices (e.g., Kellenbach, Brett, & Patterson, 2001; Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995; Simmons, Martin, & Barsalou, 2005). It is our position that to get to such knowledge from word forms, one normally goes through the two-way relays in left TP (in the case of unique entities), or at least that this is the most efficient and effective normal path, albeit perhaps not the only one. (We would be quick to acknowledge here that this framework should be taken as an heuristic, and it is undoubtedly an oversimplification, as lexical retrieval is likely to involve many other intermediate steps as suggested in the frameworks of other theorists in this area, e.g., Levelt and colleagues (Levelt, Roelofs, & Meyer, 1999).) And it follows that damage to these systems should yield a two-way defect—namely, impaired retrieval of word forms given conceptual knowledge, and impaired retrieval of conceptual knowledge given word forms. We are testing this prediction in ongoing work in our laboratory.
Acknowledgments
Supported by Program Project Grant NINDS NS19632
Footnotes
The Patient Registry provides the pool of neurological patients for all the lesion studies from our laboratory presented in this article. The Registry contains a large number of patients with focal, stable brain lesions, who have been extensively characterized from both neuropsychological (Tranel, 2007) and neuroanatomical (Frank, Damasio, & Grabowski, 1997) perspectives.
Recovery, in both neurological and neuropsychological terms, can take place beyond three months, but the most substantial changes occur within the first three months following brain damage. Moreover, most of our patients are quite a bit farther out from their brain damage at the time of data collection, and the typical time-since-lesion-onset in most of our language studies is on the order of several years (cf. Tranel, Manzel, Asp, & Kemmerer, 2008).
The rationale for using five as the criterion for “excess” in this context derives from the fact that this degree of difference (between lesion-with-deficit minus lesion-without-deficit) is highly unlikely to occur by chance (see detailed discussion in Damasio et al., 2004, p. 190). In fact, in calculations reported in Damasio et al. (2004), the difference of five corresponded to probabilities that were estimated to be smaller than 0.001 for various contrasts and categories, and we have used this threshold in several previous studies employing the same general method of lesion subtraction. The reader is also referred to a more recent discussion of this issue from a lesion probability sampling perspective, detailed in Rudrauf, Mehta, Bruss, Tranel, Damasio, and Grabowski (2008).
The effects of gender on naming performance have been directly explored (e.g., Grabowski, Damasio, Eichhorn, & Tranel, 2003). The effects are not zero, but they are small at group level and quite variable at individual level, and for the purposes of the current article, gender will not be considered as an independent variable.
Lest this seem like a foregone conclusion, it should be noted that there have been a number of strong arguments for the notion that faces constitute a “special” class of stimuli, and have properties and processing requirements that make them different from all other classes of stimuli. Moreover, there are at least some empirical data that can be taken to support this position, including the specific and reliable activation of the “fusiform face area” by face stimuli in functional imaging experiments (for varying perspectives on this debate, see Gauthier, Tarr, Skudlarski, & Gore, 1999; Kanwisher, 2000).
References
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7:84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Cohen G, Burke DM. Memory for proper names: A review. Memory. 1993;1:249–273. doi: 10.1080/09658219308258237. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Tranel D, Brandt J. Symposia on Quantitative Biology. Vol. 55. Cold Spring Harbor Laboratory Press; 1990. Neural regionalization of knowledge access: Preliminary evidence; pp. 1039–1047. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski TJ, Adolphs R, Damasio AR. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson C, Silbergeld DL, Miller JW, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fery P, Vincent E, Brédart S. Personal name anomia: A single case study. Cortex. 1995;31:191–198. doi: 10.1016/s0010-9452(13)80117-7. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal, visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: A functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Eichhorn GR, Tranel D. Effects of gender on blood flow correlates of naming concrete entities. NeuroImage. 2003;20:940–954. doi: 10.1016/S1053-8119(03)00284-2. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H2(15)O. 1. Theory and error analysis. Journal of Nuclear Medicine. 1983;24:782–289. [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Recognition and naming of famous faces in Alzheimer’s disease: A cognitive analysis. Neuropsychologia. 1993;31:775–788. doi: 10.1016/0028-3932(93)90128-m. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kellenbach MI, Brett M, Patterson K. Large, colorful, or noisy? Attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lyons F, Hanley RJ, Kay J. Anomia for common names and geographical names with preserved retrieval of names of people: A semantic memory disorder. Cortex. 2002;38:23–35. doi: 10.1016/s0010-9452(08)70636-1. [DOI] [PubMed] [Google Scholar]
- Marinković K. Spatiotemporal dynamics of word processing in the human cortex. The Neuroscientist. 2004;10:142–152. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Caramazza A, editors. A special issue of Cognitive Neuropsychology. New York: Psychology Press; 2003. The organisation of conceptual knowledge in the brain: Neuropsychological and neuroimaging perspectives. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Suzuki K, Fuji T, Miura R, Endo K, Kondo H, Yamadori A. Proper name anomia after left temporal subcortical hemorrhage. Cortex. 2005;41:39–47. doi: 10.1016/s0010-9452(08)70176-x. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Garrard P, McClelland JL, Lambon Ralph MA, Bozeat S, Hodges JR, Patterson K. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: Application to category-related naming and recognition deficits. NeuroImage. 2008;41:970–984. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, Swanson S, Hammeke T, Hermann B. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40:446–456. doi: 10.1016/s0028-3932(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Semenza C, Mondini S, Zettin M. The anatomical basis of proper name processing. A critical review. Neurocase. 1995;1:183–188. [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing: A positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. New York: Taylor and Francis; 2007. pp. 27–37. [Google Scholar]
- Tranel D, Damasio H, Damasio AR. On the neurology of naming. In: Goodglass H, Wingfield A, editors. Anomia: Neuroanatomical and cognitive correlates. New York: Academic Press; 1997a. pp. 65–90. [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997b;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Enekwechi N, Manzel K. A test for measuring recognition and naming of landmarks. Journal of Clinical and Experimental Neuropsychology. 2005;27:102–126. doi: 10.1080/138033990513663. [DOI] [PubMed] [Google Scholar]
- Tranel D, Manzel K, Asp E, Kemmerer D. Naming dynamic and static actions: Neuropsychological evidence. Journal of Physiology – Paris. 2008;102:80–94. doi: 10.1016/j.jphysparis.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Fukatsu R, Otsuki T, Okuda J, Umetsu A, Suzuki K, Tabuchi M, Yanagawa I, Nagasaka T, Kawashima R, Fukuda H, Takahashi S, Yamadori A. Neural basis of the retrieval of people’s names: Evidence from brain-damaged patients and fMRI. Journal of Cognitive Neuroscience. 2002;14:922–937. doi: 10.1162/089892902760191144. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Namiki M, Fujii T, Iijima T. Time-dependent neural activations related to recognition of people’s names in emotional and neutral face-name associative learning: an fMRI study. NeuroImage. 2003;20:784–794. doi: 10.1016/S1053-8119(03)00378-1. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Memory for famous people in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychology. 2002;16:472–480. [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computerized Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]