Abstract
The ability of elastic tissues to deform under physiological forces and to subsequently release stored energy to drive passive recoil is vital to the function of many dynamic tissues. Within vertebrates, elastic fibres allow arteries and lungs to expand and contract, thus controlling variations in blood pressure and returning the pulmonary system to a resting state. Elastic fibres are composite structures composed of a cross-linked elastin core and an outer layer of fibrillin microfibrils. These two components perform distinct roles; elastin stores energy and drives passive recoil, whilst fibrillin microfibrils direct elastogenesis, mediate cell signalling, maintain tissue homeostasis via TGFβ sequestration and potentially act to reinforce the elastic fibre. In many tissues reduced elasticity, as a result of compromised elastic fibre function, becomes increasingly prevalent with age and contributes significantly to the burden of human morbidity and mortality. This review considers how the unique molecular structure, tissue distribution and longevity of elastic fibres pre-disposes these abundant extracellular matrix structures to the accumulation of damage in ageing dermal, pulmonary and vascular tissues. As compromised elasticity is a common feature of ageing dynamic tissues, the development of strategies to prevent, limit or reverse this loss of function will play a key role in reducing age-related morbidity and mortality.
Keywords: Elastic fibres, Elastin, Fibrillin microfibrils, Biomechanics, Ageing
Introduction
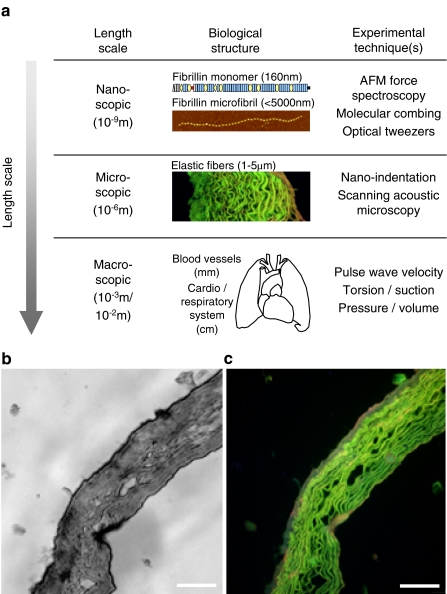
Elastic fibres are highly insoluble structures, which are composed of elastin and fibrillin microfibrils (Kielty et al. 2002) (Fig. 1). They are major components of the extracellular matrix (ECM) in dynamic tissues such as blood vessels (Davis 1993), skin (Braverman and Fonferko 1982) and the lungs (Pierce and Hocott 1960). This tissue distribution, combined with the low modulus of elasticity and high resilience of elastin (Gosline et al. 2002), allows elastic fibres to complement the tensile strength of fibrillar collagens (Kielty et al. 2002). Elastic fibre-rich dynamic tissues are therefore able to deform and store energy under normal physiological loads and to use this energy to drive recoil back to a resting state (Gosline et al. 2002). The maintenance of these mechanical properties is central to the function of dynamic tissues within the cardio-respiratory system and it has been suggested that the age-related failure of elastic fibres may underpin the apparent 100- to 120-year limit on human life expectancy (Robert et al. 2008)
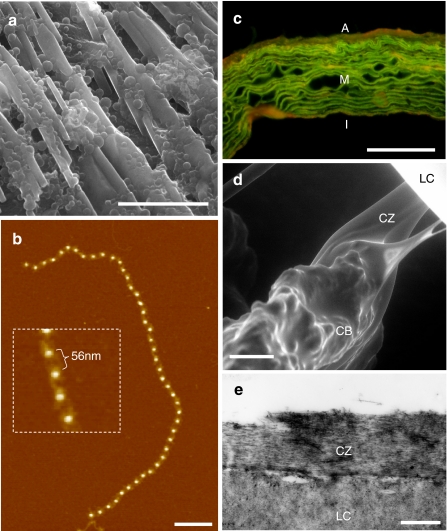
Fig. 1a–e.
Elastic fibre tissue distribution and composition. a, b The major elastic fibre components: elastin (a) and fibrillin microfibrils (b). a Environmental scanning electron microscopy (ESEM) image of the linear arrays and globules formed by coacervated recombinant tropoelastin. b AFM height image and high-resolution inset of a fibrillin microfibril isolated from young human skin (27-year-old male). The bright beads are raised 7–8 nm above the mica surface and are spaced 56 nm apart. c-e Composite elastic fibres are abundantly distributed in major arteries (c), whilst fibrillin microfibrils alone transmit forces between the ciliary muscle and the lens in the eye (d, e). c Fluorescence microscope image of haemotoxylin and eosin-stained ferret aorta. The green auto-fluorescence of elastic fibres, which are arranged into concentric lamellae in the medial (M) layer, is considerably enhanced by prior haemotoxylin and eosin staining (deCarvalho and Taboga 1996). The outer advential and inner intimal layers are indicated by A and I, respectively. d, e ESEM (d) and TEM (e) images of human ciliary zonules (CZ), which originate in the ciliary body (CB), intercalating with the lens capsule (LC) holding the lens in dynamic suspension. Scale bar 20 μm (a), 200 nm (b), 50 μm (c, d) and 500 nm (e)
Although much progress has been made in recent years in defining elastic fibre composition, many questions remain as to the precise macro-molecular structure of fibrillin microfibrils and the functional roles played by distinct elastic fibre components in both mediating tissue elasticity and maintaining tissue homeostasis. This review considers how age-related changes in the elasticity of tissues such as skin, lungs and blood vessels impact upon human morbidity and mortality, and discusses the effects of ageing on elastic fibre structure and function. Further characterisation of the molecular mechanisms which underlie age-related changes in tissue elasticity is a vital first step in the development of preventative or reparative interventions.
Elastic fibre structure, function, longevity and degradation
Elastogenesis (elastic fibre deposition)
The deposition of elastic fibres is highly regulated during development and this complex process is summarised in Fig. 2a (for reviews see Handford et al. 2000; Kielty et al. 2002, 2005; Mithieux and Weiss 2005). The three known fibrillin isoforms (fibrillin-1, -2 and -3) exhibit distinct spatial and temporal expression patterns (Corson et al. 2004; Zhang et al. 1995). Fibrillin-2 (FBN-2) is predominantly expressed during early development, whilst fibrillin-1 is the most abundant isoform in mature tissues (Ramirez and Pereira 1999). Both fibrillins are secreted from cells as profibrillin dimers or trimers which subsequently undergo N- and C-terminal processing (Cain et al. 2006; Raghunath et al. 1999; Wallis et al. 2003). Subsequent microfibril assembly, which occurs pericellularly, is critically dependent on interactions with heparin, heparan sulphate and fibronectin (Kinsey et al. 2008; Tiedemann et al. 2001). Elastin, which is secreted as the soluble precursor, tropoelastin, aggregates at the cell surface prior to transfer onto the microfibril scaffold and enzymatic conversion to the mature cross-linked form via lysyl oxidase (LOX) or LOX-like proteins (Lemaire et al. 2007; Mithieux and Weiss 2005). In the mature elastic fibre, the elastin core comprises over 90% of the volume whilst the fibrillin microfibrils are largely to confined to an outer mantle (Mecham and Davis 1994).
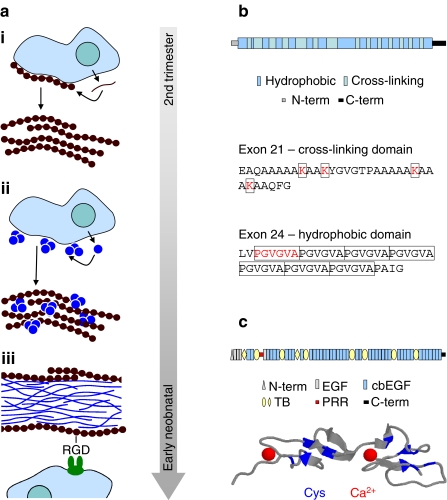
Fig. 2a–c.
Elastic fibre composition and assembly. a Assembly of fibrillin into microfibrils and the association of microfibrils with tropoelastin to form elastic fibres is a highly organised process which is limited to foetal and early neonatal development. i Secreted profibrillin is processed and assembled into pericellular microfibrils and microfibril bundles. ii Elastin globules which have assembled at the cell surface coalesce on the microfibril scaffold. iii In the core of the mature elastic fibre, ultrastructural analyses reveal twisted rope-like structures of highly cross-linked elastin (Ronchetti et al. 1998). Fibrillin microfibrils are mostly located at the microfibril periphery, where they can interact with cellular integrins via an RGD site on fibrillin-1 (Bax et al. 2003). b The structure of tropoelastin consists of alternating hydrophobic and cross-linking domains. Exon 21, for example, encodes a cross-linking domain in which pairs of lysine residues (K) are separated by two or three alanine (A) residues. In contrast, hydrophobic domains are characterised by repeating PGVGVA motifs (Keeley et al. 2002). c Fibrillin-1 is large (~320 kDa) modular glycoprotein, which, in addition to unique N- and C-terminal regions (N-term and C-term) and a potentially flexible proline-rich region (PRR), is predominantly composed of repeating eight-cysteine (also known as TB modules) and EGF-like domains, which may (cbEGF) or may not (EGF) bind calcium. The cbEGF domains play a major role in maintaining fibrillin microfibril structure. Each domain is stabilised by three cys-cys disulphide bonds (indicated in blue on the ribbon model of two contiguous fibrillin-1 cbEGF domains) and by a single bound Ca2+ (Downing et al. 1996; Wess et al. 1998)
Structure and function
Tropoelastin is an alternatively spliced, 60– to 70-kDa, highly hydrophobic protein, which is present in solution in both globular and extended forms (Mithieux and Weiss 2005; Toonkool et al. 2001). The structure of tropoelastin is characterised by repeating hydrophobic domains, which are rich in Pro, Val, Gly, Leu, Ile and Ala residues, and cross-linking domains, which are rich in Lys and Ala residues (Keeley et al. 2002) (Fig. 2b). The insolubility of cross-linked elastin precludes high-resolution structural determination by techniques such as X-ray crystallography and solution nuclear magnetic resonance, and as a result the molecular structure of cross-linked elastin and hence the mechanisms which drive elastic fibre elasticity remain to be determined (Daamen et al. 2007; Keeley et al. 2002; Urry et al. 2002). Transmission electron and atomic force microscopy (TEM and AFM) investigations, however, have revealed that the apparently amorphous elastin core is actually composed of thin rope-like filaments and globular assemblies (Ronchetti et al. 1998), whilst similar features are observed by environmental SEM in coacervated recombinant human tropoelastin (Cain et al. 2008) (Fig. 1a). There is strong evidence supporting the hypothesis that elastin is both a highly compliant and a resilient protein (Gosline et al. 2002). Gross mechanical testing studies on recombinant elastin membranes and rehydrated bovine nuchal ligaments, which had been subjected to repeated autoclaving to remove associated proteins, demonstrated that even small forces produce large extensions (Aaron and Gosline 1981), whilst stress-strain curves for extensions up to 50% demonstrated the ability of recombinant elastin peptides to recoil elastically (Keeley et al. 2002). Although the primary role of elastin appears to be mechanical, cell-signalling functions have also been identified, which include the direction of vascular and airway branching (Wendel et al. 2000) and the modulation of smooth muscle cell proliferation via the elastin-laminin receptor (Ito et al. 1997).
The fibrillins are large glycoproteins whose structures are dominated by disulphide-bonded and calcium-binding epidermal growth factor-like (cbEGF) domains (Kielty et al. 2002) (Fig. 2c). Whilst the supra-molecular conformation of fibrillin within the microfibril remains a matter of debate, with published experimental evidence favouring both 1/3 staggered (Downing et al. 1996; Lee et al. 2004) and hinged arrangements (Baldock et al. 2001; Kielty et al. 2005), it appears clear that, in the mature microfibril, an average of eight processed monomers are present within each repeat (Baldock et al. 2001; Sherratt et al. 1997). It is likely that interactions of fibrillin-1 with itself and with MAGP-1, tropoelastin and fibulin-2, as demonstrated in vitro, play a central role in elastogenesis and elastic fibre function in vivo (Jensen et al. 2001; Kielty et al. 2005; Reinhardt et al. 1996; Rock et al. 2004; Tiedemann et al. 2001). In addition to mediating matrix/matrix interactions, fibrillin microfibrils communicate with cells via an RGD (Arg-Gly-Asp) recognition site, which is bound by α5β1 and αvβ3 integrins (Bax et al. 2003). The importance of fibrillin in maintaining tissue function is highlighted by the severe ocular, skeletal and cardiovascular pathologies experienced by individuals suffering from Marfan syndrome, a heritable connective tissue disorder caused by mutations in fibrillin-1 (Robinson and Booms 2001).
In addition to their role within elastic fibres, fibrillin microfibrils also function in isolation in tissues such as the ciliary zonules which hold the lens in dynamic suspension (Fig. 1d, e). The nature of the contribution made by fibrillin microfibrils to tissue biomechanical properties remains, however, controversial. Elastin is not expressed in the tissues of invertebrates and it is thought that fibrillin microfibrils mediate elastic recoil in the low-pressure closed circulatory system of the lobster, and in the jellyfish mesoglea and sea cucumber dermis (Faury 2001; Megill et al. 2005; Thurmond and Trotter 1996). The stiffness, or conversely, the compliance, of a material is quantified by determination of the elastic modulus (also known as Young’s modulus) (Fig. 3). Gross mechanical testing of microfibril-rich invertebrate tissues suggested that, with a Young’s modulus of 0.2–1.0 MPa, the stiffness of fibrillin microfibrils was similar to that of elastin (McConnell et al. 1997; Thurmond et al. 1997). Subsequent molecular combing studies carried out in our laboratories on isolated microfibrils from vertebrate tissues, however, suggested that the Young’s modulus of microfibrils was two orders of magnitude greater (78–96 MPa) than that of elastin (Sherratt et al. 2003). From these observations we suggested that fibrillin microfibrils act to reinforce the elastic fibre and that a relatively high Young’s modulus allows microfibrils, which comprise the ciliary zonules, to transmit forces between the lens and the ciliary muscle (Burd et al. 2002; Sherratt et al. 2003). In a subsequent study, Megill and co-workers calculated a stiffness of about 0.9 MPa for the fibrillin-containing elastic fibres in the mesoglea of the hydromedusa Polyorchis penicillatus (Megill et al. 2005). Using fibre-reinforced composite models they were unable to reconcile this stiffness value of 0.9 Mpa with the reported stiffness of 78–96 MPa for individual fibrillin microfibrils. Megill and co-workers therefore suggest that the stress-strain curve of individual microfibrils is unlikely to be linear, and that microfibril stiffness will be less than our estimate at low strains and greater than our estimate at high strains. This scenario strengthens the hypothesis that fibrillin microfibrils are stiffer than elastin at the higher strains (0.22 in human aorta) which are experienced during normal physiological function (Faury 2001). The resistance of microfibrils to axial tension is further emphasised by recent X-ray diffraction studies of zonular microfibrils during extension which demonstrated that microfibril periodicity and diameter remain unchanged even at strains approaching 2 (Glab and Wess 2008). A direct experimental determination of the stress-strain response of isolated microfibrils is, however, required to resolve this issue.
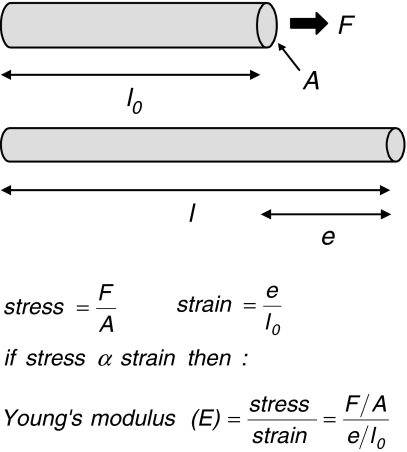
Fig. 3a, b.
Quantifying elasticity. a The elastic modulus defines the degree to which a material deforms when a tensile force is applied. Where a rod of length l0 and cross-sectional area A is stretched to a length l by a force F, the elastic modulus (E) is calculated from the stress divided by the strain. b The value of E relates to the biological function of a macromolecules; the elastic modulus of fibrillar collagen (1,200 MPa), for example, is relatively high reflecting the role of collagen fibrils in resisting tensile forces (Gosline et al. 2002), in contrast E for elastin is low (1.1 MPa) (Aaron and Gosline 1981) and a small force will produce a large extension. The elastic modulus of fibrillin microfibrils, however, remains controversial with estimates ranging from 1.0 MPa (Aaron and Gosline 1981) to 96 MPa (Sherratt et al. 2003)
Although elastin and fibrillin dominate the structure of the elastic fibre other ECM components are sporadically (decorin, biglycan, versican and emilins) or frequently [microfibril associated glycoproteins (MAGPs), latent transforming growth factor beta binding proteins (LTBPs) and fibulins] co-localised with microfibrils and/or elastin (for reviews see Gallagher et al. 2005; Hubmacher et al. 2006; Kielty et al. 2002). The MAGPs are small proteins which, in the case of MAGP-1, appear to be essential for the maintenance of microfibril structure (Lemaire et al. 2007). The carboxy-termini of MAGP-1 and -2 are thought to bind to the amino-terminal regions of fibrillin via disulphide bonds (Penner et al. 2002). Once associated with fibrillin, MAGP-1 may act to stabilise fibrillin/fibrillin interactions within the microfibril (Henderson et al. 1996; Trask et al. 2000), to mediate microfibril/elastin binding (Gibson et al. 1991) or to anchor the elastic fibre within tissues via interactions with other ECM components such as type VI collagen (Finnis and Gibson 1997). The four known LTBPs are remarkably similar in structure to the fibrillins, and the predominance of multiple contiguous cbEGF domains and the possession of apparently unique TB modules in both protein families led to the proposal that collectively the LTBPs and fibrillins should be grouped into a fibrillin super-family (Hubmacher et al. 2006; Hyytiainen et al. 2004). LTBPs appear to fulfil dual roles as both structural components of the ECM (Dallas et al. 1995) and as TGFβ trafficking molecules (Miyazono et al. 1991). Furthermore, the association of LTBPs with fibrillin microfibrils (Raghunath et al. 1998; Unsold et al. 2001) and the perturbations of normal TGFβ signalling in FBN1-mutant mice led to the hypothesis that fibrillin microfibrils play a major role in maintaining tissue homeostasis via LTBP-mediated sequestration of TGFβ (Denton and Abraham 2001; Neptune et al. 2003). Fibulins are small ECM glycoproteins which appear to play important roles during development and wound healing (for a review see Chu and Tsuda 2004). As with the LTBPs, their structure is based in contiguous cbEGF domains and three of the five fibulins (fibulin-1, -2 and -5) are known to be elastic fibre components (Kielty et al. 2002). Fibulin-1 is primarily localised to the elastin core (Kostka et al. 2001) whilst fibulin-2 and -5 are located at the microfibril/elastin interface (Nakamura et al. 2002; Reinhardt et al. 1996).
Homeostasis and elastic fibre longevity
Intracellular proteins have short life-spans: the half-lives of intracellular enzymes, for example, are usually measured in hours, whilst even the longest-lived intracellular structural proteins, such as nuclear histones, have a mean half-life of 18 days (Jennissen 1995). In contrast, the half-lives of many ECM proteins are measured in years. Aspartic acid racemization (AAR) studies estimate that the half-lives of types I and II collagen in human skin, articular cartilage and intervertebral disc are 15, 95 and 117 years, respectively (Sivan et al. 2008; Verzijl et al. 2000). The lower turnover rates and hence longer half-lives of cartilage collagens compared with dermal collagens may be a consequence of the lower cell densities and hence lower catabolic and anabolic rates which prevail in mature cartilage (Antoniou et al. 1996). The stability of the tissue collagens is matched and in many cases exceeded by the longevity of elastic fibres and their molecular components. ECM proteins in general, therefore, and structural ECM proteins of the elastic fibre system in particular, exhibit a remarkable longevity in vivo when compared with intracellular proteins and this longevity allows these proteins to gradually accumulate damage.
In the aorta, tropoelastin synthesis is under close developmental control. Elastin deposition commences in utero and reaches a maximum during early post-natal development in response to hemodynamic factors (Berry et al. 1972; Davidson et al. 1986; Bendeck and Langille 1991). Fibrillin microfibrils are thought to direct these elastogenic processes in an isoform-specific manner (Zhang et al. 1995) with FBN-2 expression preceding both tropoelastin and FBN-1 synthesis in most mammalian tissues (Ramirez and Pereira 1999). This developmental burst of tropoelastin and fibrillin expression is substantially down-regulated in humans within a few months of birth (Davidson et al. 1986; Ramirez and Pereira 1999). In mature tissues, elastin synthesis is repressed by post-transcriptional mechanisms (Zhang et al. 1999), whilst FBN-1 and FBN-2 are expressed continuously, albeit at very low levels, in adult human skin (Ashcroft et al. 1997). These observations suggest that elastic fibres, which are deposited during early development, must remain within tissues for the lifetime of the organism and there is compelling evidence, using multiple methodologies, that this is indeed the case.
In the mouse aorta, elegant auto-radiographic approaches demonstrated that tritiated valine, which was incorporated into the aortic elastic lamellae during the first month of life, remained in situ within the mature elastic fibres only for the lifetime of the animal (Davis 1993). From these studies, and from previous work in the rat, it was estimated that rodent arterial elastin, with a half-life of 27–40 years (Davis 1993; Rucker and Tinker 1977), has an expected longevity many times greater than the longevity of the host organism. AAR studies in humans indicate that aortic elastin is much more metabolically stable than the fibrillar collagens (Powell et al. 1992) and that elastin in skin, although not replaced, may undergo age-related damage (Ritz-Timme et al. 2003). The most compelling evidence for elastic fibre longevity in a human tissue was presented by Shapiro and co-workers (Shapiro et al. 1991). Using AAR techniques in combination with mass-spectrometry to assess incorporation of 14C into both the elastin and glycoprotein (fibrillin microfibril) fractions, this study demonstrated that the age of elastic fibres in the lung corresponded to the age of the individual. Taken together with the data on elastin and fibrillin synthesis, these studies provide strong evidence that, following initial synthesis during development, elastic fibre proteins function without replacement throughout the lifetime of the organism. For tissues such as the aorta, therefore, elastic fibres must, over the course of a 70-year life, maintain the ability to mechanically recoil 3 × 109 times. These stringent functional demands, coupled with their remarkable longevity, leave elastic fibres particularly vulnerable to the accumulation of age-related damage.
Degradation
The accumulation of damage by biological molecules has long been recognised as a potential contributing factor to functional decline in ageing organisms (Bailey 2001; Partridge and Gems 2002; Vijg and Campisi 2008). The low turnover of extracellular structural proteins in particular, as discussed in the previous section, exposes these macromolecular assemblies to degradation by enzymatic, chemical and biophysical mechanisms.
Remodelling and homeostasis of the ECM is mediated primarily by a large group of zinc-dependent endopeptidases, the matrix mettaloproteinases (MMPs) and their inhibitors, the TIMPs (tissue inhibitors of MMPs) (Chakraborti et al. 2003). Although up-regulation of ECM protease expression is a unifying feature of age-related inflammatory conditions such as emphysema (Robbesom et al. 2008), atherosclerosis (Robert et al. 2008) and UV-induced photoageing (Fisher et al. 1996), the constitutive expression of MMPs in non-inflamed lung, aorta and skin (Chen et al. 2005; McNulty et al. 2005; Meyer et al. 1998) may be sufficient, given the longevity of ECM assemblies, to gradually degrade proteins over many years. To date, eight MMPs have been shown to degrade elastic fibre proteins in vitro: insoluble elastin is degraded to soluble fragments by MMP-2, -7, -9, -10, -12 and -14 (Chakraborti et al. 2003; Taddese et al. 2008), whilst fibrillin microfibrils and peptides are catabolised by MMP-2,-3,-9,-12 and -13 (Ashworth et al. 1999; Tsuruga et al. 2007). In addition, both elastin and fibrillin are substrates for the serine protease neutrophil elastase (Kielty et al. 1994). The proteolytic activities of these enzymes and the structural consequences of their actions are highly variable; MMP-2 and -9, for example, fragment fibrillin microfibrils, whilst MMP-12 and -13 have a significant effect on microfibril periodicity (bead to bead distance) but not on length (Ashworth et al. 1999). Given the up-regulation of MMP expression by cultured cells in response to fibrillin fragmentation (Booms et al. 2005), microfibril degradation and protease expression may form part of a positive feedback loop which sustains chronic tissue damage. Although protease-mediated alterations in fibrillin structure are likely to have profound effects on fibrillin microfibril and hence elastic fibre function, the influence of MMPs on other microfibril associated components, such as the MAGPs, LTBPs and fibulins, remains to be characterised. Given the low substrate specificity of many MMPs, however (Chakraborti et al. 2003), and the structural similarities between the fibrillins, LTBPs and fibulins, it seems likely that, collectively, fibrillin- and elastin-degrading MMPs will be capable of degrading most elastic fibre components. In addition to their roles in directly mediating tissue remodelling via ECM degradation, MMPs also regulate ECM/cell signalling events and MMP-14 (also known as membrane type 1 MMP) has been shown to release bound TGFβ from LTBP-1 (Tatti et al. 2008).
The accumulation of oxidative damage by biological macromolecules, including lipids, proteins and nucleic acids is thought to play an important role in the ageing process (Partridge and Gems 2002; Vijg and Campisi 2008). Reactive oxygen species (ROS) including O−2, H2O2, 1O2 and •OH (Chakravarti and Chakravarti 2007), which are generated either as products of normal metabolism (Haenold et al. 2005) or by interaction with environmental factors such as UV radiation (Yaar and Gilchrest 2007), are the primary agents of protein oxidation. The influence of ROS on cellular metabolism has been the subject of intense study and it has become evident that cell-mediated perturbations in elastic fibre homeostasis, as a result of ROS-induced tropoelastin and MMP transcription (Wlaschek et al. 2001), contribute to tissue ageing. In addition to these cell-mediated anabolic and catabolic mechanisms, ROS are known to act directly on ECM collagens (Verzijl et al. 2000), but the influence of oxidation on the structure and function of elastic fibre components remains poorly understood. To date, there are two published studies which address the susceptibility of elastin to direct ROS mediated degradation: Umeda et al. (2001) characterised the oxidation and solubilisation of elastin by H2O2 in the presence of Cu2+, whilst Cantor et al. (2006) suggested that prior ROS (H2O2) exposure could enhance the susceptibility of elastic fibres to subsequent elastase-mediated degradation. These observations indicate that in vivo oxidation may, in part, be responsible for the reduced cross-linking which is characteristic of aged elastic fibres (Umeda et al. 2001). The influence of ROS on other elastic fibre components is, as yet, undefined.
In addition to degradation by proteolytic and oxidative mechanisms, the structure, and hence function, of ECM proteins in ageing tissues may be compromised by the accumulation of pathological cross-links. The structural integrity of supra-molecular ECM assemblies such as collagen fibrils and elastic fibres relies heavily on the precise enzyme-driven formation of developmentally regulated cross-links (Bailey 2001). The first stage in collagen cross-linking relies on the deamination of N- and C-terminal lysine and hydroxylysine residues by lysyl oxidase to form aldehydes (Kadler et al. 1996). The nature of the subsequently formed cross-links varies between tissues and with developmental stage; in skin, for example, divalent dehydro-hydroxylysinonorleucine (deH-HLNL) cross-links in immature tissues subsequently react with histidine to form histidino-hydroxylysinonorleucine (HHL) (Bailey 2001). In general, the mechanical strength of collagen-rich tissues increases during maturation and ageing due to the formation of non-reducible cross-links (Yamauchi et al. 1988). Elastin cross-linking, which also proceeds via the lysyl oxidase mediated deamination of lysine, occurs extensively throughout the protein and results in the formation of tetravalent desmosines and isodesmosines (Csiszar 2001; Kielty et al. 2002). Transglutaminase is thought to mediate further cross-linking events within the maturing microfibril (fibrillin-fibrillin and fibrillin-MAGP) and between microfibrils and tropoelastin (Brownaugsburger et al. 1994; Qian and Glanville 1997; Rock et al. 2004).
The developmentally regulated formation of enzyme driven intra- and inter-molecular cross-links gives way, however, to the uncontrolled accumulation of glucose and glucose-metabolite-derived cross-links in ageing tissues (Bailey 2001). These non-enzymatic cross-links undergo sequential modifications, culminating in the formation of advanced glycation end products (AGE) (Paul and Bailey 1996) which increase tissue stiffness (Sims et al. 1996), inhibit collagen assembly (Tsilibary et al. 1988) and impair collagen/cell binding (Haitoglou et al. 1992). The abundance of these pathological cross-links is positively correlated with both age and with the incidence of chronic hyperglycaemia as found in conditions such as diabetes mellitus (Bruel and Oxlund 1996; Mikulikova et al. 2008; Paul and Bailey 1996). Although the structural and functional consequences of AGE formation within ageing collagen-rich tissues have been well characterised in recent years (DeGroot 2004) less attention has been paid to the impact of AGE formation on elastic fibre function (Bailey 2001). Two recent studies have demonstrated that AGE accumulate in the elastin of both ageing human aorta (Konova et al. 2004) and yellow ligament (Chen et al. 2000), but the hypothesis that AGE formation within ECM proteins contributes to age-related cardio-vascular stiffening was questioned by Shapiro and co-workers in a study which localised AGE to smooth muscle rather than ECM-rich areas in the vasculature (Shapiro et al. 2008).
In addition to these major degradative mechanisms, the structure of elastic fibres in ageing tissues may also be altered by calcification, aspartic acid racemization, lipid accumulation and mechanical fatigue (Bailey 2001; O’Rourke and Hashimoto 2007; Robert et al. 2008). Calcium accumulates in ageing blood vessel walls and is strongly bound to the microfibrillar component of elastic fibres (Robert et al. 2008). In vitro studies have demonstrated that microfibrillar structure is highly sensitive to the concentration of calcium in the local environment (Wess et al. 1998). This bound calcium may also play a role in mediating the uptake of lipids by elastic fibres in the ageing arterial wall (for a review see Robert et al. 2008). In addition to binding extracellular ions and biomolecules, L-forms of aspartic acid within elastic fibre proteins spontaneously convert to D-forms at a predictable rate (Shapiro et al. 1991). Both elastin and fibrillin accumulate D-aspartic acid by aspartic acid racemization, but the consequences for the structure and function of the ageing elastic fibre remain poorly defined (Bailey 2001). Mechanical fatigue has also been postulated as a mechanism of age-related elastic fibre failure, with indirect evidence suggesting that the microfibril/elastin interface may be the main weak point in the composite elastic fibre (Lillie and Gosline 2007; O’Rourke and Hashimoto 2007). The following section considers the effects of this multitude of degradative mechanisms on not only the structure of the elastic fibre system in ageing skin, lungs and blood vessels but also the clinical impact which the resultant compromised tissue elasticity may, in many cases, have on human morbidity and mortality.
Elastic fibres in ageing tissues
Many of the degradative mechanisms discussed in the previous section are thought to operate systemically and, as a consequence, age-related changes in the structure of elastic fibres would be expected to impact on the functions of most dynamic tissues. However, the exposure of tissues such as the lungs and skin to unique environmental degradative mechanisms ensures that the rate and nature of age-related elastic fibre damage varies both between anatomical sites and between individuals.
Cutaneous
The elastic fibre system in skin is highly ordered (Fig. 4a). In the reticular dermis, large-diameter elastic fibres lie parallel to the skin surface. These fibres are connected to smaller-diameter elaunin fibres in the papillary dermis and eventually to oxytalan fibres which connect the papillary dermis and epidermis via the dermal-epidermal junction (DEJ) (Braverman and Fonferko 1982). The ratio of elastin to fibrillin decreases with proximity to the epidermis and the oxytalan fibres appear to be solely microfibrillar (Cotta-Periera et al. 1978; Dahlback et al. 1990). The ageing process has a profound effect on the structure and function of this elastic fibre system but, as with the ageing lung, human skin accumulates damage due to both the action of normal metabolic processes and also as a result of interactions with environmental factors. In areas exposed to ultraviolet light, acute or “extrinsic” ageing processes are superimposed on underlying chronic or “intrinsic” ageing mechanisms (El-Domyati et al. 2002). These two processes have distinct cosmetic and structural consequences for ageing skin. The smooth appearance and fine wrinkles of intrinsically aged skin are associated with a gradual fragmentation of the elastic fibre network (Braverman and Fonferko 1982; Suwabe et al. 1999; Tsuji and Hamada 1981). In contrast, mildly photoaged skin appears roughened and deeply wrinkled and is characterised histologically by the loss of fibrillin microfibrils (Watson et al. 1999) and fibulin (Kadoya et al. 2005) from the papillary dermis. In severely photoaged skin, however, abundant deposits of highly disorganised, elastic fibre material are distributed throughout the dermis (Warren et al. 1991; Watson et al. 2001; Werth et al. 1996; Yaar and Gilchrest 2007)
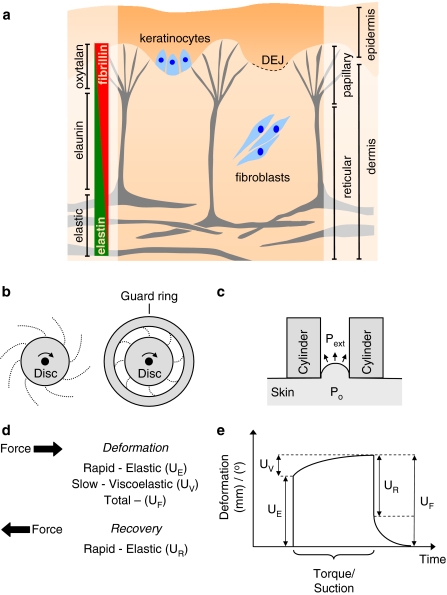
Fig. 4a–e.
Structure and mechanical function of the skin. a The elastic fibre system in skin is confined to the dermis where composite elastic fibres in the reticular dermis give way to arrays of fibrillin microfibril bundles at the DEJ. The fibrillin microfibrils of the elastic fibre system are synthesised by both keratinocytes and dermal fibroblasts. b, c Methods for testing the mechanical properties of skin include: torsion, induced by a rotating disk (b), where the area of skin may be restrained with a guard ring and suction (c), where the lowered external air pressure (Pext) in a cylinder causes skin deformation due to the internal pressure of the tissue (Po). d, e Both methods induce a total deformation (UF) which is the result of an initial rapid (UE) and a slower viscoelastic (UV) deformation. Release of the torque or suction is followed by a rapid recovery (UR)
Early attempts to quantify the effects of ageing on the mechanical properties of skin were hampered by: the use of terminology which was open to mis-interpretation (Doubal and Klemera 2002), the diversity of both mechanical tests (Diridollou et al. 1998; Rodrigues 2001) and anatomical sites (Escoffier et al. 1989), and most importantly by the heterogeneity and anisotropy of the skin layers themselves (Diridollou et al. 1998; Pierard 1999). The mechanical response of skin to the application of tensile and torsional forces and to deformations induced by indentation and suction have all been reported in the literature (see Rodrigues 2001 for an excellent review). Of these techniques, torsional approaches, which may be used in vivo, have the advantage of applying mechanical stresses parallel to the plane of the skin, thereby minimising the influence of tissue anisotropy and underlying tissue structures on the measured mechanical parameters (Escoffier et al. 1989). The device rotates a disk which has been adhered to the skin and records the torque (rotational force) and degree of rotation (Fig. 4b). By introducing guard rings of variable diameter, it has been proposed that the depth of mechanical deformation can be controlled (Batisse et al. 2002). Since their introduction in the 1970s (Finlay 1970) torsional approaches have been applied to the study of both intrinsic (Escoffier et al. 1989) and extrinsic ageing (Agache et al. 1980; Batisse et al. 2002; Sanders 1973) and a commercial device, the Dermal Torque Meter is now in use (Batisse et al. 2002). Suction methods which use a negative pressure to deform the skin (Fig. 4c) are perhaps the most widely used (Diridollou et al. 1999; Pierard 1999; Rodrigues 2001; Smalls et al. 2006; Takema and Imokawa 1998; Takema et al. 1994). Diridollou and co-workers suggest that this technique enables the measurement of mechanical responses which originate primarily from the dermis rather than sub-cutaneous structures (Diridollou et al. 1998). Commercially developed devices include the Cutometer, which measures skin deformations optically, and the Dermaflex A, which measures vertical skin displacements by changes in capacitance (Diridollou et al. 1998; Smalls et al. 2006). Interpretation of the biomechanical properties measured by these techniques requires quantitation of skin thickness. Early attempts to measure skin thickness using callipers (Agache et al. 1980) have largely been superseded by the use of ultrasound (Diridollou et al. 1999; Escoffier et al. 1989; Takema et al. 1994).
Applying either suction or torsional forces to skin induces a rapid elastic deformation (UE), followed by a slower viscoelastic deformation (UV) until finally the total skin deformation (UF) is reached (Fig. 4d, e) (Escoffier et al. 1989; Leveque et al. 1980; Rodrigues 2001; Takema et al. 1994). Removing the force allows the skin to recover and the ratio of the recovery to initial deformation quantifies the ability of the skin to elastically recoil. Both the ratio of the rapid elastic recovery to the elastic deformation (where UR/UE is defined as the elastic function) and the ratio of the rapid elastic recovery to the total skin deformation (where UR/UF is defined as relative elastic recovery) have been quoted in the literature (Escoffier et al. 1989; Rodrigues 2001; Smalls et al. 2006). The ability of skin to recoil is significantly reduced by both the intrinsic (Escoffier et al. 1989; Smalls et al. 2006; Takema et al. 1994) and extrinsic ageing processes (Agache et al. 1980; Smalls et al. 2006; Staloff et al. 2008; Takema et al. 1994) and by exposure to UV-A and UV-B radiation in vitro (Takema and Imokawa 1998). In most studies, skin stiffness [as determined from a decrease in elastic (UE) or total deformation (UF)] also increased significantly in both intrinsically (Escoffier et al. 1989; Smalls et al. 2006) and extrinsically aged tissue (Agache et al. 1980; Smalls et al. 2006; Takema et al. 1994) and in UV radiation-exposed tissues (Takema and Imokawa 1998). In contrast to intrinsically aged skin, which is characterised histologically by the loss of elastic fibre components, extrinsically aged skin is characterised by the gain of disorganised elastotic material. Despite these striking differences in ECM composition, the elastic moduli of both tissues are significantly increased compared with both young and UV-protected skin (Escoffier et al. 1989; Agache et al. 1980). It appears, therefore, that alterations in inter- or intra-molecular architecture, rather than ECM composition alone, must play a key role in mediating age-related loss of elasticity in the skin. As a consequence, it is unlikely that attempts to characterise the age-related mechanisms which underlie loss of tissue elasticity (both recoil and extensibility) by solely quantifying changes in the amount of individual elastic fibre proteins will prove successful. Our preliminary studies, for example, suggest that age-related alterations in fibrillin microfibril structure can have a profound effect on the tensile strength of these macro-molecular assemblies (Sherratt et al. 2006, 2007).
Aberrant protein proteolysis, oxidation and cross-linking have all been implicated in age-related degradation of the dermal elastic fibre network. Exposure of skin or skin-derived cells to UVR, for example, is known to upregulate the expression of many elastic fibre degrading enzymes, including MMP-2, -3, -9, -12 and -13 and the serine protease neutrophil elastase (Fisher et al. 1996; Kim et al. 2006; Rijken et al. 2005; Saarialho-Kere et al. 1999). ROS generation may mediate elastic fibre damage in skin either indirectly, via protease upregulation (Yaar and Gilchrest 2007), or directly via interaction with the sulphur containing amino acids methionine and cysteine (Haenold et al. 2005). The immuno-histochemical identification of protein carbonyl end products in the upper dermis suggests that direct, ROS mediated, protein oxidation occurs in both acutely and chronically photoaged skin (Sander et al. 2002). Finally, aberrant elastin cross-linking appears to be a feature of both photoaged and intrinsically aged skin (Jeanmaire et al. 2001; Mizutari et al. 1997).
Pulmonary
The exposure of human lungs to environmental pollutants, in particular cigarette smoke, complicates the study of ageing within the pulmonary system (Teramoto et al. 1999). Characterisation of the mechanical and molecular effects of intrinsic ageing in the lungs has been carried out in humans with known medical histories and in animal models. In these systems, the pulmonary system undergoes progressive changes in mechanical properties with age which ultimately compromise lung function and therefore diminish quality of life. In the aged lung, the loss of elasticity simulates emphysema (Knudson et al. 1977; Turner et al. 1968), and both forced expiratory volume and forced vital capacity are reduced (Meyer et al. 1998). This reduction in tissue elasticity (both compliance and recoil), which has been well characterised in both human (Lai-Fook and Hyatt 2000; Turner et al. 1968) and rodent lungs (Janssens et al. 1999; Nagase et al. 1994), plays an important role in increasing the risk of mortality in the ageing population as a result of acute pulmonary diseases (Janssens et al. 1999; Meyer et al. 1998). The mechanical properties of the lung are highly complex and depend on: the relative mechanical properties of the constituent ECM components (Fredberg and Kamm 2006), the arrangement of these molecules within the alveolar wall, and the geometrical construction of the alveolus itself (Kitaoka et al. 2007; Wilson and Bachofen 1982). Dilatation of the alveolus, which is thought to be a universal feature of the ageing mammalian lung (Hyde et al. 1977; Pinkerton et al. 1982; Verbeken et al. 1992), correlates with increases in alveolar wall thickness in most (Escolar et al. 1997; Snider et al. 1985; Verbeken et al. 1992), but not all human and rodent studies (Escolar et al. 1994, 1993). These gross morphological changes occur without significant mass loss (Escolar et al. 1994; Pinkerton et al. 1982). Whilst the lung, therefore, experiences age-related alterations in both structure and function, it has not proven possible to relate these morphological and mechanical changes to simple variations in ECM composition.
The ECM of the lung is dominated by type I collagen fibrils and elastic fibres (Pierce and Hocott 1960). Most studies of the ageing lung have failed to identify any change in collagen content (Escolar et al. 1997; Lang et al. 1994; Pierce and Hocott 1960; Takubo et al. 1999; Yamamoto et al. 2003), although age-related increases have been observed in both rodent (Goldstein 1982; Huang et al. 2007) and human systems (Derrico et al. 1989). Similarly, the concentration of elastin and/or elastic fibres in human and rodent lungs has been reported to remain unchanged (Andreotti et al. 1983; Takubo et al. 1999; Yamamoto et al. 2003), to increase (Escolar et al. 1994, 1997; Fitzpatrick and Hospelhorn 1962; Pierce and Hocott 1960) and to decrease with age (Derrico et al. 1989; Huang et al. 2007). There are three potential explanations for the lack of a consensus regarding changes in ECM composition in the ageing lung. First, there are many experimental difficulties involved in the complete extraction and purification of large, insoluble and increasingly aberrantly cross-linked ECM proteins from ageing tissues (Bailey 2001). Second, the accumulation of chemical and morphological changes by long-lived ECM proteins may influence morphometric analyses by changing histological staining and/or antibody binding affinities. Third, the respiratory system is exposed to environmental effects such as smoking and air pollutants which are known to influence both lung structure and function (Teramoto et al. 1999). Even where attempts have been made to control for these environmental factors using rodent models and to control for differential protein extraction using histological approaches, elastic fibre content in the ageing rat lung may still appear to increase (Escolar et al. 1997), decrease (Huang et al. 2007) or remain unchanged (Yamamoto et al. 2003). As the structural and functional consequences of intrinsic ageing appear to be invariant within mammalian lung, these observations support the hypothesis that age-related changes in the molecular and supramolecular structures of ECM components (John and Thomas 1972), rather than their relative tissue concentrations, underlie gross changes in lung mechanics. Relatively few studies have addressed the change in elastic fibre morphology or composition with age, but in the rat model, increases in elastic fibre diameter are thought to reflect elastic fibre degeneration (Escolar et al. 1994, 1997). It has been established that even apparently normal human lung contains detectable levels of proteolytic enzymes with elastolytic abilities (Meyer et al. 1998). Low-grade chronic inflammation may therefore contribute to enzyme- or ROS-driven age-related elastic fibre remodelling (Lambeth 2007). The formation of AGE within ageing rat lung collagen suggests that ECM glycation may also play a role in mediating age-related pulmonary pathologies (Bellmunt et al. 1995).
In addition to the deleterious changes in lung function induced by intrinsic ageing processes, environmentally-linked pulmonary diseases such as chronic obstructive pulmonary disorder (COPD) and emphysema become increasingly prevalent with age (Viegi et al. 2001). COPD is characterised by a loss of elastin from the alveolar wall (Merrilees et al. 2008) and the ensuing progressive emphysema induces by alveolar enlargement, perenchymal destruction and loss of lung elasticity (Robbesom et al. 2008). Two potential mechanisms are thought to play a role in the development of emphysema; first, a chronic imbalance between proteases, including neutrophil elastase, MMP-2 and -9, and protease inhibitors such as secretory leukoprotease inhibitor (Gadek et al. 1984; Meyer et al. 1998); and second, ROS-mediated DNA and protein damage as a result of infections or exposure to environmental pollutants (Repine et al. 1997). Histologically, elastic fibres in the emphysematous lung appear fragmented, with a reduced microfibrillar component and evidence of disorganised elastin deposition (Fukuda et al. 1989). Evidence for the involvement of fibrillin in the pathogenesis of emphysema comes from the observation of emphysematous lesions in connective tissues disorders such as neonatal (Jacobs et al. 2002; Milewicz and Duvic 1994) and adult (Bolande and Tucker 1964; Sayers et al. 1975) Marfan syndrome and the tight skin mouse model of hereditary emphysema (Gardi et al. 1989). Whilst even in the early stages of human emphysema fragmentation of fibrillin microfibril bundles is evident (Robbesom et al. 2008).
In summary, deterioration of lung function is an important factor in age-related morbidity and mortality and elastic fibre degeneration appears to play a central role in many age-related pulmonary disorders. The evidence points towards the cumulative damage of long-lived elastic fibre proteins as both a cause of altered mechanical function and as a trigger for further cell-mediated damage via aberrant cell-signalling mechanisms. Understanding the molecular causes and effects of age-related pulmonary disorders will require detailed study of the degradative mechanisms which produce microfibril degeneration, and the functional consequences of these macro-molecular changes.
Vascular
Age-related alterations in the mechanical properties of the vascular system have profound effects on human morbidity and mortality (for recent reviews see Greenwald 2007; Mitchell 2008; O’Rourke and Hashimoto 2007). Arteries and veins are composed of three distinct layers, the tunica intima, tunica media, and tunica adventia (Quaglino and Pasquali-Ronchetti 2002). Within arteries, elastic fibres are concentrated at the layer boundaries (in the internal and external elastic laminae) and in the medial layer where elastic lamellae alternate with smooth muscle cells (Kielty et al. 2007). The number of elastic lamellae decreases from 40–70 in conducting arteries such as the aorta, to fewer than ten in the smaller resistance arteries. The compliance and elasticity of the major arteries plays a key role in cardiac function; driving systemic blood flow via the storage of elastic tensile energy in the aorta (Greenwald 2007) and converting pulsatile to steady blood flow (O’Rourke and Hashimoto 2007). Age-related reductions in arterial compliance (known as arteriosclerosis) lead to increases in systolic blood pressure in the aorta, a major risk factor for the development of heart failure (Mitchell 2008; O’Rourke and Hashimoto 2007). It is still unclear, however, whether vascular hypertension is a cause, or symptom, of this arterial stiffening (Arribas et al. 2006; McEniery et al. 2007). In addition to heart failure, chronic hypertension is implicated as a major risk factor in the development of strokes (Nilsson 2005), renal failure (Lariviere and Lebel 2003) and aortic aneurysms (Lederle et al. 2008). The fundamental role of elastic fibre components in maintaining arterial function is underlined by the severe clinical consequences of both fibrillin and tropoelastin mutations (Kielty et al. 2002; Robinson and Booms 2001). Mutations in fibrillin-1, for example, cause Marfan syndrome, a heritable connective tissue disorder which is associated with ocular, skeletal, pulmonary and vascular defects. It is these vascular pathologies which are primarily responsible for the markedly reduced life expectancy experienced by Marfan patients in the absence of modern medical interventions [32 ± 16 years (Silverman et al. 1995)]. Dilatation, and ultimately, dissection, of the aortic root and/or ascending aorta being the main cause of death (Robinson and Booms 2001). Mutations in elastin can have an equally severe impact on morbidity and mortality giving rise to supravalvular aortic stenosis (SVAS), which in turn can cause left ventricular hypertrophy and ultimately congestive heart failure (Milewicz et al. 2000).
The stiffness of central arteries, as determined by in vivo methods such as transesophageal echocardiography (Pearson et al. 1994) and pulse wave velocity (O’Rourke et al. 1968; Ruitenbeek et al. 2008) or by in vitro mechanical testing approaches (Bruel and Oxlund 1996; Learoyd and Taylor 1966), increases with age independently of confounding factors such as atherosclerosis or hypertension. The contribution of individual tissue components to this age-related progression of arterial stiffening is, however, less well defined (Fig. 5). Arterial collagen concentration has been reported to: steadily increase, to increase only after the age of 50 and to remain unchanged with age (Cattell et al. 1996; Hosoda et al. 1984; Maurel et al. 1987). Although elastin concentration in ageing arteries has been reported to decrease (Hosoda et al. 1984; Maurel et al. 1987), in the specific case of normotensive individuals, aortic elastin concentration appears to increase (Cattell et al. 1996). Cattell and co-workers highlight the need to distinguish between changes in the absolute amount of a tissue component versus changes in its concentration (Cattell et al. 1996). In their study, aortic collagen and elastin concentrations increased with age, whilst the absolute amounts decreased. The authors attribute this discrepancy to the age-related differential loss of other tissue components. There is mounting evidence to suggest that ECM organisation, rather than relative composition mediates arterial mechanical properties. The collagen/elastin ratio, for example, is a poor predictor of relative tissue mechanical properties (Cox 1981) and the highly organised structure of elastic lamellae becomes increasingly disrupted in the ageing aorta (Avolio et al. 1998; Bruel and Oxlund 1996). Theoretical modelling of the artery wall during extension suggests that altered collagen fibril-fibril interactions, rather than changes in the compliance of individual fibrils, underlies age-related arterial stiffening (Zulliger and Stergiopulos 2007). In the same study, the authors present evidence that alterations in the microstructure of elastic fibre are likely to play an important role in determining tissue mechanical properties and that, as a consequence, the model of elastin as an isotropic solid is over-simplified. The proteolytic, oxidative and cross-linking mechanisms which are thought to compromise the structure of elastic fibres in skin and the lungs are also thought to be major factors in arterial ageing (Watanabe et al. 1996; Zieman et al. 2005). In addition to these chemical and biochemical mechanisms, the extreme mechanical demands made on tissues such as the aorta may promote the mechanical failure of elastic fibres (O’Rourke and Hashimoto 2007).
Fig. 5a–c.
Measuring biomechanical properties at increasing length scales. a In general, the elastic moduli of the constituent ECM molecules are higher than elastic moduli of the connective tissues themselves (Akhtar et al. 2009). In order, therefore, to understand the mechanical role played by age-related changes in molecular abundance, distribution and structure it is necessary to characterise the mechanical properties of tissues at the nano-scopic, micro-scopic and macro-scopic lengths scales. b, c Scanning acoustic microscopy (SAM) image of an unfixed cryo-sectioned ferret aorta (b) and a fluorescence microscopy image of the same section post-stained with haematoxylin and eosin (c). Variations in wavespeed in the SAM image (which are correlated with material stiffness) closely match the distribution of elastic fibres imaged in the fluorescence microscope. Scale bar 100 μm
Conclusions: repairing and preventing elastic fibre degradation
Although collagenous structures, such as tendons, may become increasingly compliant with age (Onambele et al. 2006) elastic fibre-rich tissues, such as skin, blood vessels, and lungs, in general, lose their compliance in older individuals. The repair of damaged elastic fibres within these tissues remains a difficult challenge for biomedicine, but in tissues such as photoaged skin, topical treatment with all-trans retinoic acid has been shown to promote the deposition of fibrillin-1 but not of type I collagen (Watson et al. 2008). In the vasculature, whilst there is a great deal of interest in the potential of in vitro tissue engineering approaches (for a review see Kielty et al. 2007), Merrilees and co-workers have demonstrated the ability of an ECM glycoprotein (verscian) to stimulate elastic fibre deposition in vivo (Merrilees et al. 2002). Perhaps the best hope, however, lies with preventing the damage from occurring; elastic fibres have a widespread tissue distribution, where they fulfil vital mechanical and biochemical roles and are uniquely long-lived and hence susceptible the accumulation of age-related damage. This combination of factors suggests that any preventative strategies which succeed in ameliorating the adverse effects of protein glycation and low grade inflammation (Vijg and Campisi 2008), for example, are likely to have profound effects on elastic fibre structure/function in ageing tissues and hence on human morbidity and mortality.
Acknowledgements
I am indebted to Prof. Cay Kielty and Dr. Nigel Hodson for providing the microscopic images of coacervated tropoelastin, and to Drs. Carolyn Jones and Riaz Akhtar for the micrographs of human ciliary zonules and ferret aorta. I would also like to thank Drs. Helen Graham and David Holmes for reading and commenting on the manuscript and Research into Ageing for supporting this work.
References
- Aaron BB, Gosline JM. Elastin as a random-network elastomer: a mechanical and optical analysis of single elastin fibers. Biopolymers. 1981;20:1247–1260. [Google Scholar]
- Agache PG, Monneur C, Leveque JL, Derigal J. Mechanical-properties and youngs modulus of human-skin in vivo. Arch Dermatol Res. 1980;269:221–232. doi: 10.1007/BF00406415. [DOI] [PubMed] [Google Scholar]
- Akhtar R, Schwarzer N, Sherratt MJ, Watson REB, Graham HK, Trafford AW, Mummery PM, Derby B (2009) Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J Mater Res 24:638–646 [DOI] [PMC free article] [PubMed]
- Andreotti L, Bussotti A, Cammelli D, Aiello E, Sampognaro S. Connective-tissue in aging lung. Gerontology. 1983;29:377–387. doi: 10.1159/000213148. [DOI] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc - Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas SM, Hinek A, Gonzalez MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111:771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Kielty CM, Horan MA, Ferguson MWJ. Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J Pathol. 1997;183:80–89. doi: 10.1002/(SICI)1096-9896(199709)183:1<80::AID-PATH1104>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Biochem J. 1999;340:171–181. [PMC free article] [PubMed] [Google Scholar]
- Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Baldock C, Koster AJ, Ziese U, Rock MJ, Sherratt MJ, Kadler KE, Adrian Shuttleworth C, Kielty CM. The supramolecular organization of fibrillin-rich microfibrils. J Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batisse D, Bazin R, Baldeweck T, Querleux B, Leveque JL. Influence of age on the wrinkling capacities of skin. Skin Res Technol. 2002;8:148–154. doi: 10.1034/j.1600-0846.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha(5)beta(1) and alpha(v)beta(3) integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- Bellmunt MJ, Portero M, Pamplona R, Muntaner M, Prat J. AGE-related fluorescence in rat lung collagen. Lung. 1995;173:177–185. doi: 10.1007/BF00175658. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Langille BL. Rapid accumulation of elastin and collagen in the aortas in sheep in the immediate perinatal-period. Circ Res. 1991;69:1165–1169. doi: 10.1161/01.res.69.4.1165. [DOI] [PubMed] [Google Scholar]
- Berry CL, Looker T, Germain J. Nucleic-acid and scleroprotein content of developing human aorta. J Pathol. 1972;108:265. doi: 10.1002/path.1711080402. [DOI] [PubMed] [Google Scholar]
- Bolande RP, Tucker AS. Pulmonary emphysema and other cardiorespiratory lesions as part of Marfan abiotrophy. Pediatrics. 1964;33:356. [PubMed] [Google Scholar]
- Booms P, Pregla R, Ney A, Barthel F, Reinhardt DP, Pletschacher A, Mundlos S, Robinson PN. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum Genet. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Fonferko E. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol. 1982;78:434–443. doi: 10.1111/1523-1747.ep12507866. [DOI] [PubMed] [Google Scholar]
- Brownaugsburger P, Broekelmann T, Mecham L, Mercer R, Gibson MA, Cleary EG, Abrams WR, Rosenbloom J, Mecham RP. Microfibril-associated glycoprotein binds to the carboxyl-terminal domain of tropoelastin and is a substrate for transglutaminase. J Biol Chem. 1994;269:28443–28449. [PubMed] [Google Scholar]
- Bruel A, Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996;127:155–165. doi: 10.1016/s0021-9150(96)05947-3. [DOI] [PubMed] [Google Scholar]
- Burd HJ, Judge SJ, Cross JA. Numerical modelling of the accommodating lens. Vision Res. 2002;42:2235–2251. doi: 10.1016/s0042-6989(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- Cain SA, Raynal B, Hodson N, Shuttleworth A, Kielty CM. Biomolecular analysis of elastic fibre molecules. Methods. 2008;45:42–52. doi: 10.1016/j.ymeth.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Shteyngart B, Cerreta JM, Ma SR, Turino GM. Synergistic effect of hydrogen peroxide and elastase on elastic fiber injury in vitro. Exp Biol Med. 2006;231:107–111. doi: 10.1177/153537020623100113. [DOI] [PubMed] [Google Scholar]
- Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245:73–84. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Chakravarti DN. Oxidative modification of proteins: age-related changes. Gerontology. 2007;53:128–139. doi: 10.1159/000097865. [DOI] [PubMed] [Google Scholar]
- Chen JR, Takahashi M, Kushida K, Suzuki M, Suzuki K, Horiuchi K, Nagano A. Direct detection of crosslinks of collagen and elastin in the hydrolysates of human yellow ligament using single-column high performance liquid chromatography. Anal Biochem. 2000;278:99–105. doi: 10.1006/abio.1999.4412. [DOI] [PubMed] [Google Scholar]
- Chen Z, Seo JY, Kim YK, Lee SR, Kim KH, Cho KH, Eun HC, Chung JH. Heat modulation of tropoelastin, fibrillin-1, and matrix metalloproteinase-12 in human skin in vivo. J Invest Dermatol. 2005;124:70–78. doi: 10.1111/j.0022-202X.2004.23550.x. [DOI] [PubMed] [Google Scholar]
- Chu ML, Tsuda T. Fibulins in development and heritable disease. . Birth Defects Res C Embryo Today. 2004;72:25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Cotta-Periera G, Guerro Rodriguez F, Bittencourt-Sampaio S. Oxytalan, elaunin and elastic firbes in human skin. J Invest Dermatol. 1978;66:143–148. doi: 10.1111/1523-1747.ep12481882. [DOI] [PubMed] [Google Scholar]
- Cox RH. Basis for the altered arterial-wall mechanics in the spontaneously hyptertensive rat. Hypertension. 1981;3:485–495. doi: 10.1161/01.hyp.3.4.485. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Progress in Nucleic Acid Research and Molecular Biology. 70. San Diego: Academic Press; 2001. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Daamen WF, Veerkamp JH, Hest JCM, Kuppevelt TH. Elastin as a biomaterial for tissue engineering. Biomaterials. 2007;28:4378–4398. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Dahlback K, Ljungquist A, Lofberg H, Dahlback B, Engvall E, Sakai LY. Fibrillin immunoreactive fibers constitute a unique network in the human dermis—immunohistochemical comparison of the distributions of fibrillin, vitronectin, amyloid-p component, and orcein stainable structures in normal skin and elastos. J Invest Dermatol. 1990;94:284–291. doi: 10.1111/1523-1747.ep12874430. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth-factor-beta binding-protein in storage of latent TGF-beta in the extracellular-matrix and as a structural matrix protein. J Cell Biol. 1995;131:539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JM, Hill KE, Alford JL. Developmental changes in collagen and elastin biosynthesis in the porcine aorta. Dev Biol. 1986;118:103–111. doi: 10.1016/0012-1606(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Davis EC. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry. 1993;100:17–26. doi: 10.1007/BF00268874. [DOI] [PubMed] [Google Scholar]
- deCarvalho HF, Taboga SR. Fluorescence and confocal laser scanning microscopy imaging of elastic fibers in hematoxylin-eosin stained sections. Histochem Cell Biol. 1996;106:587–592. doi: 10.1007/BF02473274. [DOI] [PubMed] [Google Scholar]
- DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4:301–305. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–511. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Derrico A, Scarani P, Colosimo E, Spina M, Grigioni WF, Mancini AM. Chnages in the alveolar connective-tissue of the aging lung - an immunohistochemcial study. Virchows Arch A Pathol Anat Histopathol. 1989;415:137–144. doi: 10.1007/BF00784351. [DOI] [PubMed] [Google Scholar]
- Diridollou S, Berson M, Vabre V, Black D, Karlsson B, Auriol F, Gregoire JM, Yvon C, Vaillant L, Gall Y, Patat F. An in vivo method for measuring the mechanical properties of the skin using ultrasound. Ultrasound Med Biol. 1998;24:215–224. doi: 10.1016/s0301-5629(97)00237-8. [DOI] [PubMed] [Google Scholar]
- Diridollou S, Vienne MP, Alibert M, Aquilina C, Briant A, Dahan S, Denis P, Launais B, Turlier V, Dupuy P. Efficacy of topical 0.05% retinaldehyde in skin aging by ultrasound and rheological techniques. Dermatology. 1999;199:37–41. doi: 10.1159/000051377. [DOI] [PubMed] [Google Scholar]
- Doubal S, Klemera P. Visco-elastic response of human skin and aging. J. Am Aging Assoc. 2002;25:115–117. doi: 10.1007/s11357-002-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing AK, Knott V, Werner JM, Cardy CM, Campbell ID, Handford PA. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996;85:597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- Escoffier C, Rigal J, Rochefort A, Vasselet R, Leveque JL, Agache PG. Age-related mechanical properties of human skin: an in vivo study. J Invest Dermatol. 1989;93:353–357. [PubMed] [Google Scholar]
- Escolar JD, Gallego B, Tejero C, Escolar MA. Changes occuring with increasing age in the rat lung: morphometrical study. Anat Rec. 1994;239:287–296. doi: 10.1002/ar.1092390307. [DOI] [PubMed] [Google Scholar]
- Escolar JD, Mateos J, Alfaro E, Escolar MA, Minana C, Roche P. Goodpasture’s syndrome in aging. An experimental study on the rat. 1. Histol Histopathol. 1993;8:599–608. [PubMed] [Google Scholar]
- Escolar JD, Tejero C, Escolar MA, Montalvo F, Garisa R. Architecture, elastic fiber, and collagen in the distal air portion of the lung of the 18-month-old rat. Anat Rec. 1997;248:63–69. doi: 10.1002/(sici)1097-0185(199705)248:1<63::aid-ar7>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol. 2001;49:310–325. doi: 10.1016/s0369-8114(01)00147-x. [DOI] [PubMed] [Google Scholar]
- Finlay B. Dynamic mechanical testing of human skin in-vivo. J Biomech. 1970;3:557–568. doi: 10.1016/0021-9290(70)90040-0. [DOI] [PubMed] [Google Scholar]
- Finnis ML, Gibson MA. Microfibril-associated glycoprotein-1 (MAGP-1) binds to the pepsin-resistant domain of the alpha 3(VI) chain of type VI collagen. J Biol Chem. 1997;272:22817–22823. doi: 10.1074/jbc.272.36.22817. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M, Hospelhorn VD. Studies on human pulmonary connective tissue. 1. Amino acid composition of leastins isolated by alkaline digestion. J Lab Clin Med. 1962;60:799–810. [PubMed] [Google Scholar]
- Fredberg JJ, Kamm RD. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol. 2006;68:507–541. doi: 10.1146/annurev.physiol.68.072304.114110. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Masuda Y, Ishizaki M, Masugi Y, Ferrans VJ. Morphogenesis of abnormal elastic fibers in lungs of patients with panacinar and centriacinar emphysema. Hum Pathol. 1989;20:652–659. doi: 10.1016/0046-8177(89)90152-4. [DOI] [PubMed] [Google Scholar]
- Gadek JE, Fells GA, Zimmerman RL, Crystal RG. Role of connective-tissue proteases in the pathogenesis of chronic inflammatory lung-disease. Environ Health Perspect. 1984;55:297–306. doi: 10.1289/ehp.8455297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: friend or foe? Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gardi C, Martorana PA, Desanti MM, Vaneven P, Lungarella G. A biochemcial and morphological investigation of the early development of genetic emphysema in tight-skin mice. Exp Mol Pathol. 1989;50:398–410. doi: 10.1016/0014-4800(89)90048-8. [DOI] [PubMed] [Google Scholar]
- Gibson MA, Sandberg LB, Grosso LE, Cleary EG. Complementary-DNA cloning establishes microfibril-associated glycoprotein (MAGP) to be a discrete component of the elastin-associated microfibrils. J Biol Chem. 1991;266:7596–7601. [PubMed] [Google Scholar]
- Glab J, Wess T. Changes in the molecular packing of fibrillin microfibrils during extension indicate intrafibrillar and interfibrillar reorganization in elastic response. J Mol Biol. 2008;383:1171–1180. doi: 10.1016/j.jmb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Goldstein RH. Response of the aging hamster lung to elastase injury. Am Rev Respir Dis. 1982;125:295–298. doi: 10.1164/arrd.1982.125.3.295. [DOI] [PubMed] [Google Scholar]
- Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic Proteins: Biological Roles and Mechanical Properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- Haenold R, Wassef DM, Heinemann SH, Hoshi T. Oxidative damage, aging and anti-aging strategies. Age. 2005;27:183–199. doi: 10.1007/s11357-005-2915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitoglou CS, Tsilibary EC, Brownlee M, Charonis AS. Altered cellular interactions between endotehlial-cells and nonenzymaticallu glucosylated laminin type-IV collagen. J. Biol Chem. 1992;267:12404–12407. [PubMed] [Google Scholar]
- Handford PA, Downing AK, Reinhardt DP, Sakai LY. Fibrillin: from domain structure to supramolecular assembly. Matrix Biol. 2000;19:457–470. doi: 10.1016/s0945-053x(00)00100-1. [DOI] [PubMed] [Google Scholar]
- Henderson M, Polewski R, Fanning JC, Gibson MA. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J Histochem Cytochem. 1996;44:1389–1397. doi: 10.1177/44.12.8985131. [DOI] [PubMed] [Google Scholar]
- Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary-artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- Huang KW, Mitzner W, Rabold R, Schofield B, Lee H, Biswal S, Tankersley CG. Variation in senescent-dependent lung changes in inbred mouse strains. J Appl Physiol. 2007;102:1632–1639. doi: 10.1152/japplphysiol.00833.2006. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Tiedemann K, Reinhardt DP. Fibrillins: from biogenesis of microfibrils to signaling functions. Curr Top Dev Biol. 2006;75:93–123. doi: 10.1016/S0070-2153(06)75004-9. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Robinson NE, Gillespie JR, Tyler WS. Morphometry of distal air spaces in lungs of aging dogs. J Appl Physiol. 1977;43:86–91. doi: 10.1152/jappl.1977.43.1.86. [DOI] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Ito S, Ishimaru S, Wilson SE. Effect of coacervated alpha-elastin on proliferation of vascular smooth muscle and endothelial cells. 44th Annual Meeting of the American College of Angiology. Las Vegas: Westminster; 1997. pp. 289–297. [DOI] [PubMed] [Google Scholar]
- Jacobs AM, Toudjarska I, Racine A, Tsipouras P, Kilpatrick MW, Shanske A. A recurring FBN1 gene mutation in neonatal Marfan syndrome. Arch Pediatr Adolesc Med. 2002;156:1081–1085. doi: 10.1001/archpedi.156.11.1081. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- Jeanmaire C, Danoux L, Pauly G. Glycation during human dermal intrinsic and actinic ageing: an in vivo and in vitro model study. Br J Dermatol. 2001;145:10–18. doi: 10.1046/j.1365-2133.2001.04275.x. [DOI] [PubMed] [Google Scholar]
- Jennissen HP. Ubiquitin and the enigma of intracellular protein-degradation. Eur J Biochem. 1995;231:1–30. [PubMed] [Google Scholar]
- Jensen SA, Reinhard DP, Gibson MA, Weiss AS. Protein Interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J Biol Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- John R, Thomas J. Chemical compositions of elastins isolated from aortas and pulmonary tissues of humans of different ages. Biochem J. 1972;127:261–269. doi: 10.1042/bj1270261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Sasaki T, Kostka G, Timpl R, Matsuzaki K, Kumagai N, Sakai LY, Nishiyama T, Amano S. Fibulin-5 deposition in human skin: decrease with ageing and ultraviolet B exposure and increase in solar elastosis. Br J Dermatol. 2005;153:607–612. doi: 10.1111/j.1365-2133.2005.06716.x. [DOI] [PubMed] [Google Scholar]
- Keeley FW, Bellingham CM, Woodhouse KA. Elastin as a self-organizing biomaterial: use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin. Philos Trans R Soc B Biol Sci. 2002;357:185–189. doi: 10.1098/rstb.2001.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Marson A, Baldock C. Fibrillin microfibrils. Adv Protein Chem. 2005;70:405–436. doi: 10.1016/S0065-3233(05)70012-7. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Stephan S, Sherratt MJ, Williamson M, Shuttleworth CA. Applying elastic fibre biology in vascular tissue engineering. Philos Trans R Soc B Biol Sci. 2007;362:1293–1312. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Woolley DE, Whittaker SP, Shuttleworth CA. Catabolism of intact fibrillin microfibrils by neutrophil elastase, chymotrypsin and trypsin. FEBS Lett. 1994;351:85–89. doi: 10.1016/0014-5793(94)00818-3. [DOI] [PubMed] [Google Scholar]
- Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun HC, Chung JH. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J Lipid Res. 2006;47:921–930. doi: 10.1194/jlr.M500420-JLR200. [DOI] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Kitaoka H, Nieman GF, Fujino Y, Carney D, DiRocco J, Kawase I. A 4-dimensional model of the alveolar structure. J Physiol Sci. 2007;57:175–185. doi: 10.2170/physiolsci.RP000807. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Clark DF, Kennedy TC, Knudson DE. Effect of aging alone on the mechanical-properties of normal adult human lung. J Appl Physiol. 1977;43:1054–1062. doi: 10.1152/jappl.1977.43.6.1054. [DOI] [PubMed] [Google Scholar]
- Konova E, Baydanoff S, Atanasova M, Velkova A. Age-related changes in the glycation of human aortic elastin. Exp Gerontol. 2004;39:249–254. doi: 10.1016/j.exger.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kostka G, Giltay R, Bloch W, Addicks K, Timpl R, Fassler R, Chu ML. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol Cell Biol. 2001;21:7025–7034. doi: 10.1128/MCB.21.20.7025-7034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Fook SJ, Hyatt RE. Effects of age on elastic moduli of human lungs. J Appl Physiol. 2000;89:163–168. doi: 10.1152/jappl.2000.89.1.163. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJS, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49:319–326. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol. 2003;81:607–621. doi: 10.1139/y03-012. [DOI] [PubMed] [Google Scholar]
- Learoyd BM, Taylor MG. Alterations with age in the viscoelastic properties of human arterial walls. Circ Res. 1966;18:278–292. doi: 10.1161/01.res.18.3.278. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Larson JC, Margolis KL, Allison MA, Freiberg MS, Cochrane BB, Graettinger WF, Curb JD. Abdominal aortic aneurysm events in the women’s health initiative: cohort study. BMJ. 2008;337:5. doi: 10.1136/bmj.a1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SSJ, Knott V, Jovanovic J, Harlos K, Grimes JM, Choulier L, Mardon HJ, Stuart DI, Handford PA. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure. 2004;12:717–729. doi: 10.1016/j.str.2004.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire R, Bayle J, Mecham RP, Lafyatis R. Microfibril-associated MAGP-2 stimulates elastic fiber assembly. J Biol Chem. 2007;282:800–808. doi: 10.1074/jbc.M609692200. [DOI] [PubMed] [Google Scholar]
- Leveque JL, Rigal JD, Agache PG, Monneur C. Influence of aging on the in vivo extensibility of human-skin at a low stress. Arch Dermatol Res. 1980;269:127–135. doi: 10.1007/BF00406532. [DOI] [PubMed] [Google Scholar]
- Lillie MA, Gosline JM. Limits to the durability of arterial elastic tissue. Biomaterials. 2007;28:2021–2031. doi: 10.1016/j.biomaterials.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Maurel E, Shuttleworth CA, Bouissou H. Interstitial collagens and aging in human aorta. Virchows Arch A Pathol Anat Histopathol. 1987;410:383–390. doi: 10.1007/BF00712757. [DOI] [PubMed] [Google Scholar]
- McConnell CJ, DeMont ME, Wright GM. Microfibrils provide non-linear elastic behaviour in the abdominal artery of the lobster Homarus americanus. J Physiol. 1997;499:513–526. doi: 10.1113/jphysiol.1997.sp021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34:665–671. doi: 10.1111/j.1440-1681.2007.04657.x. [DOI] [PubMed] [Google Scholar]
- McNulty M, Spiers P, McGovern E, Feely J. Aging is associated with increased matrix metalloproteinase-2 activity in the human aorta. Am J Hypertens. 2005;18:504–509. doi: 10.1016/j.amjhyper.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Davis EC. Elastic fiber structure and assembly. New York: Academic Press; 1994. [Google Scholar]
- Megill WM, Gosline JM, Blake RW. The modulus of elasticity of fibrillin-containing elastic fibres in the mesoglea of the hydromedusa Polyorchis penicillatus. J Exp Biol. 2005;208:3819–3834. doi: 10.1242/jeb.01765. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Ching PST, Beaumont B, Hinek A, Wight TN, Black PN. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir Res. 2008;9:9. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican V3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Mikulikova K, Eckhardt A, Kunes J, Zicha J, Miksik I. Advanced glycation end-product pentosidine accumulates in various tissues of rats with high fructose intake. Physiol Res. 2008;57:89–94. doi: 10.33549/physiolres.931093. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Duvic M. Severe neonatal marfan-syndrome resulting from a de-novo 3-bp insertion into the fibrillin gene on chromosome-15. Am J Hum Genet. 1994;54:447–453. [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Urbán Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role for the latent TGF-beta-1 binding prtoein in the assembly and secretion of TGF-beta-1. EMBO J . 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Ono T, Ikeda K, Kayashima K, Horiuchi S. Photo-enhanced modification of human skin elastin in actinic elastosis by N-epsilon-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J Invest Dermatol. 1997;108:797–802. doi: 10.1111/1523-1747.ep12292244. [DOI] [PubMed] [Google Scholar]
- Nagase T, Fukuchi Y, Teramoto S, Matsuse T, Orimo H. Mechanical interdependence in relation to age: effects of lung volume on airway-resitance in rats. J Appl Physiol. 1994;77:1172–1177. doi: 10.1152/jappl.1994.77.3.1172. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Nilsson PM. Reducing the risk of stroke in elderly patients with hypertension - A critical review of the efficacy of antihypertensive drugs. Drugs Aging. 2005;22:517–524. doi: 10.2165/00002512-200522060-00005. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Blazek JV, Morreels CL, Krovetz LJ. Pressure wave transmission along human aorta. Changes with age and in arterial degenerative disease. Circ Res. 1968;23:567–579. doi: 10.1161/01.res.23.4.567. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–1310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Pearson AC, Guo RQ, Orsinelli DA, Binkley PF, Pasierski TJ. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic-wall size, thickness, and stiffness. Am Heart J. 1994;128:344–351. doi: 10.1016/0002-8703(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Penner AS, Rock MJ, Kielty CM, Shipley JM. Microfibril-associated glycoprotein-2 interacts with fibrillin-1 and fibrillin-2 suggesting a role for MAGP-2 in elastic fiber assembly. J Biol Chem. 2002;277:35044–35049. doi: 10.1074/jbc.M206363200. [DOI] [PubMed] [Google Scholar]
- Pierard GE. EEMCO guidance to the in vivo assessment of tensile functional properties of the skin. Part 1: Relevance to the structures and ageing of the skin and subcutaneous tissues. Skin Pharmacol Appl Skin Physiol. 1999;12:352–362. doi: 10.1159/000029897. [DOI] [PubMed] [Google Scholar]
- Pierce JA, Hocott JB. Studies on the collagen and elastin content of the human lung. J Clin Invest. 1960;39:8–14. doi: 10.1172/JCI104030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, Barry BE, Oneil JJ, Raub JA, Pratt PC, Crapo JD. Morphologic changes in the lung during the lifespan of Fischer 344 rats. Am J Anat. 1982;164:155–174. doi: 10.1002/aja.1001640206. [DOI] [PubMed] [Google Scholar]
- Powell JT, Vine N, Crossman M. On the accumulation of D-aspartate in elastin and other proteins of the aging aorta. Atherosclerosis. 1992;97:201–208. doi: 10.1016/0021-9150(92)90132-z. [DOI] [PubMed] [Google Scholar]
- Qian RQ, Glanville RW. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry. 1997;36:15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Pasquali-Ronchetti I. Morphology and chemical composition of connective tissue: the cardiovascular system. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. New York: Wiley; 2002. pp. 121–144. [Google Scholar]
- Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschodrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112:1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Unsold C, Kubitscheck U, Bruckner-Tuderman L, Peters R, Meuli M. The cutaneous microfibrillar apparatus contains latent transforming growth factor-beta binding protein-1 (LTBP-1) and is a repository for latent TGF-beta-1. J Invest Dermatol. 1998;111:559–564. doi: 10.1046/j.1523-1747.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Pereira L. The fibrillins. Int J Biochem Cell Biol. 1999;31:255–259. doi: 10.1016/s1357-2725(98)00109-5. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Sasaki T, Dzamba BJ, Keene DR, Chu ML, Gohring W, Timpl R, Sakai LY. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J Biol Chem. 1996;271:19489–19496. doi: 10.1074/jbc.271.32.19489. [DOI] [PubMed] [Google Scholar]
- Repine JE, Bast A, Lankhorst I, deBacker W, Dekhuijzen R, Demedts M, vanHerwaarden C, vanKlaveren R, Lammers JW, Larsson S, Lundback B, Petruzelli S, Postma D, Riise G, Vermeire P, Wouters E, Yernault JC, vanZandwijk N. Oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- Rijken F, Kiekens RCM, Bruijnzeel PLB. Skin-infiltrating neutrophils following exposure to solar-simulated radiation could play an important role in photoageing of human skin. Br J Dermatol. 2005;152:321–328. doi: 10.1111/j.1365-2133.2004.06335.x. [DOI] [PubMed] [Google Scholar]
- Ritz-Timme S, Laumeier I, Collins MJ. Aspartic acid racemization: evidence for marked longevity of elastin in human skin. Br J Dermatol. 2003;149:951–959. doi: 10.1111/j.1365-2133.2003.05618.x. [DOI] [PubMed] [Google Scholar]
- Robbesom AA, Koenders M, Smits NC, Hafmans T, Versteeg EMM, Bulten J, Veerkamp JH, Dekhuijzen PNR, Kuppevelt TH. Aberrant fibrillin-1 expression in early emphysematous human lung: a proposed predisposition for emphysema. Mod Pathol. 2008;21:297–307. doi: 10.1038/modpathol.3801004. [DOI] [PubMed] [Google Scholar]
- Robert L, Robert AM, Fulop T. Rapid increase in human life expectancy: will it soon be limited by the aging of elastin? Biogerontology. 2008;9:119–133. doi: 10.1007/s10522-007-9122-6. [DOI] [PubMed] [Google Scholar]
- Robinson PN, Booms P. The molecular pathogenesis of the Marfan syndrome. Cell Mol Life Sci. 2001;58:1698–1707. doi: 10.1007/PL00000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Marson A, Shuttleworth CA, Weiss AS, Kielty CM. Molecular basis of elastic fiber formation: critical interactions and a tropoelastin-fibrillin-1 cross-link. J Biol Chem. 2004;279:23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- Rodrigues L. EEMCO guidance to the in vivo assessment of tensile functional properties of the skin. Part 2: Instrumentation and test modes. Skin Pharmacol Appl Skin Physiol. 2001;14:52–67. doi: 10.1159/000056334. [DOI] [PubMed] [Google Scholar]
- Ronchetti IP, Alessandrini A, Contri MB, Fornieri C, Mori G, Quaglino D, Valdre U. Study of elastic fiber organization by scanning force microscopy. Matrix Biol. 1998;17:75–83. doi: 10.1016/s0945-053x(98)90126-3. [DOI] [PubMed] [Google Scholar]
- Rucker RB, Tinker D. Structure and metabolism of arterial elastin. Int Rev Exp Pathol. 1977;17:1–47. [PubMed] [Google Scholar]
- Ruitenbeek AG, Cammen TJM, Meiracker AH, Mattace-Raso FUS. Age and blood pressure levels modify the functional properties of central but not peripheral arteries. Angiology. 2008;59:290–295. doi: 10.1177/0003319707305692. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U, Kerkela E, Jeskanen L, Hasan T, Pierce R, Starcher B, Raudasoja R, Ranki A, Oikarinen A, Vaalamo M. Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage. J Invest Dermatol. 1999;113:664–672. doi: 10.1046/j.1523-1747.1999.00731.x. [DOI] [PubMed] [Google Scholar]
- Sander CS, Chang H, Salzmann S, Muller CSL, Ekanayake-Mudiyanselage S, Elsner P, Thiele JJ. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- Sanders R. Torsional elasticity of human skin in vivo. Pflugers Arch. 1973;342:225–260. doi: 10.1007/BF00591373. [DOI] [PubMed] [Google Scholar]
- Sayers CP, Goltz RW, Mottaz J. Pulmonary elastic tissue in generalized elastolysis (Cutis-Laxa) and Marfans-Syndrome_ light and electron microscopic study. J Invest Dermatol. 1975;65:451–457. doi: 10.1111/1523-1747.ep12608193. [DOI] [PubMed] [Google Scholar]
- Shapiro BP, Owan TE, Mohammed SF, Meyer DM, Mills LD, Schalkwijk CG, Redfield MM. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation. 2008;118:1002–1010. doi: 10.1161/CIRCULATIONAHA.108.777326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt MJ, Baldock C, Haston JL, Holmes DF, Jones CJP, Shuttleworth CA, Wess TJ, Kielty CM. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol. 2003;332:183–193. doi: 10.1016/s0022-2836(03)00829-5. [DOI] [PubMed] [Google Scholar]
- Sherratt MJ, Bastrilles JY, Bowden JJ, Watson REB, Griffiths CEM. Age-related deterioration in the mechanical function of human dermal fibrillin microfibrils. Br J Dermatol. 2006;155:240–241. [Google Scholar]
- Sherratt MJ, Holmes DF, Shuttleworth CA, Kielty CM. Scanning transmission electron microscopy mass analysis of fibrillin-containing microfibrils from foetal elastic tissues. Int J Biochem Cell Biol. 1997;29:1063–1070. doi: 10.1016/s1357-2725(97)00028-9. [DOI] [PubMed] [Google Scholar]
- Sherratt MJ, Watson REB, Griffiths CEM. Molecular structure and function in ageing elastic fibres. Age. 2007;29:112–112. [Google Scholar]
- Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–160. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946–951. doi: 10.1007/BF00403914. [DOI] [PubMed] [Google Scholar]
- Sivan SS, Wachtel E, Tsitron E, Sakkee N, Ham F, DeGroot J, Roberts S, Maroudas A. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J Biol Chem. 2008;283:8796–8801. doi: 10.1074/jbc.M709885200. [DOI] [PubMed] [Google Scholar]
- Smalls LK, Wickett RR, Visscher MO. Effect of dermal thickness, tissue composition, and body site on skin biomechanical properties. Skin Res Technol. 2006;12:43–49. doi: 10.1111/j.0909-725X.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Snider GL, Kleinerman JL, Thurlbeck WM, Bengali ZH. The definition of emphysema: report of a National Heart, Lung-and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- Staloff IA, Guan E, Katz S, Rafailovitch M, Sokolov A, Sokolov S. An in vivo study of the mechanical properties of facial skin and influence of aging using digital image speckle correlation. Skin Res Technol. 2008;14:127–134. doi: 10.1111/j.1600-0846.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Suwabe H, Serizawa A, Kajiwara H, Ohkido M, Tsutsumi Y. Degenerative processes of elastic fibers in sun-protected and sun-exposed skin: immunoelectron microscopic observation of elastin, fibrillin-1, amyloid P component, lysozyme and alpha(1)-antitrypsin. Pathol Int. 1999;49:391–402. doi: 10.1046/j.1440-1827.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- Taddese S, Weiss AS, Neubert RHH, Schmelzer CEH. Mapping of macrophage elastase cleavage sites in insoluble human skin elastin. Matrix Biol. 2008;27:420–428. doi: 10.1016/j.matbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Takema Y, Imokawa G. The effects of UVA and UVB irradiation on the viscoelastic properties of hairless mouse skin in vivo. Dermatology. 1998;196:397–400. doi: 10.1159/000017931. [DOI] [PubMed] [Google Scholar]
- Takema Y, Yorimoto Y, Kawai M, Imokawa G. Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol. 1994;131:641–648. doi: 10.1111/j.1365-2133.1994.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Takubo Y, Hirai T, Muro S, Kogishi K, Hosokawa M, Mishima M. Age-associated changes in elastin and collagen content and the proportion of types I and III collagen in the lungs of mice. Exp Gerontol. 1999;34:353–364. doi: 10.1016/s0531-5565(99)00017-0. [DOI] [PubMed] [Google Scholar]
- Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta 1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Matsuse T, Ouchi Y. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;14:1454–1455. [PubMed] [Google Scholar]
- Thurmond FA, Koob TJ, Bowness JM, Trotter JA. Partial biochemical and immunologic characterization of fibrillin microfibrils from sea cucumber dermis. Connect Tissue Res. 1997;36:211–222. doi: 10.3109/03008209709160221. [DOI] [PubMed] [Google Scholar]
- Thurmond FA, Trotter JA. Morphology and biomechanics of the microfibrillar network of sea cucumber dermis. J Exp Biol. 1996;199:1817–1828. doi: 10.1242/jeb.199.8.1817. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Batge B, Muller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly. J Biol Chem. 2001;276:36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- Toonkool P, Regan DG, Kuchel PW, Morris MB, Weiss AS. Thermodynamic and hydrodynamic properties of human tropoelastin. Analytical ultracentrifuge and pulsed field-gradient spin-echo NMR studies. J Biol Chem. 2001;276:28042–28050. doi: 10.1074/jbc.M103391200. [DOI] [PubMed] [Google Scholar]
- Trask BC, Trask TM, Broekelmann T, Mechame RP. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol Biol Cell. 2000;11:1499–1507. doi: 10.1091/mbc.11.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilibary EC, Charonis AS, Reger LA, Wohlhueter RM, Furcht LT. The effect of nonenzymatic glucosylation on the binding of the main noncollagneous NC1 domain to type-IV collagen. J Biol Chem. 1988;263:4302–4308. [PubMed] [Google Scholar]
- Tsuji T, Hamada T. Age-related changes in human dermal elastic fibers. Br J Dermatol. 1981;105:57–63. doi: 10.1111/j.1365-2133.1981.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Tsuruga E, Irie K, Yajima T. Fibrillin-2 degradation by matrix metalloproteinase-2 in periodontium. J Dent Res. 2007;86:352–356. doi: 10.1177/154405910708600410. [DOI] [PubMed] [Google Scholar]
- Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968;25:664–671. doi: 10.1152/jappl.1968.25.6.664. [DOI] [PubMed] [Google Scholar]
- Umeda H, Nakamura F, Suyama K. Oxodesmosine and isooxodesmosine, candidates of oxidative metabolic intermediates of pyridinium cross-links in elastin. Arch Biochem Biophys. 2001;385:209–219. doi: 10.1006/abbi.2000.2145. [DOI] [PubMed] [Google Scholar]
- Unsold C, Hyytiainen M, Bruckner-Tuderman L, Keski-Oja J. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J Cell Sci. 2001;114:187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, Xu J, Parker T. Elastin: a representative ideal protein elastomer. Philos Trans R Soc B Biol Sci. 2002;357:169–184. doi: 10.1098/rstb.2001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Vandewoestijne KP. The senile lung—comparison with nomral and emphysematous lungs. 1. Structural aspects. Chest. 1992;101:793–799. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JWJ, Lafeber F, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Viegi G, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease (COPD) Respiration. 2001;68:4–19. doi: 10.1159/000050456. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis DD, Putnam EA, Cretoiu JS, Carmical SG, Cao SN, Thomas G, Milewicz DM. Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones. J Cell Biochem. 2003;90:641–652. doi: 10.1002/jcb.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R, Gartstein V, Kligman AM, Montagna W, Allendorf RA, Ridder GM. Age, sunlight, and facial skin: a histologic and quantitative study. J Am Acad Dermatol. 1991;25:751–760. doi: 10.1016/s0190-9622(08)80964-4. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sawai T, Nagura H, Suyama K. Age-related alteration of cross-linking amino acids of elastin in human aorta. Tohoku J Exp Med. 1996;180:115–130. doi: 10.1620/tjem.180.115. [DOI] [PubMed] [Google Scholar]
- Watson REB, Craven NM, Kang SW, Jones CJP, Kielty CM, Griffiths CEM. A short-term screening protocol, using fibrillin-1 as a reporter molecule, for photoaging repair agents. J Invest Dermatol. 2001;116:672–678. doi: 10.1046/j.1523-1747.2001.01322.x. [DOI] [PubMed] [Google Scholar]
- Watson REB, Griffiths CEM, Craven NM, Shuttleworth CA, Kielty CM. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999;112:782–787. doi: 10.1046/j.1523-1747.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- Watson REB, Long SP, Bowden JJ, Bastrilles JY, Barton SP, Griffiths CEM. Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. Br J Dermatol. 2008;158:472–477. doi: 10.1111/j.1365-2133.2007.08364.x. [DOI] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Werth VP, Shi X, Kalathil E, Jaworsky C. Elastic fiber-associated proteins of skin in development and photoaging. Photochem Photobiol. 1996;63:308–313. doi: 10.1111/j.1751-1097.1996.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Wess J, Purslow PP, Sherratt MJ, Ashworth J, Shuttleworth CA, Kielty CM. Calcium determines the supramolecular organization of fibrillin-rich microfibrils. J Cell Biol. 1998;141:829–837. doi: 10.1083/jcb.141.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TA, Bachofen H. A model for mechanical strcutre of the alveolar duct. J Appl Physiol. 1982;52:1064–1070. doi: 10.1152/jappl.1982.52.4.1064. [DOI] [PubMed] [Google Scholar]
- Wlaschek M, Tantcheva-Poor I, Naderi L, Ma WJ, Schneider A, Razi-Wolf Z, Schuller J, Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63:41–51. doi: 10.1016/s1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Tanaka A, Kanamaru A, Tanaka S, Tsubone H, Atoji Y, Suzuki Y. Morphology of aging lung in F344/N rat: alveolar size, connective tissue, and smooth muscle cell markers. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:538–547. doi: 10.1002/ar.a.10172. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Woodley DT, Mechanic GL. Aging and cross-linking of skin collagen. Biochem Biophys Res Commun. 1988;152:898–903. doi: 10.1016/s0006-291x(88)80124-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MC, Pierce RA, Wachi H, Mecham RP, Parks WC. An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor beta 1. Mol Cell Biol. 1999;19:7314–7326. doi: 10.1128/mcb.19.11.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- Zulliger MA, Stergiopulos N. Structural strain energy function applied to the ageing of the human aorta. J Biomech. 2007;40:3061–3069. doi: 10.1016/j.jbiomech.2007.03.011. [DOI] [PubMed] [Google Scholar]