Abstract
Different brain mechanisms seem to mediate wanting and liking for the same reward. This may have implications for the modular nature of mental processes, and for understanding addictions, compulsions, free will and other aspects of desire. A few wanting and liking phenomena are presented here, together with discussion of some of these implications.
I. Desires that deviate from what is best
The phenomenon of incentive salience might be introduced by viewing it as a particular form of desire. This form does not necessarily conform to traditional views of desire that posit individuals to want only what they judge to be best or most pleasant. Incentive salience can be thought of as one module or type of desire, which operates by deterministic rules and has its own brain mechanisms, amidst other modules. This draws on the idea of mind as containing multiple modules, which is certainly common at least in psychology and neuroscience as well as I think in philosophy (Ainslie, 1992; Berridge, 2004; Damasio, 1999; Davidson, Scherer, & Goldsmith, 2003; Fodor, 1983; LeDoux, 1996; Pinker, 1997).
II. Incentive salience as a “wanting” module
Incentive salience is a “wanting” module (Berridge, 2007; Berridge & Aldridge, 2008; Robinson & Berridge, 1993). It has evolved to add a visceral omph to mental desires. “Wanting” corresponds best to decision utility, in the terminology of Kahneman and colleagues, and could be a mechanism for decisions that is distinct from other forms of utility (Berridge & Aldridge, 2007, in press; Kahneman, Wakker, & Sarin, 1997). That is, “wanting” for an outcome is distinguishable from both experienced utility (hedonic impact or “liking” the outcome) and forecast or predicted utility (expecting in advance to like an eventual outcome). This is part of what makes “wanting” a unique module and quite different from wanting (no quotation marks) in the usual sense of the word as a conscious, cognitive desire. That is why my colleagues and I put quotation marks around “wanting” when writing about incentive salience. Ordinarily, incentive salience “wanting” is congruent with other forms of desire such as cognitive wanting, and is also congruent with the hedonic pleasure or “liking” of the outcome. But dissociations can occur among all of these utility forms, and when they do incentive salience is revealed as a distinct module of desire.
How does ordinary wanting in the usual sense of desires differ from “wanting”? Ordinary cognitive desires involve explicit thoughts of the target or reward. In cognitive desires, or wanting in the ordinary sense without quotation marks, you know what you want, or at least think you do, you expect to like the wanted target, and you may have some idea of how you intend to get it. Such desires are guided by explicit memories of how nice the target was in the past, or if never before experienced then at least on imagination of what it would be like to experience.
By contrast, none of this cognition need be part of incentive salience “wants”. Incentive salience is a percept-bound type of “wanting”, which typically occurs as relatively brief peaks upon encountering a reward or a physical reminder of the reward (a cue). Incentive salience does not require a clear cognition of what is wanted, and does not even need to be consciously experienced as a feeling of wanting, at least in some cases (though when it is brought into consciousness it can considerably intensify feelings of desire). Perhaps a reason for the difference is that incentive salience is mediated chiefly by subcortical brain mechanisms, whereas cognitive forms of desire are more dependent on higher cortex-based brain systems. Incentive salience may have evolved using early brain systems as a distinct “wanting” module to pursue particular innate incentives. Possibly it gave an elementary form of goal directedness, which could guide behavior in the right direction in advance of experiencing the goals. Later in evolution “wanting” may have become harnessed to serve “liking” and learned features of reward, so that most incentive salience in our lives today is probably attributed to learned reward cues.
Incentive salience as a module is only one type of wanting. It is not the one we are most aware of in daily life nor the type of desire that has been the greatest focus of philosophers. But incentive salience is important in daily life, as it is needed to color conscious desires with motivational power, to make them compelling spurs to action. It may be a crucial component of our most intense and visceral desires, and especially important in the pathological intensity of some addictions and compulsive desires.
Incentive salience can be viewed as a motivational transformation of a brain signal corresponding to the perceived object of desire or its mental representation (Berridge, 2007; Berridge & Aldridge, 2008; Robinson & Berridge, 1993). When attributed to a stimulus representation, incentive salience transforms the mere sensory shape, smell or sound into an attractive and attention-riveting incentive. Once attributed, the incentive percept becomes difficult to avoid noticing, the eyes naturally move toward the incentive, it captures the gaze and becomes motivationally attractive, and the rest of the body may well follow to obtain it.
III. Psychological features of “wanting”
How can incentive salience be recognized? There are several distinguishing psychological features that help it be recognized even in animal experiments as well as in human daily life. Incentive salience gives a “motivational magnet” property to stimuli it is attributed to, and makes those stimuli attractive, “wanted”, and potently able to elicit approach. Incentive salience also triggers momentary peaks of intense motivation to obtain a cued reward. Such features (reward cues becoming motivational magnets, cues as objects of desire, peaks of cue-triggered “wanting” for the actual reward) allow us to recognize incentive salience in behavioral neuroscience experiments with animals as well as in people.
In the neuroscience experiments, we manipulate the brains of rats or mice in painless ways that alter the operation of underlying substrates for “wanting”, using techniques such as microinjection of tiny drug droplets into a targeted brain structure. Such microinjections remain painless because they are made through a permanently implanted cannula that was previously fixed in place when the rat was under surgical anesthesia.
When activated by a brain microinjection, the chief features of incentive salience that become enhanced are cue-triggered “wanting” for rewards, the potency of reward cues as motivational-magnet, and their potency as conditioned reinforcers. These features are described below.
IV. Cue-triggered “wanting”: temporary peaks of desire
One feature of incentive salience is to endow reward-related cues (in experiments these are Pavlovian conditioned stimuli or CSs) with an ability to trigger powerful peaks of “wanting” for their own associated reward. For example, the scent of food may suddenly make you ravenous as lunchtime approaches even if you were not feeling particularly hungry moments before that cue occurred. As suggested by Olav Gjelsvik in his Workshop commentary, the arrival of a dessert trolley at your restaurant table may make you succumb at that moment to the temptation to indulge. The tinkling sound of an email arriving in your computer inbox may trigger a sudden urge to check the message. In all such cases, cue-triggered “wanting” occurs as a temporary peak, bound to a particular encounter with a cue or to a vivid mental image of the reward. Peaks of cue-triggered “wanting” are sudden, intense, temporary, reversible and repeatable.
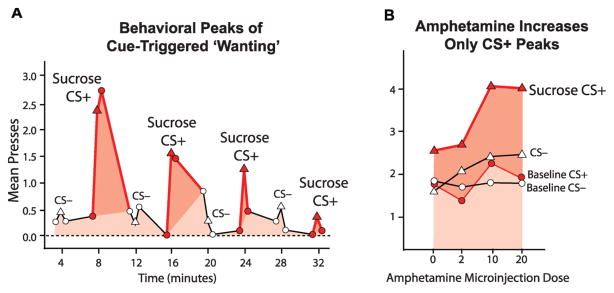
In behavioral neuroscience experiments, we’ve dramatically amplified cue-triggered “wanting” by stimulating brain mesolimbic dopamine systems (Figure 1). The most specific way to do that is by placing a tiny droplet of amphetamine drug in the nucleus accumbens. Amphetamine causes the ends of dopamine-containing neurons to release extra amounts of dopamine onto their target neurons. The psychological result is typically to increase the incentive salience that cues trigger for their reward without altering other components of desire or reward. For example, in an illustrative experiment by Cindy Wyvell, brain dopamine activation selectively increased the peaks of “wanting” for a sugary reward that were triggered by encounters with its cue (Wyvell & Berridge, 2001). Each cue was the sound of a beeping tone that on previous occasions had predicted a sugar pellet reward (the cue is sometimes called a Pavlovian CS or conditioned stimulus in literature on such experiments).
Figure 1. Irrational cue-triggered “wanting”.
Transient irrational “wanting” comes and goes with the cue (left). Amphetamine microinjection in nucleus accumbens magnifies “wanting” for sugar reward—but only in presence of reward cue (CS+). Cognitive expectations and ordinary wanting are not altered (reflected in baseline lever pressing in absence of cue and during irrelevant cue, (CS−) (right). Modified from Wyvell and Berridge, 2000.
The sound of the cue triggered the rat to engage in a burst of effort in pressing on a lever that had previously earned it sugar pellets. Each CS cue lasted about 30 seconds, and the peak of enhanced “wanting” lasted not much longer. More stable forms of desire that continued to motivate working for the reward in the absence of the cue were not enhanced by amphetamine microinjections (including cognitive expectancies of the reward or habitual goal-seeking responses that continue stably whether the cue is present or not). So in a half-hour session, as the cue came and went several times, bursts of cue-triggered “wanting” appeared as mountain peaks resting on a plateau of stable motivation. The effect of mesolimbic dopamine activation was to selectively make the mountain peaks of “wanting” taller without changing the elevation of the plateau on which they sat, and the higher the amphetamine dose, the higher the peaks of cue-triggered “wanting” became (Figure 1). Each peak of cue-triggered “wanting” lasted less than a minute after the cue, regardless of height. Of course the exact temporal features in this example are peculiar to rats, and human “wanting” peaks might well last longer if powered by sustained imagery about the reward. But the example helps show how cue-bound incentive salience depends closely on percepts even when the brain is activated more constantly. “Wanting” occurs as a temporary burst linked to the cue presence, and mesolimbic brain systems need a cue on which to act in order to motivate. In the experiment described, a rat’s brain would have been constantly flooded with dopamine after an amphetamine microinjection, yet its “wanting” came and went with the coming and going of the physical cue for sugar. Incentive salience thus reflects a synergy between brain states of mesolimbic activation and particular events happening in the world. Both must be present to trigger the “want”. These constraints of operation help show the psychological boundaries of the “wanting” module and reveal it as just one among several psychological mechanisms of desire—albeit a powerful one when present.
V. Cues as motivational magnets: good enough to eat
A second feature of incentive salience is that its attribution to a reward-related stimulus may make that stimulus “wanted”, even if the stimulus is just the cue. The Pavlovian CS stimulus becomes an attractive “motivational magnet” itself, in addition to triggering “wanting” for its hedonic reward, although the CS cue is only a learned predictor for the reward with no intrinsic value of its own. In a sense, a cue can even become “good enough to eat”. Stephen Mahler in our lab has shown that activating incentive salience makes a rat more intensely approach, handle, and even try to “consume” a metal object whose presence predicts sugar, with nibbles and sniffs of the metal cue that are similar to movements used in eating actual sugar (Mahler & Berridge, 2009). Cues for other rewards become attractive in their own ways. Crack cocaine addicts, for instance, have been reported to compulsively “chase ghosts” when they have no cocaine, that is to scrabble around on the floor after tiny whitish specks under the table, even if they know the specks are more likely to be sugar than cocaine (Rosse et al., 1993). They are chasing a visual cue for cocaine: white specks. Such motivational magnet features of attractive cues are made much more potent by activation of mesolimbic systems, such as the amygdala or nucleus accumbens. Motivational magnet features of reward cues may account for a host of phenomena in which individuals become pulled toward reward cues acting as beacons to guide brain motivation systems.
VI. Conditioned reinforcers: working to obtain the cue
A related mark of a motivational magnet is that individuals may “want” to possess that cue, just as they “want” its hedonic reward. Animals in an activated brain state will work harder to obtain a CS or cue that is attributed with incentive salience, just as they would work to obtain the actual reward. This is sometimes called conditioned reinforcement. For example, rats will learn to press a lever or poke their nose into a hole simply in order to obtain a sound or other cue-event or object that has been previously associated with a sugar reward or a cocaine reward. Activating their mesolimbic brain systems makes them work much harder at it. People too may sometimes “want” cues for particular rewards, as when a miser wishes to handle and count the physical money as coins or notes in the hand, motivated to feel these mere cues or symbols, above and beyond possessing the monetary wealth. And perhaps to be motivated to obtain mere symbols is not unknown even among academics.
VII. Brain bases of incentive salience
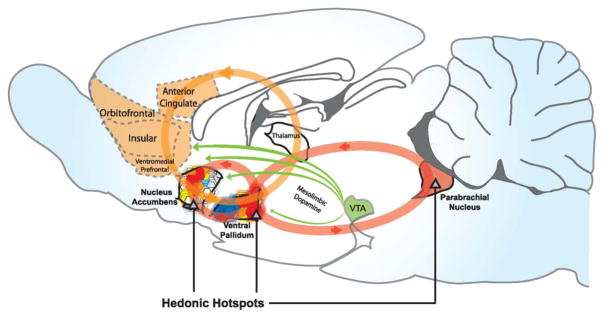
Incentive salience is generated chiefly by subcortical brain circuits, especially a circuit called the mesocorticolimbic dopamine system or simply the mesolimbic dopamine system (Figure 2). As the name implies, its most prominent neurotransmitter is dopamine, although other neurotransmitters also are important including opioids (natural brain neurochemicals similar to poppy-derived opiate drugs like opium, heroin or morphine), glutamate and GABA. We have often used dopamine and opioid activations in mesolimbic structures to turn on incentive salience in our laboratory. The dopamine neurons project from the midbrain (this origin gives the “meso” portion of the name) where their neuronal cell bodies are, sending axon fibers upwards to nucleus accumbens, other parts of striatum, amygdala and prefrontal neocortex (these targets give the “limbic” portion). Opioid neurons live within these structures themselves, as well as entering in from deep brain sites. Amygdala and cortex neurons project down to nucleus accumbens, forming a common interchange there. Nucleus accumbens in turn projects downwards to ventral pallidum and eventually up again into prefrontal cortex, and also again back down to midbrain, where the process can start over, forming recursive loops for reward-related signals.
Figure 2. Hedonic hotspots and hedonic circuits.
Hedonic hotspots are shown in nucleus accumbens, ventral pallidum, and brainstem parabrachial nucleus where opioid or other signals cause amplification of core “liking” reactions to sweetness. Reprinted by permission from Smith, Mahler, Pecina, & Berridge, 2009.
Manipulations of dopamine neurotransmission has proven especially useful in manipulating and isolating incentive salience to reveal its psychological features, because of all neurotransmitters in this system dopamine activates “wanting” most selectively as a psychological function. Manipulations that selectively boost brain mesolimbic dopamine signals tend to specifically increase “wanting”, without increasing other reward aspects such as “liking” or hedonic pleasure, or other motivation aspects such as cognitive desires. In addition to activating dopamine systems by direct drug administration, it is also possible to make the systems permanently hyper-reactive by inducing what is called neural sensitization via a repeated series of binges with addictive drugs. Neural sensitization of mesolimbic systems by drugs causes a similarly selective but enduring enhancement of cue-triggered “wanting”. Thus, it is a useful shorthand to think of brain dopamine systems as powerfully controlling “wanting” (though other neural stages of the mesocorticolimbic circuit are involved too).
VIII. Addiction and incentive-sensitization
Human drug addiction may be a special illustration of intense “wanting” that results from permanent sensitization of mesocorticolimbic systems (Robinson & Berridge, 1993; Robinson & Berridge, 2003). Sensitized “wanting” may rise to quite irrational levels. That is, the intensity of cue-triggered “wanting” to take drugs for brain-sensitized addicts could outstrip their “liking” even for pleasant drugs, outstrip their expectation of how much they will like the drugs, and outlast any feelings of withdrawal if they stop. Brain-sensitized addicts may be unable to give a reason for their drug taking in such a case. Indeed, there is no reason, there is only a cause for why they “want” so much.
Sensitization of mesolimbic systems arises as a permanent change that addictive drugs can produce in the brains of susceptible individuals. Sensitization increases the neurochemical responsiveness of these neurons and can even change their physical shapes. Individual susceptibility is determined by genetic factors, hormonal factors, previous drug experiences, and previous experiences with major stresses in life. Sensitization is also influenced by drug dose and the speed with which it reaches the brain, and is facilitated most when the drugs are taken in binges. Neural sensitization of mesolimbic dopamine systems means that the brain system becomes hyperreactive to drugs. The system is not constantly hyperactive in a stable fashion, but it can be put temporarily into a hyperactive state by reaction to the drug again or to related cues: it is hyper-reactive to particular stimuli. Sensitization of mesolimbic systems may create compulsive levels of “wanting” for drugs or other addictive incentives. A sensitized brain responds with extra incentive salience to reward cues just as a brain that has been drugged with amphetamine does—even if the sensitized brain has no drug on board at that moment. A sensitized addict’s brain, on encountering the right drug cue, would irrationally “want” the cued reward at that moment because of excessive incentive salience—even if the person cognitively expected not to like it very much and eventually did not like it much in the end. And crucially, sensitization may last years after an individual stops taking any drugs.
IX. “Liking”: Hedonic hotspots for generating pleasure
Different from “wanting” is “liking” or the core process of hedonic pleasure. In our hands, brain manipulations that cause “liking” almost always cause “wanting” too. This perhaps mirrors their close association in daily life. But many manipulations that cause “wanting”, as described above, fail to cause a match in “liking”. The brain appears relatively recalcitrant to stimulation of pleasure, unless exactly the right hedonic systems are activated.
What are the neural bases of pleasure “liking” itself? A much more restricted brain circuit appears to mediate hedonic “liking” rather than incentive “wanting” (Peciña, Smith, & Berridge, 2006; Smith et al., 2009). The generation of pleasure “liking” is more restricted neurochemically: opioid stimulation but not dopamine stimulation in some limbic strutures can enhance “liking” (whereas “wanting” is enhanced by both). “Liking” is also more restricted anatomically: enhanced by opioid “hotspots” but not by the rest of the same limbic structures (even if the entire structure can enhance “wanting”). And “liking” generation is also more restricted as a brain circuit, requiring unanimous activation of multiple hotspots simultaneously (whereas “wanting” can be enhanced by a single hotspot). In short, enhancement of pleasure “liking” is restricted and fragile, and brain pleasure systems are relatively recalcitrant to activation compared to “wanting” systems. Consequently, our limbic mechanisms may consign us more often to states of desire than of pleasure.
Hedonic hotspots are what my colleagues and I call the anatomically small pleasure-generating islands of brain tissue contained within the larger sea of a limbic structure, such as nucleus accumbens or ventral pallidum (Figures 2 and 3). The signature feature of these hedonic hotspots is that they can generate increases in pleasure “liking” reactions when stimulated with appropriate neurochemical microinjections. The size of each hotspot discovered so far is only a cubic millimeter in volume of the brain of a rat. In the brain of a person a hotspot would be expected to be about a cubic centimeter in volume, extrapolating on the basis of the difference between rats and humans in whole brain size.
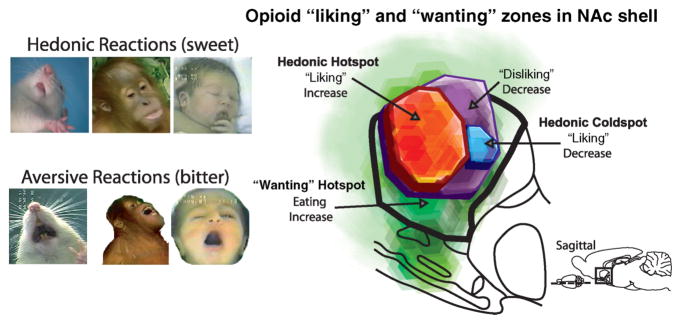
Figure 3. Taste “liking” reactions and map of a hedonic hotspot.
Positive “liking” reactions to pleasant sweet tastes shared by human newborn, young orangutan, and adult rat (tongue protrusion; left top), and aversive “disliking” reactions to unpleasant bitter tastes (gape; left bottom). Opioid hotspots and coldspots in the nucleus accumbens (medial shell region shown in sagittal view; right). The entire medial shell mediates opioid-stimulated increases in “wanting” for food reward. Only a cubic-millimeter sized hedonic hotspot generates increases in “liking” for the same opioid stimulation. A small hedonic “coldspot” suppresses “liking” reactions to sucrose, whereas a larger zone suppresses “disliking” reactions to quinine. Reprinted by permission from Smith et al., 2009.
In order to recognize a pleasure-generating hedonic hotspot, a technique we have used is to make microinjections of a pleasure-enhancing drug into or around the hotspot. We then deliver a sweet sensory pleasure into the mouth of a rat, because a sugary liquid infusion elicits natural affective “liking” reactions that can help reveal the intensity of the sensory pleasure at that moment. “Liking” reactions include natural facial expressions to a sensory pleasure. In human babies, for example, sweet tastes elicit positive facial “liking” expressions such as rhythmic tongue protrusions and licking of the lips, whereas bitter tastes instead elicit negative “disliking” expressions such as gapes (Steiner, Glaser, Hawilo, & Berridge, 2001). Essentially the same facial reactions have evolved in apes, monkeys and rats as in humans. Whether human, ape or rat, the brain seems to use the same mechanisms to generate sensory “liking”, and we count on that similarity to find the brain bases of pleasure.
When a true hedonic mechanism is stimulated, sweetness “liking” expressions become increased. Microinjection of drugs into hotspots enhance “liking” for sweetness if they stimulate receptors for opioid receptors that detect heroin-like neurochemicals, or receptors for endocannabinoid receptors that detect marijuana-like neurochemicals. But no “liking” enhancement occurs if the same drug microinjections are moved outside the boundaries of the hedonic hotspots, though if still within the nucleus accumbens or ventral pallidum. Many of the microinjections still stimulate intense “wanting” in the remainder of these structures, but they no longer stimulate “liking” outside the hotspot. Localization of the “liking” function defines the anatomical boundaries of hedonic hotspots.
There are several hedonic hotspots scattered across the brain, as an archipelago of interacting islands (Figure 2). The entire archipelago appears to become activated as a single integrated circuit to amplify sensory pleasures. The various hotspots must cooperate together to do this. For example, activation of one hotspot with an opioid microinjection automatically recruits activation in other hotspots in different brain structures (Smith & Berridge, 2007). Pleasure magnification requires unanimity among all opioid hotspots in nucleus accumbens and ventral pallidum (Smith & Berridge, 2007). Pleasure will not be enhanced by a hotspot opioid activation if unanimity is prevented by simultaneously suppressing another hotspot with an opioid opposing drug. Although opioid stimulation of either hotspot would usually be sufficient to enhance “liking”, it cannot do so if the larger circuit is prevented from joining in the activation. However, “wanting” stimulation of “wanting” still persists after either hotspot is activated, even if “liking” enhancement has been prevented. Thus partial activation of the limbic circuit is enough to generate desire, whereas pleasure generation requires the whole.
Up to this point in the paper, my goal has been to describe some of the affective neuroscience findings that gave rise to the notions of “wanting” and “liking”. In the remaining portion, I would like to switch focus to potential implications of these ideas. In particular, I will turn to a few philosophical issues regarding desire that were raised at the Oslo workshop, and try to say something about what “liking” and “wanting” might mean for those issues.
X. Intentionality in incentive salience?
Intentionality seems to be an important part of many philosophical treatments of desire. Most cognitive desires have intentionality in the form of an explicit object or event that is the goal of the desire. Cognitive mental representations can imagine the desired object, its look or feel, or remember what it was like on previous occasions. Basically, one knows what one wants, and one always wants some goal in particular.
Incentive salience, by contrast, has a changeable relation to intentionality. “Wanting” as I see it is intentional in some cases, but not in others. At its most unintentional, incentive salience may detach from the object of desire and be attributed too widely among stimuli, spewing indiscriminant “wanting” in directions that are inappropriate or completely general. The entire world can brighten up in a motivational sense on such occasions, taking on diffuse incentive properties. Thus intentionality is not intrinsic to “wanting”, but depends on mechanisms that focus the attribution of incentive salience to particular targets.
By contrast, consider cases of “wanting” that do have intentionality. Cue-triggered “wanting” for a particular hedonic reward is quite intentional, such as when a drug cue makes an addict want to take drugs again or a food cue makes you think of lunch. These each have specific goals, at least as objects in the world (though they may or may not be recognized subjectively as cognitive goals in the mind of the individual). The cue triggers “wanting” for its own appropriate reward goal. If that reward is not present, the intentional focus must be mediated by a mental representation of the absent object of desire (either via cognitive imagery or at the very least via a learned Pavlovian representation).
However, it must be acknowledged that even cue-triggered “wanting” can detach to some degree from the actual associated reward. Experiments have shown this detachment by using manipulations to reduce the value of the actual reward from what was remembered before. In such cases, the “wanting” may persist or widen inappropriately, as mentioned above, and at least a partial widening of “wanting” is not uncommon.
A less reasonable form of intentionality seems involved when the cue itself becomes “wanted” as a motivational magnet, rather than only the actual pleasant reward. When cues become the focus of desire there is a slight distortion in the targeting of intentionality. No reason exists to desire the cue, there is merely a psychological associative history and a neural mechanism that makes it desired. In some addiction situations, an individual may become so obsessively focused on the attractive cue that opportunities are lost to obtain the actual reward it is associated with. In experiments when the mesocorticolimbic system for incentive salience is activated, animals may persistently approach and gnaw on their cue even if doing so delays or loses receipt of the actual reward.
“Wanting” of a cue as motivational magnet can detach further from intentional wanting of the reward in some instances when the actual reward is devalued, such as by satiety or pairing with aversive events. Similarly to the persistence of cue-triggered “wanting” after goal devaluation, a cue may still sometimes be “wanted” and worked for even after its associated reward is no longer valuable.
Least intentional of all may be the cases of truly indiscriminate attribution of incentive salience we began with, in which nearly everything becomes simultaneously “wanted” at once. Certain forms of brain mesolimbic activation may cause diffuse “wanting” attribution to all stimuli that are present. A brain electrode activation or a drug microinjection in some limbic structure, and perhaps brain sensitization in some cases of human addiction might activate the system to a very high degree, and simultaneously disrupt associative mechanisms that usually focus “wanting” on a particular target. As a result, incentive salience in such cases may be attributed broadly to many different stimuli at the same time. At the extreme, essentially everything perceived at that moment might become more attractive and “wanted”. For example, some people who have been implanted with brain stimulation electrodes in their mesolimbic systems have been reported to describe the entire room as “brightening” in a motivational sense, so that they perceive everyone present as more interesting, more socially attractive and even more romantically or sexually attractive, and at that moment they feel motivated to do quite a number of things. Such indiscriminate “wanting” is powerfully motivational, but when “everything” is “wanted”, then nothing in particular is. Does such an unfocused desire have intentionality at all?
XI. Unconscious aspects of incentive salience “wants”
Another way in which incentive salience diverges from intentionality, and from the ordinary sense of desire, is that “wanting” need not always be conscious. Examples of unconscious core “wanting” have been demonstrated in people ranging from drug addicts to ordinary college students.
For instance, my former colleague Piotr Winkielman, who is now at the University of California at San Diego, asked college students to view a computer screen on which they were told faces would be flashed for ½ second, in which the student’s task was to identify the gender of the face as woman or man (Winkielman & Berridge, 2004). Unbeknownst to the participants, subliminally fast faces with happy or angry emotional expressions were also sometimes flashed on the screen extremely briefly (1/60th second each). These brief flashes could not be consciously seen nor the faces recognized later. Finally, the students were also told they would subsequently be asked to judge a new fruit-flavored beverage that was under development by a beverage company, and they were given a pitcher of the drink to pour, taste and rate before the end of the session.
All the students reported their own hedonic mood and emotional feelings before and after viewing the computer screen. Their reports completely failed to show any sign of the happy or angry faces they had subliminally “seen”. They did not feel any increase in positive or negative mood after seeing an emotional facial expression once they had finished their gender identification task. Yet when presented a few minutes subsequently with the novel beverage, students found the drink 50% more attractive after seeing the subliminal happy face, pouring and drinking more and rating it more highly. Further, they expressed willingness to pay 4 times more for the drink if it were sold when asked after the happy faces than after the angry faces.
We think the subliminal happy faces activated incentive mesolimbic circuits of “wanting” in the brains of students who viewed them, which persisted for some minutes undetected as students evaluated their own mood. The “wanting” surfaced only when an appropriate target was finally presented in the form of a hedonically laden sweet stimulus that they could taste and choose to ingest or not. Similarly, drug addicts who have been given an intravenous cocaine dose too low to produce detectable physiological effects after pressing a button for it, have been reported to say that the injection feels empty and completely devoid of any cocaine at all, yet the addicts still work substantially more to receive more of the same “empty” dose.
Such instances of unconscious “wanting” suggest that incentive salience can at least sometimes operate underneath conscious awareness. Mesolimbic “wanting” may run in parallel with ordinary (and more cortex-mediated) wanting. Usually they point in the same direction, but in cases of unconscious “wanting”, only one of the mechanisms seems to be in operation. These cases may lack recruitment of the additional brain and psychological mechanisms needed under more usual conditions to translate the core “wanting” process into a cognitive and conscious desire, so that both motivate toward the same target. From the philosophical perspective, doesn’t an unconscious “want” seem difficult to reconcile with intentionality in the usual sense? To the degree that an unconscious “want” can be assigned to a malleable target, it does not have an explicit object of desire. It has only a stimulus target, which may to some degree depend on chance in the form of what happens to turn up next.
XII. Impatience and urgency: a role for action salience?
Another interesting issue raised at the Oslo workshop was the distinction between impatience and urgency that was highlighted by Jon Elster (Elster, 2008). Impatience is desire for an outcome right now, by Elster’s view, whereas urgency is a desire to act right now. Impatience and urgency often are intertwined, but it is possible for them to occur separately (Elster, 2008; Sperber, 2008). Similar distinctions have been made by psychologists interested in motivation and stress (Carver, 2005).
Could either impatience and urgency be related to incentive salience in any way? It seems reasonable to speculate that incentive salience might on occasion contribute to impatience, by making an outcome more intensely “wanted”. It is less clear whether urgency to act relates to incentive salience at all, at least at first sight. Incentive salience as my colleagues and I have typically conceived it is a property attributed to physical stimuli and their neuropsychological counterparts: stimulus perceptions, and mental memories and images of absent objects or events. By contrast, actions are not physical objects or stimulus sensory events, except in so far as they produce sensation feedback during the act.
Still, I’d like to suggest that there may be a role for a mechanism in action similar to incentive salience, which might be considered to contribute to urgency. I call this notion action salience. My colleagues and I have often wondered whether an action-attributed form of incentive salience might attach to action representations in the brain (e.g., motor programs), just as “wanting” attaches to stimulus representations. If so, the transformation of the action representation might give incentive properties to an action. As a consequence one might urgently “want” to act. The urge to act may be evolutionarily old, and shared by animals and humans. Mink, seals and polar bears may “want” to swim, rodents “want” to run, gnaw, and build nests, and so on, and even people may have their own set of urgent actions (Elster, 2008; Glickman & Schiff, 1967; Mason, Cooper, & Clarebrough, 2001).
This possibility gains some physiological plausibility from considering that there is substantial neurobiological overlap between brain dopamine systems of action and of desire. The two brain systems are surprisingly intermingled: so surprisingly that neuroscientists have been hard pressed to separate their two functions in experimental evidence, and in many experiments the functions refuse to separate at all. To the degree they can be separated, here is the textbook difference between them. A “nigrostriatal” system of dopamine projections exists just above the mesolimbic system, and is implicated in action. The nigrostriatal action system is famous for its involvement in Parkinson’s disease, and involves similar loops of neural circuitry. For example, dopamine neurons project from midbrain to striatum; striatum sends its output down to a pallidus target. The pallidus projects to thalamus and from there to cortex, which sends loops back to striatum, and so on. The two systems even merge in some respects (for example, signals in the mesolimbic system migrate up through spiralling circuits into the nigrostriatal system) so that there is no strict division between them as they function. Could particular dopamine activations of nigrostriatal circuits make actions “wanted” in the same sense that mesolimbic activations make objects “wanted”? If so, action salience would be simply the action mirror image of incentive salience, and “wanting” might contribute as a partial cause to create a compelling urge to act—now!
XIII. Temporal discounting
Temporal discounting is an important and widespread phenomenon in decision-making (Ainslie, 1992; Ainslie, 2001; Frederick, Loewenstein, & O’Donoghue, 2002). Understanding of discounting has been led in large part by the path-breaking work of George Ainslie (Ainslie, 2008), who described his most recent thinking in Oslo, and which has demonstrated that people and animals alike have a strong hyperbolic tendency to place excessive value on immediately available rewards in favor of significantly better alternatives that are more distant in the future.
Hyperbolic discounting is essentially an outside description of choice for temporal outcomes. It does not necessarily entail a specific psychological process or brain mechanism, as any number of different processes and mechanisms could be involved (Ainslie, 2008; Gjelsvik, 2008). But could “wanting” ever play a role? I think that mesolimbic mechanisms of incentive salience could at most contribute only to a limited set of instances of discounting choices. But to those sharply delineated instances, incentive salience might be a quite powerful contributor. Recent neuroscience results suggest “wanting” could contribute most to temporal discounting when two conditions are simultaneously met: 1) mesolimbic dopamine systems are activated, and 2) a cue is present for the immediate reward.
The simultaneous requirement of immediate cues and mesolimbic activation for a “wanting” contribution to discounting is suggested by evidence from the laboratory of my colleague at the University of Michigan, J. Wayne Aldridge (Tindell, Berridge, Zhang, Peciña, & Aldridge, 2005; Berridge & Aldridge, 2008). His team recorded the firing of limbic neurons in an output station of mesocorticolimbic circuits that coded the incentive salience of reward cues. Examining the effect of activating mesolimbic dopamine systems with drugs or with prior sensitization, the team found that neuronal “wanting” signals were intensely enhanced by mesolimbic activation—but only for a cue that signaled immediate reward. Another earlier cue that predicted reward at greater temporal distance was not nearly as enhanced in signal by dopamine activations (even though the earlier cue was only a little bit more separated in time from the same reward).
Those neural results suggest that brain dopamine activation may specifically enhance cue-triggered “wanting” for an immediately available reward, much more than for an even moderately more distant reward. Cues for immediate reward are especially powerful at eliciting desire and addictive relapse. For a recovering alcoholic, for example, the clink of the ice cubes in the glass and smell of the alcohol that are associated with the drug may become able to trigger compulsively high “wanting”. Even if the person has successfully resisted more temporally-distant cues such as the sight of the tavern door or the bottle on the shelf, the clink, or especially, smell, may break through the resolution to abstain and precipitate drinking again. Immediate cues become especially dangerous. And if a recovering addict tries to take “just one hit”, the limbic data of Aldridge and colleagues suggest that the combined neuronal enhancements of inebriation and sensitization may add together into a one-two punch of doubly extreme “wanting”. Such results may possibly help to provide a brain-based explanation of why hyperbolic discounting especially describes the choices of sensitized addicts, the inebriated, or even ordinary people who are in a momentary “hot” state that recruits mesocorticolimbic circuits (Ainslie, 2001, 2008; Frederick et al., 2002).
XIV. Desire versus dread: a hidden overlap
An enormous divide appears to exist between desire for an attractive incentive and dread of a frightening threat. They are hedonically opposite outcomes: one positive in valence and the other negative. But desire and dread may share something surprisingly in common at mechanistic levels, in the form of a shared affective building block. One possibility for overlap was highlighted by George Ainslie at the Oslo workshop (Ainslie, 2008), and is described in his article in this issue. Another possibility is found in recent results from our laboratory at Michigan, which promote the idea that desire and dread may involve a paradoxical sharing of motivational mechanisms (Reynolds & Berridge, 2008).
The shared mechanism of desire and dread takes the form of a mesocortical generation of motivational salience whose affective valence can be flipped back and forth between positive and negative. Even in the same individual and in the same hour, both can be activated together by a brain manipulation, and it is possible to convert one into the other. The shared psychological feature of motivation salience is essentially incentive salience with the positive valence drained out. What that leaves is an intrinsically nonvalenced salience that can be used in the service of either motivation, and as equally applied to a negatively valenced threat as to a positively valenced salience. Applied to a threat, this produces fearful salience: a frightening stimulus is attention-grabbing and compels a motivated response of avoidance, almost as an incentive stimulus grabs attention and compels approach, but the threat is perceived as sinister and repellant, and perhaps requiring attack.
Either incentive salience or fear can be activated as one chooses in rats by tapping an appropriate emotional key in a limbic keyboard, done through a cortical signal-blocking microinjection placed in the nucleus accumbens. Part of the nucleus accumbens is arranged as an emotional keyboard, front to back, that organizes incoming signals about the outside world (Reynolds & Berridge, 2008). Tapping keys in the front of this structure elicits strong desires, for example stimulating intense eating and creating a desire to return to the place where the keys were tapped. Tapping keys in the back conversely elicits strong fear (antipredator reactions to the sight or touch of people, sometimes accompanied by fearful squealing and escape attempts). Desire and dread are opposites, but we have found that taps in the middle of the accumbens keyboard reliably elicit both together. This observation made us wonder whether a shared motivational salience mechanism might contribute to generate both, so that they could so closely overlap in mechanism. If so, we hoped it might be possible to flip an intermediate neural key in accumbens back and forth between generating fear and generating desire, as a flexible psychological building block, depending on the psychological context in which it was activated (Reynolds & Berridge, 2008).
Sheila Reynolds examined the shared mechanism possibility by testing the effects of the microinjections in situations where the ambience could be flipped: either a soothing environment (the rat’s own home: dark, quiet and familiar) or a stressful environment (a room with bright lights and where a loud discordant soundtrack played of the punk rock musician Iggy Pop). The rats much preferred their home to Iggy Pop if given the choice. But if not given a choice, and simply plopped in one or the other environment after a brain microinjection, the environment determined what the brain building block became built into. Sheila Reynolds found that many middle keys in accumbens could switch between desire and dread, depending on which environment the rat was in as the microinjection acted. Some accumbens sites flipped completely, changing from pure fear-generators to pure desire-generators, or vice versa. This flip is consistent with the counter-intuitive possibility that a single shared psychological operation exists as a truly flexible building block within desire and dread.
It is beyond me to say if philosophical implications are raised by the potential existence of a shared kernel in desire and dread, but I should be very interested to know whether philosophers discern any noteworthy issues in this phenomenon.
XV. Compulsive “wanting” versus free will
Incentive salience is a powerful mechanism to produce motivational compulsions, at least if activated to extremely high levels. Impatient “wants” powered by mesocorticolimbic activation are hard to resist, and may power compulsive addictions. The deterministic nature of incentive salience raises seems potentially incongruent with notions of human agency and free will (Camerer, 2006; Holton, 2004).
Do incentive salience mechanisms carry consequences for concepts of free will? On this topic I defer to those better qualified philosophically to evaluate this issue who participated in the Oslo workshop, such as Richard Holton and Jennifer Hornsby. Here I offer only a brief and admittedly naïve opinion: no. Scientific knowledge of “wanting” mechanisms should not necessarily change the philosophical status of free will concepts. Philosophers of free will have always recognized the existence of temptations in the form of independent or involuntary desires that act to subvert the intentions of a will to abstain from those temptations. A temptation remains a temptation regardless of its brain mechanism. Identifying mesolimbic mechanisms of incentive salience as a substrate pinpoints a brain basis and clarifies certain psychological operating rules, but does not seem to me to substantially change the situation as far as a philosopher need be concerned. If philosophy has ever successfully reconciled temptation to free will, then it might well succeed again in a reconciliation with incentive salience (Holton, 2004; Holton, 2008; Hornsby, 2004, 2008).
Perhaps a step in this direction was illustrated by Holton’s discussion in the Oslo workshop of the potential compartmentalization of free will into some domains versus other domains which are less able to be controlled (Holton, 2008). Activation of incentive salience potentially might tilt or nudge an individual between these domains. As Holton put it in his workshop essay, “an agent is less free to F the harder it is to follow though on a decision to F” (Holton, 2008). “Wanting” makes it harder to do F if F requires abstaining from acting upon a salient incentive. Harder is just a matter of degree, as the mechanism of “wanting” is always graded in intensity. In some individuals and in some conditions harder may indeed approach very high levels so as to legitimately be called compulsive. Just how hard it is to resist a compulsive “want” at the highest intensities is still an open question, but no doubt it might be hard indeed, so hard that most of us might fail if put to the test again and again. Still, in principle, incentive salience seems no different from ideas of temptation that philosophers have wrestled with for ages.
The techniques for exerting self control over temptations that Holton and others describe seem usefully applicable also to incentive salience, and could be employed for example even by an addict to help resist sensitized “wants”. The very modularity of incentive salience may open opportunities for its control by other facets of mind, through the boundary conditions that limit its realm of operation. Precommitment to an alternative course of action that excludes giving in to the tempting incentive is one tactic considered by Holton. Perhaps equally important for coping with sensitized cue-triggered “wanting” might be to arrange as much as possible to avoid encountering the tempting reward and its cues (Elster, 2003; Holton, 2004; Holton, 2008; Metcalfe & Mischel, 1999). It is difficult to control brain mesolimbic states, but avoiding particular physical and sensory triggers (rewards and their cues) may be crucial and a feasible tactic to avoid overturning of precommitment strategies. Avoiding imagery of the reward stimulus and cues is admittedly a trickier problem (Wegner, 2002).
Thus although psychological and neural mechanisms of incentive salience are inherently deterministic, perhaps philosophers can reconcile them with modern concepts of free will and agency. While that task is not easy, nothing seems changed in principle by the modern identification of brain mechanisms for temptations that philosophers have known to exist all along.
XVI. Conclusion
In sum, “wanting” a reward is distinct from “liking” the same reward. The two usually converge, but can sometimes diverge. When dissociations occur, most notably, “wanting” can become unjustifiably high, as in addiction. The identity of incentive salience as a distinct module within desire creates conditions under which in some cases incentive salience creates a compulsive “wanting” that exceeds the expected goodness of the outcome, persists in the face of a sincere cognitive intention to do the opposite, and is not matched by actual pleasure of the outcome in the end. Incentive salience can detach from intentionality in the form of cognitive goals, and attach to percepts that are associated with incentives, sometimes pulling “wanting” in odd directions. Incentive salience might also attach to particular actions, as well as to stimuli, creating urges to act that are as motivationally compelling as any external incentive. Finally, incentive salience provides a mechanism for temptation, but perhaps not a new challenge to philosophers of free will. I am grateful for the opportunity to have participated in the Oslo Workshop that combined the approaches of philosophy, neuroscience and psychology. Building bridges between the disciplines, as the workshop helped to do, would seem to have much to offer for carrying these issues further.1
Footnotes
I thank Olav Gjelsvik and other organizers of the Oslo Workshop on Liking and Wanting, and all the participants, for a stimulating and enjoyable experience in colleagueship. I also thank Jay Schulkin and Barbara Smuts for helpful comments on an earlier version of this manuscript. The laboratory experiments described in this paper were made possible by grants from the US National Institutes of Health.
References
- Ainslie G. Picoeconomics. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Ainslie G. Breakdown of Will. Cambridge; New York: Cambridge University Press; 2001. [Google Scholar]
- Ainslie G. Pleasure and aversion: Challenging the dichotomy. Paper presented at the University of Oslo Philosophy workshop on wanting and liking.2008. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology and Behaviour. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Decision utility, the brain and pursuit of hedonic goals. Social Cognition. 2008;26(5):621–46. doi: 10.1521/soco.2008.26.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer CF. Wanting, liking, and learning: Neuroscience and paternalism. The University of Chicago Law Review. 2006 Winter;73:87–110. [Google Scholar]
- Carver CS. Impulse and constraint: Perspectives from personality psychology, convergence with theory in other areas, and potential for integration. Personality and Social Psychology Review. 2005;9(4):312–33. doi: 10.1207/s15327957pspr0904_2. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. 1. New York: Harcourt Brace; 1999. [Google Scholar]
- Davidson RJ, Scherer KR, Goldsmith HH. Handbook of Affective Sciences. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- Elster J. Don’t burn your bridge before you come to it: Some ambiguities and complexities of precommitment. Texas Law Review. 2003;81(7):1751–87. [Google Scholar]
- Elster J. Impatience and urgency. Paper presented at the University of Oslo Philosophy workshop on wanting and liking..2008. [Google Scholar]
- Fodor JA. Modularity of Mind: An Essay on Faculty Psychology. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: A critical review. Journal of Economic Literature. 2002;40(2):351–401. [Google Scholar]
- Gjelsvik O. Commentary on Ainslie’s ‘Pleasure and aversion: Challenging the conventional dichotomy’. Paper presented at the University of Oslo Philosophy workshop on wanting and liking.2008. [Google Scholar]
- Glickman SE, Schiff BB. A biological theory of reinforcement. Psychological Review. 1967;74:81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- Holton R. Rational resolve. Philosophical Review. 2004;113(4):507–35. [Google Scholar]
- Holton R. Choice and effort. Paper presented at the University of Oslo Philosophy workshop on wanting and liking..2008. [Google Scholar]
- Hornsby J. Agency and actions. In: Hyman J, Steward H, editors. Agency and Action. Cambridge: Cambridge University Press; 2004. pp. 1–23. [Google Scholar]
- Hornsby J. Commentary on Holton’s ‘Choice and effort’. Paper presented at the University of Oslo Philosophy workshop on wanting and liking.2008. [Google Scholar]
- Kahneman D, Wakker PP, Sarin R. Back to Bentham? Explorations of experienced utility. The Quarterly Journal of Economics. 1997;112:375–405. [Google Scholar]
- LeDoux J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon & Schuster; 1996. [Google Scholar]
- Mahler S, Berridge K. Which cue to ‘want’? Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. Journal of Neuroscience. 2009;29(20):6500–13. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GJ, Cooper J, Clarebrough C. Frustrations of fur-farmed mink. Nature. 2001;410(6824):35–36. doi: 10.1038/35065157. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106(1):3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pinker S. How the Mind Works. New York: Norton; 1997. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature and Neuroscience. 2008;11(4):423–25. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54(1):25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Fay-McCarthy M, Collins JP, Jr, Risher-Flowers D, Alim TN, Deutsch SI. Transient compulsive foraging behavior associated with crack cocaine use. American Journal of Psychiatry. 1993;150(1):155–56. doi: 10.1176/ajp.150.1.155. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27(7):1594–605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: Generating sensory pleasure in the brain. In: Kringelbach M, Berridge KC, editors. Pleasures of the Brain. Oxford: Oxford University Press; 2009. pp. 27–49. [Google Scholar]
- Sperber D. Commentary on Elster’s ‘Impatience and urgency’. Paper presented at the University of Oslo Philosophy workshop on wanting and liking.2008. [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neuroscience and Biobehavioral Reviews. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: Amplification by mesolimbic sensitization and amphetamine. European Journal of Neuroscience. 2005;22(10):2617–34. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Wegner DM. The Illusion of Conscious Will. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Winkielman P, Berridge KC. Unconscious emotion. Current Directions in Psychological Science. 2004;13(3):120–23. [Google Scholar]
- Wyvell CL, Berridge KC. Incentive-sensitization by previous amphetamine exposure: Increased cue-triggered ‘wanting’ for sucrose reward. Journal of Neuroscience. 2001;21(19):7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]