Abstract
The most widely recognised consequence of normal age-related changes in biological timing is the sleep disruption that appears in old age and diminishes the quality of life. These sleep disorders are part of the normal ageing process and consist primarily of increased amounts of wakefulness and reduced amounts of deep sleep. Changes in the amplitude and timing of the sleep-wake cycle appear to represent, at least in part, a loss of effective circadian regulation of sleep. Understanding alterations in the characteristics of stimuli that help to consolidate internal rhythms will lead to recommendations to improve synchronisation in old age. Converging evidence from both human and animal studies indicate that senescence is associated with alterations in the neural structure thought to be primarily responsible for the generation of the circadian oscillation, the suprachiasmatic nuclei (SCN). Work has shown that there are changes in the anatomy, physiology and ability of the clock to reset in response to stimuli with age. Therefore it is possible that at least some of the observed age-related changes in sleep and circadian timing could be mediated at the level of the SCN. The SCN contain a circadian clock whose activity can be recorded in vitro for several days. We have tested the response of the circadian clock to a number of neurochemicals that reset the clock in a manner similar to light, including glutamate, N-methyl-D-aspartate (NMDA), gastrin-releasing peptide (GRP) and histamine (HA). In addition, we have also tested agents which phase shift in a pattern similar to behavioural ‘non-photic’ signals, including neuropeptide Y (NPY), serotonin (5HT) and gamma-aminobutyric acid (GABA). These were tested on the circadian clock in young and older mice (approximately 4 and 15 months old). We found deficits in the response to specific neurochemicals but not to others in our older mice. These results indicate that some changes seen in the responsiveness of the circadian clock to light with age may be mediated at the level of the SCN. Further, the responsiveness of the circadian clock with age is attenuated to some, but not all stimuli. This suggests that not all clock stimuli loose their effectiveness with age, and that it may be possible to compensate for deficits in clock performance by enhancing the strength of those stimulus pathways which are intact.
Keywords: Circadian, Brain slice, Suprachiasmatic
Introduction
The disruption of sleep evident in older individuals may be a symptom of loss of effective synchronisation. The impact of this deficit has measurable consequences. There are medical implications from the disruption in chemical, metabolic and hormonal cycles, as well as consequences for mood and performance. By understanding the mechanisms of normal age-related alterations in clock function we may be able to make recommendations of interventions that will improve the quality of life for individuals of advanced age
Age-related phase advances of circadian rhythms are consistent with the hypothesis that the pacemaker is altered in animals of advanced age. Studies indicate that ageing is associated with a decreased responsiveness to the phase shifting effects of both light (photic) and behavioural (non-photic) stimuli. Although age-related changes in phase shifts of circadian rhythms have been documented, little is known about the physiological basis underlying these alterations (Hofman and Swaab 2006). Such phase shifting responses are likely to contribute to the synchronisation of the circadian pacemaker to the environment.
Age-related changes in daily rhythms may involve alterations along the input pathway for synchronising signals, in the central pacemaker itself, or in the output pathways which communicate timing information to areas governing overt rhythms. A growing body of evidence obtained both in humans and animals shows that ageing is associated with alterations in the neural structure thought to be primarily responsible for the generation of the circadian oscillation, the suprachiasmatic nuclei (SCN). Reports indicate that both the anatomy and physiology of the SCN are altered in advanced age, with the most dramatic changes being found in the brains of demented patients (Harper et al. 2008). Thus, the observation that clock-controlled properties of overt rhythms change during senescence, and the fact that the SCN itself shows pronounced changes in advanced age, make it likely that at least some of the age-related changes are due to alterations in the pacemaker mechanism itself (Aujard et al. 2001).
The hypothalamic SCN are the site of an endogenous circadian pacemaker in mammals (Rusak and Zucker 1979), and this maintains clock-function in vitro. The SCN generate a self-sustained oscillation which may be measured in the hypothalamic brain slice preparation as a rhythm in firing rate. This time of the peak in firing is consistent with multi-unit recordings from rats conducted in vivo (Inouye and Kawamura 1982). The peak recorded from rodents held under the same light/dark cycle is highly congruent (Biello et al. 1997a), and can be measured for three to four circa 24-h cycles (Prosser and Gillette 1989). Measures of neurochemical-induced changes in peak firing times using this model have been integral to our understanding of input pathways to the SCN (Brown and Piggins 2007).
Light is the primary synchronising agent in the natural environment. It resets the circadian clock with a phase response curve characterised by phase delays in the early night and phase advances in the late night. Photosensitive ganglion cells convey signals directly to the SCN via the retinohypothalamic tract (RHT) (Johnson et al. 1988). Converging evidence suggests that glutamate is the main transmitter of the RHT (Ebling 1996), used to transmit photic information to the circadian clock. The phase of the peak in SCN cell firing rate in vitro is reset by application of glutamate in a manner that resembles the light phase response curve in vivo, with phase-delay shifts in the early evening, followed by phase advances in the late evening. Some actions of glutamate are mediated via the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors, and resetting in response to light is blocked by NMDA receptor antagonists (Colwell et al. 1990). Resetting to stimulation of the SCN by glutamate can be mimicked in the mouse in vitro slice preparation by application of NMDA (Soscia and Harrington 2004).
In addition, other transmitters such as histamine (HA) and gastrin-releasing peptide (GRP), which can reset the circadian clock in a manner similar to light may also participate in synchronisation.
HA is involved in the control of arousal and the sleep wake system. Further, it may be involved in photic synchronisation of the circadian clock (Haas et al. 2008). In the brain of the mammal, all HA is synthesised in the tuberomammillary of the hypothalamus, and fibres innervate most areas of the central nervous system, including the SCN (Panula et al. 1989). HA can reset the circadian clock in both rats and hamsters (Harrington et al. 2000). In hamsters, these shifts are dependent on NMDA receptor activation, and HA can potentiate NMDA currents within the hamster SCN (Meyer et al. 1998).
GRP is synthesised by neurons in the ventrolateral SCN. The peptide and activation of the associated receptor also resets the circadian clock in vitro and in vivo in a pattern resembling that of photic stimulation (McArthur et al. 2000; Albers et al. 1995; Piggins et al. 1995). In hamsters this mechanism involves the NMDA receptor (Kallingal and Mintz 2006). It is, therefore, possible that GRP or receptors for GRP are involved in photic entrainment of circadian rhythms.
While light is the most potent synchroniser, non-photic cues (both arousal and pharmacological treatments) can also entrain circadian rhythms. These stimuli reset in a pattern very different from that seen in response to photic signals, and resetting is characterised by significant phase advances during the day, and smaller, more variable phase delays during the night (Smith et al. 1992). This non-photic shifting is, in part, modulated by several neurochemicals found within the rodent circadian clock. These include neuropeptide Y (NPY), serotonin (5HT) and gamma-aminobutyric acid (GABA).
The SCN are innervated by NPY-containing cells from the intergeniculate leaflet of the lateral geniculate nucleus, some of which are retinorecipient (Pickard et al. 1987).
This transmitter produces a characteristic non-photic phase response curve when administered both in vivo and in vitro (Biello et al. 1994, 1997b). These shifts are blocked by depolarization and involve protein kinase C (Biello et al. 1997b).
The raphe hypothalamic tract arises from serotonergic neurons in the median raphe. Electrical stimulation of the median raphe phase shifts circadian activity rhythms in a non-photic pattern, producing the most significant phase changes during the day, and smaller phase delays during the night (Meyer-Bernstein and Morin 1999). This is similar to the effect of systemic injections of serotonin agonists in hamsters (Bobrzynska et al. 1996) or mice (Horikawa and Shibata 2004; but see Antle et al. 2003). The rhythm in firing rate in vitro can also be reset by serotonin agonists in a dose-dependent manner in hamster, rat and mouse (Biello and Dafters 2001; Prosser 2001, 2003).
Finally, GABA is also an important neurotransmitter in the circadian system (Moore and Speh 1993). The SCN receive extensive GABAergic innervation (Buijs et al. 1994) and nearly all of the neurones in the SCN are GABA-producing (Moore and Speh 1993). Many phase shifting stimuli are thought to involve GABA in their actions on the circadian clock, and it is likely that it plays a significant role in regulating entrainment. GABA agonists have been shown to phase shift the circadian clock when administered systemically (Ralph and Menaker 1989), centrally (Huhman et al. 1995), and in vitro (Tominaga et al. 1994) in a phase-dependent manner.
There was early evidence that changes in the electrophysiological properties of the pacemaker emerged with age (Satinoff et al. 1993) and that this reflected changes in behaviour. Presumably this alteration in the SCN itself has consequences for the ability of phase resetting and synchronising stimuli to act on the circadian clock. However, little published evidence evaluates the relative impact of ageing on different transmitter systems. Here we compare the phase shifting effects of a number of phase shifting agents thought to be involved in entrainment. These include transmitters which are thought to act both within the photic and non-photic pathways. If there are alterations in the function of the circadian clock at the level of the SCN, we would expect to see changes in reponse to these phase shifting stimuli. Further, if resetting occurs in response to some stimuli but not others, this would suggest there are disruptions to particular phase shifting pathways, and not the ability of the circadian clock itself to reset.
Experimental procedures
Animals and tissue preparation Younger (4–5 months) or older (15–17 months), adult, male C57BL/6 mice (University of Glasgow) were housed in one of two opposite photoperiods, with a light:dark schedule of 12:12 h. Zeitgeber time (ZT) was defined as ZT 12 being the projected time of lights off in the animal room. Mice were administered an overdose of halothane anaesthesia and decapitated during the phases when this manipulation does not induce phase shifts (Gillette 1986); in most cases between ZT 2 and 5. Hypothalamic slices (500 μm) containing the SCN were placed in a gas-fluid interface slice chamber (Medical Systems BSC with Haas top), continuously bathed (1 ml/min) in artificial cerebrospinal fluid (ACSF) containing 125.2 mM NaCl, 3.8 mM KCl, 1.2 mM KH2PO4, 1.8 mM CaCl2, 1 mM MgSO4, 24.8 mM NaHCO3, 10 mM glucose. ACSF (pH 7.4) was supplemented with an antibiotic (gentamicin, 0.05 gm/l) and a fungicide (amphotericin, 2 mg/l) and maintained at 34.5°C. Warm, humidified 95% oxygen:5% carbon dioxide was continuously provided.
Electrophysiological recordings Extracellular single-unit activity of SCN cells was detected with glass micropipette electrodes filled with ACSF, advanced through the slice using a hydraulic microdrive. The electrode was placed into regions of the SCN at random, alternating between the left and right SCN. The signal was fed into an amplifier for further amplification and filtering, and was continuously monitored by an oscilloscope and audio monitor. Firing rate and interspike interval data were analysed using Spike (Cambridge Electronic Design, Cambridge, UK) data acquisition software and a customised program for calculation of descriptive statistics. The average spontaneous firing rate and the ZT for each single unit encountered was recorded for 4–5 min by an experimenter blind to all treatments.
Drugs and treatments Since this was the first time a number of these neurochemicals had been tested in the mouse, they were administered as either a microdrop, or within a bath, in the same way previously published work had shown were able to phase shift the rodent SCN (see references for drug applications below). Drugs were dissolved to the desired concentrations in ACSF (Biello et al. 1997b) and applied as a 200-nl microdrop to the SCN area of the slice or as a 1-h bath application centered around the ZT time of administration (relative to the animals’ previous light:dark cycle). Microdrops were administered using a Hamilton 1-μl syringe, at least 1 h after dissection, on the same day as slice preparation. Drugs were warmed to 34.5°C and applied at approximately ZT 6 or ZT 15. Recordings were typically performed for 8–12 h during ZT 0–12 or ZT 12–24 of the second or third 24 h in vitro. Several slices from both young and old mice were monitored continuously for 48 h from ZT 0 of the first day in vitro. Slices with less than four cells recorded during any 1-h period were not included in analysis.Drugs were all obtained within the UK. Glutamate, NMDA, HA, 8-OH-DPAT, and muscimol, were all obtained from Sigma Aldrich (Dorset), while GRP was obtained from Bachem (Saffron Waldon), and NPY from Calbiochem (Nottingham).Concentrations of drug applications were as follows:Neurochemicals that phase shift in a pattern similar to light:
- Glutamate
10−3 M microdrop (Biello et al. 1997a)
- NMDA
10 μM microdrop (Soscia and Harrington 2004)
- GRP
10−7 μM microdrop (McArthur et al. 2000)
- HA
1 mM bath (Harrington et al. 2000)
Neurochemicals that phase shift in a pattern similar to behavioural stimuli:
Data analysis Initially the average firing rate of each cell recorded from one slice was plotted against the ZT of the recording. Slices without significant differences across firing rate data grouped into 1-h (ZT) bins (p < 0.05; ANOVA) were not used for further analysis. If there were significant differences, data were smoothed by 1-h running means with a 15-min lag. The time corresponding to the maximum of the smoothed data was used as the time of the peak firing. Phase shifts were measured relative to the average time of peak firing of age appropriate control slices treated with ACSF at either ZT 6 or ZT 15. Significant differences between groups (p < 0.05) were determined by a one-way ANOVA followed by a Tukey-Kramer post hoc test correcting for multiple comparisons, or a t-test. Differences between treatment conditions were determined within each age group (comparing peak time in age appropriate untreated slices with drug treatments), and then the two age groups were analysed comparatively along with data from microdrop treated slices [for example, comparing four groups treated at ZT 6: (1) ACSF in young mice at ZT 6, (2) young NPY at ZT 6, (3) ACSF in older mice at ZT 6, (4) older NPY at ZT 6]. All results are reported as mean ± standard error of the mean (SEM).
Results
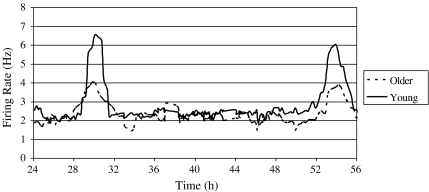
Control slices Electrical activity in untreated, control slices from younger mice and older mice both peaked near the middle of the projected light phase on days two and three following slice preparation (young: n = 10, mean peak (mp) = 6.1 ± 0.1; old: n = 7, mp = 5.9 ± 0.2). Peaks in slices that received applications of ACSF at ZT 6 or 15 (ZT 6: young, n = 5, mp = 5.9 ± 0.1; older, n = 3, mp = 6.0 ± 0.1; ZT 15: young, n = 6, mp = 5.9 ± 0.1; older, n = 4, mp = 6.1 ± 0.1) did not significantly differ from those in untreated slices. While there was no difference in peak times between untreated or ACSF-treated slices taken from younger and older mice, there was a significant difference in amplitude [t(33) = 12.32, p < 0.001; see Fig. 1 and Table 1].
Fig. 1.
Frequency of SCN cells firing rates represented by a 1-h running mean with a 15-min lag over time for two individual slices recorded from a young (5 months) and older (15 months) mouse. ZT 12 is defined as the time of lights off in the animal’s previous light:dark cycle, so the above trace shows data obtained from recordings over 32 h in vitro, and allows visualisation of two peak times in both slices. As evident from the representative traces shown here, there was no difference between the mean peak times for untreated slices from young and older mice, although there was a significant difference in amplitude such that slices recorded from older mice showed attenuated amplitude
Table 1.
Number and amplitude of all slices recorded within each condition. Phase shifts were determined relative to peak times of control slices receiving applications of ACSF at either ZT 6 or 15
| Treatment | Young mice | Older mice | ||||
|---|---|---|---|---|---|---|
| n | Mean amplitude (Hz) | Mean phase shift (h) | n | Mean amplitude (Hz) | Mean phase shift (h) | |
| Photic stimuli | ||||||
| Glutamate | 8 | 5.9 ± 0.2 | −3.4 ± 0.3 | 6 | 4.2 ± 0.2 | −2.0 ± 0.2 |
| NMDA | 5 | 6.2 ± 0.2 | −4.2 ± 0.2 | 4 | 4.5 ± 0.2 | −3.0 ± 0.1 |
| GRP | 6 | 6.1 ± 0.2 | −2.1 ± 0.2 | 7 | 4.0 ± 0.1 | −1.8 ± 0.2 |
| HA | 7 | 6.3 ± 0.2 | −2.9 ± 0.2 | 6 | 4.2 ± 0.2 | −1.4 ± 0.2 |
| Non-photic simtuli | ||||||
| NPY | 8 | 6.3 ± 0.2 | 2.3 ± 0.2 | 8 | 4.5 ± 0.2 | 2.6 ± 0.2 |
| 5HT agonist | 9 | 6.1 ± 0.1 | 3.4 ± 0.2 | 8 | 4.7 ± 0.3 | 1.4 ± 0.3 |
| GABA agonist | 7 | 5.9 ± 0.2 | 3.1 ± 0.3 | 8 | 4.5 ± 0.1 | 1.2 ± 0.2 |
| Controls | ||||||
| Untreated | 10 | 6.2 ± 0.2 | NA | 7 | 4.6 ± 0.3 | NA |
| ACSF ZT 6 | 5 | 6.1 ± 0.2 | NA | 3 | 4.5 ± 0.3 | NA |
| ACSF ZT 15 | 6 | 6.1 ± 0.1 | NA | 4 | 4.2 ± 0.2 | NA |
Neurochemicals that phase shift in a pattern similar to light (see Fig. 2)
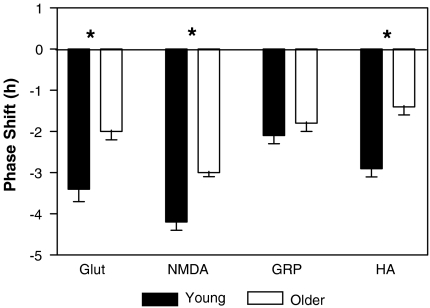
Fig. 2.
Phase shifts of the pattern of SCN firing rates in young (5 months) and older (15 months) mice to at ZT 14 to glutamate (glut), NMDA, GRP and HA. These neurochemicals all phase shift the firing rate of the suprachiasmatic nucleus in a photic pattern, and show phase delays when applied early in the night at ZT 15. Mean phase delays ± the standard error of the mean. Asterisks indicate significant differences between phase shifts seen in young and older mice
Glutamate A one-way ANOVA showed that applications of glutamate (10−3 M) reset the peak rhythm in firing rate with respect to ACSF-treated control slices [F(3,20) = 160.5, p < 0.001]. Younger mice treated early in the projected night (ZT 15), showed a delay in the peak of the rhythm (n = 8, mp = 9.3 ± 0.1). Post-hoc Tukey’s tests showed that this same application of glutamate in older mice did not produce as large phase delays (n = 6, mp = 8.1 ± 0.2; p < 0.001).
NMDA Microdrop applications of NMDA (10 μM) to the SCN delayed the peak in firing rate [F(3,15) = 304.0, p < 0.001]. Younger mice early in the projected night (ZT 15), showed a delayed the peak in the rhythm of the SCN (n = 5, mp = 10.1 ± 0.1). Phase delays to the same dose of NMDA in older mice were less (n = 4, mp = 9.1 ± 0.1; p < 0.001).
GRP When GRP was administered as a microdrop, it produced phase delays in the peak firing rate which were different from control slices [F(3,19) = 225.3, p < 0.001], but indistinguishable between younger and older mice (young, ZT 8.0 ± 0.1 h, n = 6; older, ZT 7.8 ± 0.1 h, n = 7; p > 0.05).
HA The 1 mM bath application of HA centred around ZT 15 produced phase delays in both young and older mice [F(3,19) = 90.12, p < 0.001]. Younger mice delayed by almost 3 h (n = 7, mp = 8.8 ± 0.1 h). When older animals were treated in the same way they did not show as great a delay (n = 6, mp = 7.5 ± 0.2 h, p < 0.001).
Neurochemicals that phase shift in a pattern similar to behavioural stimuli (see Fig. 3)
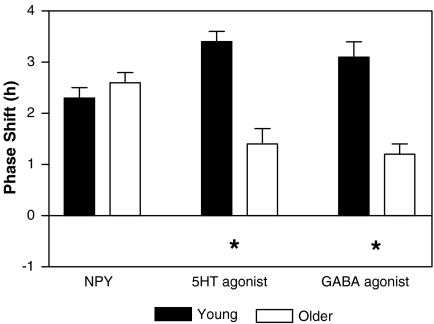
Fig. 3.
Phase shifts of the pattern of suprachiasmatic nucleus firing rates in young (5 months) and older (15 months) mice to at ZT 6 to neuropeptide Y (NPY), 8-OH-DPAT (serotonin agonist) and muscimol (GABA agonist). These neurochemicals all phase shift the firing rate of the suprachiasmatic nucleus in a non-photic pattern, showing phase advances when applied in the middle of the day at ZT 6. Mean phase delays ± the standard error of the mean. Asterisks indicate significant differences between phase shifts seen in young and older mice
NPY When treated with a microdrop of NPY during the day at ZT 6 mouse SCN slices showed phase advances in their peaks [F(3,22) = 488.2, p < 0.001]. Older mice still retained the ability to advance in response to NPY application (young mice, n = 8, mp = 3.6 ± 0.1 h; older mice, n = 8, mp = 3.4 ± 0.2) such that Tukey’s test showed there was no significant difference between them (p > 0.05).
8-OH DPAT A one-way ANOVA showed that microdrop application of 8-OH-DPAT at ZT6 reset the firing rate rhythm both in young or older mice [F(3,23) = 62.97, p < 0.001]. This administration robustly advanced the young mouse SCN (n = 9, mp = 2.5 ± 0.2 h). Older mice reset significantly less to the serotonin agonist (n = 8, mp = 4.6 ± 0.2 h, p < 0.001).
Muscimol When slices were treated at ZT 6 on day 1 in vitro with 10 μM, the newly shifted peak showed a phase advance [F(3,21) = 34.5, p < 0.001]. In younger mice the peak occurred at 2.8 ± 0.1 h (n = 7). Older mice reset significantly less to the GABA agonist (n = 8, mp = 4.8 ± 0.2 h, p < 0.01).
Discussion
Earlier work has show disruption to a number of circadian parameters in C57BL/6 mice with senescence in vivo (Valentinuzzi et al. 1997). This is the first report of changes to the phase shifting ability of neurotransmitters within the mouse SCN in vitro with age. It is in agreement with the previous literature, which suggested that at least some of the age-related changes seen in circadian timing and sleep in vivo could be attributed to changes within the SCN itself. Initially, Satinoff et al. (1993) showed that rhythms in firing rate recorded from the aged rat SCN had lower amplitude and were less organised than those recorded from young rats. Later, Li and Satinoff (1998) showed it was possible to improve circadian rhythms in older rats with poor circadian rhythms by implanting fetal SCN tissue into the third ventricle. Our own work here takes this further, suggesting that changes at the level of the SCN in older rodents contribute to alterations in the response to some but not all stimuli involved in synchronising the circadian clock.
This is significant as it suggests that there is not a general decline in pacemaker function with age, no overall deterioration in the ability of clock cells to synchronise successfully in order to affect a phase shift. We see here that the resetting in response to a number of stimuli in older mice is indistinguishable from that seen in younger mice. This indicates that while there is a general decrease seen in the amplitude of the circadian rhythm in firing rate with age, the pacemaker is still able to reset successfully to stimuli. Further, as the ability to phase shift is maintained through ageing to some agents which reset in both the photic or the non-photic pattern, this suggests that there is not a general breakdown of the physiological or molecular mechanisms mediating one particular pattern of resetting. Therefore, each transmitter system must be investigated individually to understand at what point in the signal transduction pathway, and/or output systems there are disruptions.
Previous work has shown that hamsters and mice show a decreased response to light pulses with age (Zhang et al. 1996; Benloucif et al. 1997). Further, the reduction in lens transmittance, the histological appearance of the RHT projection or volume of SCN they innervated could not account for these changes (Zhang et al. 1998). Molecular work within the SCN has indicated that aging significantly reduces photic induction of c-fos mRNA or FOS protein in mice, rats and hamsters (Zhang et al. 1996). In addition, photic activation of cyclic-AMP response element binding protein (CREB) and induction of Per1 within the SCN are both reduced with age in hamsters (Zhang et al. 1996; Kolker et al. 2003). Our work suggests that these molecular changes seen in response to photic stimuli with age may be due to altered action of glutamate at the NMDA receptor during senescence.
Phase resetting to HA was also decreased in mice. There are age-related changes seen in HA receptor mRNA levels in the mouse brain (Terao et al. 2004), although this may not account for the changes we observed as HA is thought to alter circadian rhythms by actions on the NMDA receptor (Harrington et al. 2000). This would fit well with our other data showing a decreased response to NMDA within the aged mouse SCN in vitro. While HA appears to be involved in the control of sleep and arousal, the role of this transmitter in circadian timing is not well understood. In addition to changes in circadian timing with age, animals also show changes in sleep parameters. Further investigations into the substrates mediating the change in reponse to HA with age, may inform our understanding of interactions between the circadian timing mechanism and the factors controlling homeostatic sleep factors.
Photic stimulation increases FOS in GRP cells within the ventral SCN in the rat (Earnest et al. 1993). This suggests that these peptidergic cells may process photic information, and participate in entrainment. Although both phase shifts to light, and FOS expression after photic stimulation is decreased within the SCN with age, we saw no age-related decline in the response to GRP. As phase shifts to GRP in the hamster are believed to require NMDA receptor activation (Kallingal and Mintz 2006), this may be evidence of a species difference in the mechanism of action.
In addition to attenuating phase shifts to light in older animals, resetting in response to some non-photic stimuli have been shown to be decreased in hamsters (Van Reeth et al. 1992; Penev et al. 1995; Duncan and Deveraux 2000, but see Mrosovsky and Biello 1994). Some of these signals are thought to be mediated by NPY, and SCN content of this peptide is decreased with age in rats (Sahu et al. 1998). Still, NPY shifted the SCN of older mice as robustly as shown in younger animals. This supports earlier unpublished findings from administration of NPY in vivo (Duncan unpublished, cited in Duncan and Franklin 2007). Further, while phase shifts to NPY in the rat and hamster may require actions at GABAa receptors (Huhman et al. 1995; Gribkoff et al. 1998 but see Biello et al. 1997a, b) our work here would suggest that resetting to NPY in the mouse may not, as phase shifts to the GABAa agonist muscimol was decreased with age, while phase shifts to NPY remain unaltered. Phase shifts to NPY are thought to be mediated by the Y2 receptor (Golombek et al. 1996; Huhman et al. 1996), while attenuation of photic stimuli by NPY are mediated by the Y5 receptor (Gamble et al. 2005; Yannielli et al. 2004). Further work should investigate changes in NPY receptor expression with age in the SCN, and also if the actions of NPY on phase shifts to photic stimuli during the night change with age.
Older hamsters do not reset to serotonin agonists in the same way as younger hamsters in vivo (Penev et al. 1995), although it is possible this not mediated at the level of the SCN (Duncan et al. 2004). Still, even in hamsters there are actions of serotonin on the circadian system mediated at the level of the SCN (Weber et al. 1998), and serotonin agonists reset the mouse SCN in vitro (Prosser 2003). The mechanism by which serotonin agonists reset the circadian clock in vitro is still unclear, and may involve the 5HT1A, 5HT5A and 5HT7 receptor subtypes (Sprouse et al. 2005; Guscott et al. 2005). The agonist 8-OH-DPAT can act on both the 5HT1A and 5HT7 subtype. While age-related changes in response to serotonin agonists have been reported, it is possible that this is not mediated by changes in the 5HT1A and 5HT7 receptor subtypes within the SCN, as work has shown that there are no age-related changes in the expression of mRNA for either the 5HT1A or 5HT7 receptor with age in the hamster SCN (Duncan et al. 1999; Duncan and Franklin 2007). Further work should investigate not only serotonin receptor numbers but also function within the mouse SCN. In addition, basal levels of serotonin receptor stimulation can determine the ability of agonists to reset the SCN in vitro (Prosser et al. 2006). While this would predict that reduced endogenous serotonin signalling in the SCN in vitro would result in larger phase shifts to exogenously applied agonists, it may also suggest that processes interfering with receptor internalisation or reinsertion may be altered in the aged SCN.
We also saw age-related changes in the response to GABA in the mouse SCN. This is important as GABA is the main neurochemical used to mediate synaptic transmission within the SCN (Moore and Speh 1993; Buijs et al. 1994). Agonists of GABAa receptors phase advance the in vitro SCN of hamsters during the day (Tominaga et al. 1994). There is evidence of age-related changes within the GABAergic network of mice, such that the ventral area of the SCN shows reduced GABAA spontaneous currents (Nygard et al. 2005; Nygard and Palomba 2006). While the expression of genes associated with the expression of GABAergic transmission were not altered with senescence, reductions were seen in GABAergic presynaptic terminals of aged mice in the SCN (Palomba et al. 2008). GABA is known to play a part in the regulation of amplitude and the synchronisation of neuronal firing rate activity within the SCN (Aton et al. 2006). In addition, blocking GABA in the SCN in vitro can disrupt coupling between dorsal and ventral regions of the SCN (Albus et al. 2005).
Our work supports other experimental studies showing that the circadian system remains responsive to some types of stimuli and not others in advanced age. This has implications for both pharmacological and behavioural interventions to improve synchronisation in aged subjects. It may be possible to enhance activity in an individual pathway in an attempt to compensate for decreased activity in another. For example, NPY and serotonin both participate in synchronisation to non-photic stimuli and interact with each other in the SCN (Prosser 1998). While the resetting actions of serotonin are decreased with age, those of NPY remain as in youth. Further, some transmitters may work in subtle combination to affect synchronisation. For example resetting to glutamate (thought to be the major transmitter mediating photic phase shifts) can be attenuated by NPY (Biello et al. 1997a; Lall and Biello 2003), or behavioural interventions thought to rely on this transmitter system (Ralph and Mrosovsky 1992). Therefore, while it is still unclear how these transmitters participate in entrainment, we would predict that altering the relative contribution of each signal either behaviourally or pharmacologically could be used to enhance synchronisation in older animals.
In addition, this is the first report of the phase shifting ability of several of these neurotransmitters in younger mice. With the exception of NPY, NMDA and DPAT which have shown to reset the mammalian circadian clock of the mouse in a manner similar to hamsters and/or rats (Guscott et al. 2005; Soscia and Harrington 2004), the other transmitters have not been shown to be effective as resetting agents within the mouse SCN. Testing these agents in mice is particularly important as much circadian work now takes advantage of developed molecular tools only available in the mouse model.
The fact that each individual transmitter produces similar phase shifts to those seen in hamsters, rats and mice, reflects how well the circadian clock is conserved across species. In addition, the similarities in the timing and direction of the in vitro shift of so many transmitters to their behavioural effects in vivo shows that an in vitro model of circadian resetting can be a useful initial step to establishing the second messenger systems and intra-SCN communication necessary for their mechanism of action.
Age-related disruptions in sleep in humans and locomotor activity in non-human primates have been well documented (Wu and Swaab 2007; Aujard et al. 2006). Recent data suggest that these disruptions are due to weaker circadian regulation of sleep and wakefulness rather than alterations in the homeostatic mechanisms governing the sleep/wake cycle (Cajochen et al. 2006). Our data suggest that at least some of the observed disruption with age may be due to alterations in the activity of neurotransmitters crucial for synchronisation of the circadian clock.
Acknowledgements
We are grateful to Research into Ageing (UK) and the Wellcome Trust (UK) for financial support.
References
- Albers HE, Gillespie CF, Babagbemi TO, Huhman KL. Analysis of the phase shifting effects of gastrin releasing peptide when microinjected into the suprachiasmatic region. Neurosci Lett. 1995;191:63–66. doi: 10.1016/0304-3940(95)11559-1. [DOI] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. AGABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Antle MC, Ogilvie MD, Pickard GE, Mistlberger RE. Response of the mouse circadian system to serotonin IA/2/7 agonists in vivo: surprisingly little. J Biol Rhythms. 2003;18(2):145–158. doi: 10.1177/0748730403251805. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABAand Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci USA. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neurosci. 2001;106(2):255–261. doi: 10.1016/S0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Aujard F, Cayetanot F, Bentivoglio M, Perret M. Age-related effects on the biological clock and its behavioural output in a primate. Chronobiol Int. 2006;23:451–460. doi: 10.1080/07420520500482090. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/S0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Biello SM, Dafters RI. MDMA and fenfluramine alter the response of the circadian clock to a serotonin agonist in vitro. Brain Res. 2001;920:202–209. doi: 10.1016/S0006-8993(01)03070-0. [DOI] [PubMed] [Google Scholar]
- Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neurosci. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-X. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other’s phase shifts in the suprachiasmatic nucleus in vitro. Neurosci. 1997;77:1049–1058. doi: 10.1016/S0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Schak KM, Harrington ME. Circadian phase shifts to neuropeptide Y in vitro: cellular communication and signal transduction. J Neurosci. 1997;17(21):8468–8475. doi: 10.1523/JNEUROSCI.17-21-08468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrzynska K, Godfrey MH, Mrosovsky N. Serotonergic stimulation and nonphotic phase-shifting in hamsters. Physiol Behav. 1996;59:221–230. doi: 10.1016/0031-9384(95)02130-2. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82(5):229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Hou YX, Shinn S, Renaud LP. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994;340:381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23:461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-U. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Deveraux AW. Age-related changes in circadian responses to dark pulses. Am J Physiol Reg Int Comp Physiol. 2000;279(2):R586–R590. doi: 10.1152/ajpregu.2000.279.2.R586. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Franklin KM. Expression of 5-HT7 receptor mRNA in the hamster brain: effect of aging and association with calbindin-D28K expression. Brain Res. 2007;1143:70–77. doi: 10.1016/j.brainres.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Short J, Wheeler DL. Comparison of the effects of aging on 5-HT 7 and 5-HT 1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res. 1999;829:39–45. doi: 10.1016/S0006-8993(99)01311-6. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Grear KE, Hoskins MA. Aging and SB-269970-A, a selective 5-HT 7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2004;1008:40–48. doi: 10.1016/j.brainres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, DiGiorgio S, Olschowka JA. Light induces expression of fos-related proteins within gastrin-releasing peptide neurons in the rat suprachiasmatic nucleus. Brain Res. 1993;627:205–209. doi: 10.1016/0006-8993(93)90322-E. [DOI] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/S0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Ehlen JC, Albers HE. Circadian control during the day and night: role of neuropeptide YY5 receptors in the suprachiasmatic nucleus. Brain Res Bull. 2005;65(6):513–519. doi: 10.1016/j.brainresbull.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. Neuroreport. 1996;7(7):1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Pol AN, Yoccal FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18(8):3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beera MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowskia JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharm. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME, Biello SM, Panula P. Effects of histamine on circadian rhythms and hibernation. Biol Rhythms Res. 2000;31(3):374–390. doi: 10.1076/0929-1016(200007)31:3;1-K;FT374. [DOI] [Google Scholar]
- Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5(1):33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Shibata S. Phase-resetting response to (+)8-OH-DPAT, a serotonin 1A/7 receptor agonist, in the mouse in vivo. Neurosci Lett. 2004;368(2):130–134. doi: 10.1016/j.neulet.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Babagbemi TO, Albers HE. Bicuculline blocks neuropeptide y-induced phase advances when microinjected in the suprachiasmatic nucleus of syrian-hamsters. Brain Res. 1995;675(1–2):333–336. doi: 10.1016/0006-8993(95)00018-L. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Gillespie CF, Marvel CL, Albers HE. Neuropeptide Y phase shifts circadian rhythms in vivo via a Y-2 receptor. Neuroreport. 1996;7(7):1249–1252. doi: 10.1097/00001756-199605170-00005. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Characteristics of a circadian pacemaker in the suprachiasmatic nucleus. J Comp Physiol. 1982;146:153–160. doi: 10.1007/BF00610233. [DOI] [Google Scholar]
- Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera-toxin. Brain Res. 1988;462(2):301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Glutamatergic activity modulates the phase shifting effects of gastrin-releasing peptide and light. Eur J Neurosci. 2006;24:2853–2858. doi: 10.1111/j.1460-9568.2006.05165.x. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Lall GS, Biello SM. Attenuation of circadian light induced phase advances and delays by neuropeptide Y and a neuropeptide YY1/Y5 receptor agonist. Neuroscience. 2003;119(2):611–618. doi: 10.1016/S0306-4522(02)00811-4. [DOI] [PubMed] [Google Scholar]
- Li H, Satinoff E (1998) Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am J Physiol 275(6)2:R1735–R1744 [DOI] [PubMed]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J Neurosci. 2000;20(14):5496–5502. doi: 10.1523/JNEUROSCI.20-14-05496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JL, Hall AC, Harrington ME. Histamine phase shifts the hamster circadian pacemaker via an NMDA dependent mechanism. J Biol Rhythms. 1998;13:288–295. doi: 10.1177/074873098129000129. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Electrical stimulation of the median or dorsal raphe nuclei reduces light-induced FOS protein in the suprachiasmatic nucleus and causes circadian activity rhythm phase shifts. Neurosci. 1999;92(1):267–279. doi: 10.1016/S0306-4522(98)00733-7. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-A. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Biello SM. Nonphotic phase-shifting in the old and the cold. Chronobiol Int. 1994;11(4):232–252. doi: 10.3109/07420529409067792. [DOI] [PubMed] [Google Scholar]
- Nygard M, Palomba M. The GABAergic network in the suprachiasmatic nucleus as a key regulator of the biological clock: does it change during senescence? Chronobiol Int. 2006;23(1–2):427–435. doi: 10.1080/07420520500545938. [DOI] [PubMed] [Google Scholar]
- Nygard M, Hill RH, Wikstrom MA, Kristensson K. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res Bull. 2005;65:149–154. doi: 10.1016/j.brainresbull.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Palomba M, Nygard M, Florenzano F, Bertini G, Kristensson K, Bentivoglio M. Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J Biol Rhythms. 2008;23(3):220–231. doi: 10.1177/0748730408316998. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neurosci. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Penev PD, Zee PC, Wallen EP, Turek FW. Aging alters the phase-resetting properties of a serotonin agonist on hamster circadian rhythmicity. Am J Physiol. 1995;268:R293–R298. doi: 10.1152/ajpregu.1995.268.1.R293. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythms. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 1998;80(1):31–41. doi: 10.1016/S0006-8993(98)00808-7. [DOI] [PubMed] [Google Scholar]
- Prosser RA. Glutamate blocks serotonergic phase advances of the mammalian circadian pacemaker through AMPA and NMDA receptors. J Neurosci. 2001;21:7815–7822. doi: 10.1523/JNEUROSCI.21-19-07815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Serotonin phase-shifts the mouse suprachiasmatic circadian clock in vitro. Brain Res. 2003;966:110–115. doi: 10.1016/S0006-8993(02)04206-3. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Lee HM, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neurosci. 2006;142(2):547–555. doi: 10.1016/j.neuroscience.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. 1. Involvement of GABAa-benzodiazepine and GABAb receptors. J Neurosci. 1989;9(8):2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–359. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian-rhythms. Physiol Rev. 1979;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra PS, Crowley WR, Kalra SP. Evidence that hypothalamic neuropeptide Y secretion decreases in aged male rats: implications for reproductive aging. Endocrinology. 1998;122:2199–2203. doi: 10.1210/endo-122-5-2199. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, McArthur AJ, Medanic M, Gillette MU. Do the Suprachiasmatic Nuclei oscillate in old rats as they do in young ones. Am J Physiol. 1993;265(5):R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Smith RD, Turek FW, Takahashi JS. Two families of phase-response curves characterize the resetting of the hamster circadian clock. Am J Physiol. 1992;262:R1149–R1153. doi: 10.1152/ajpregu.1992.262.6.R1149. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Harrington ME. Neuropeptide Y attenuates NMDAinduced phase shifts in the SCN of NPY Y1 receptor knockout mice in vitro. Brain Res. 2004;1023:148–153. doi: 10.1016/j.brainres.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Li X, Stock J, McNeish J, Reynolds L. Circadian rhythm phenotype of 5-HT7 receptor knockout mice: 5-HT and 8-OH-DPAT induced phase advances of SCN neuronal firing. J Biol Rhythms. 2005;20:122–131. doi: 10.1177/0748730404273432. [DOI] [PubMed] [Google Scholar]
- Terao A, Steininger TL, Morairty SR, Kilduff TS. Age-related changes in histamine receptor mRNA levels in the mouse brain. Neurosci Lett. 2004;355:81–84. doi: 10.1016/j.neulet.2003.10.061. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Shibata S, Hamada T, Watanabe S. GABAA receptor agonist muscimol can reset the phase of neural activity rhythm in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1994;166:81–84. doi: 10.1016/0304-3940(94)90845-1. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheelrunning activity in C57BL/6 mice. Am J Physiol Reg Int Comp Physiol. 1997;273:1957–1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Reeth O, Zhang Y, Zee PC, Turek FW. Aging alters feedback effects of the activity rest cycle on the circadian clock. Am J Physiol. 1992;263:R981–R986. doi: 10.1152/ajpregu.1992.263.4.R981. [DOI] [PubMed] [Google Scholar]
- Weber ET, Gannon RL, Rea MA. Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J Biol Rhythms. 1998;13(3):209–218. doi: 10.1177/074873098129000057. [DOI] [PubMed] [Google Scholar]
- Wu Y-H, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Brewer JM, Harrington ME. Blockade of the NPY Y5 receptor potentiates circadian responses to light: complementary in vivo and in vitro studies. Eur J Neurosci. 2004;19:891–897. doi: 10.1111/j.0953-816X.2004.03098.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neurosci. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brainard GC, Zee PC, Pinto LH, Takahashi JS, Turek FW. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/S0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]