Abstract
The co-inhibitory receptor killer-cell lectin like receptor G1 (KLRG1) is expressed on NK cells and antigen-experienced T cells and has been postulated to be a marker of senescence. Whilst KLRG1 has frequently been used as a marker of cellular differentiation, data are emerging indicating that KLRG1 plays an inhibitory role. In this review we examine evidence highlighting this view of KLRG1 with emphasis on the functional defects that arise during T cell differentiation with age that may, in part, be actively maintained by inhibitory receptor signalling.
Keywords: KLRG1, Inhibitory, ITIM, Highly differentiated T cell
Introduction
The immune system undergoes a dramatic restructuring with age, leading to a decline in immune responses and an increased vulnerability of old individuals. The incidence and severity of infectious diseases, such as pneumonia (LaCroix et al. 1989), meningitis (Gorse et al. 1984), sepsis (Chattopadhyay and Al-Zahawi 1983), urinary tract infections (Ackermann and Monroe 1996), infection with respiratory syncytial virus (Barker and Mullooly 1980) or influenza (Sprenger et al. 1993) all increase with age. Indeed the mortality rate of older adults suffering urinary tract infections or tuberculosis is ten-fold higher than that of young adults (Yoshikawa 1997). This waning immunity in old age results from defects in numerous different leukocyte populations with the dysfunction being most pronounced in T cells. This T cell immune decline is marked by a dramatic decline in the number of naïve T cells as a result of a thymic atrophy (Douek et al. 1998; Linton and Dorshkind 2004). This reduced thymic output leads to the peripheral expansion of naïve and memory T cells to regenerate the T cell pool, which in turn leads to the accumulation of oligoclonally expanded, functionally impaired T cells (Akbar and Fletcher 2005; Messaoudi et al. 2004). These age-associated changes contribute to the inability of the aged immune system to respond to new antigenic challenge and mount optimum responses to vaccination (Goronzy et al. 2001).
Phenotypic changes to T cells during aging
There are numerous reports cataloging the phenotypic and functional changes to human T cells that occur during ageing (Table 1). Old individuals show an increased proportion of T cells that are highly differentiated, with similar phenotypic changes occurring in both CD4+ and CD8+ T cells during differentiation. However, the rate at which these changes happen varies within each subset, with age-related changes being more pronounced on CD8+ T cells due to a greater homeostatic stability of CD4+ T cells (Effros et al. 1994; Czesnikiewicz-Guzik et al. 2008; Goronzy et al. 2007). These highly differentiated cells have functional defects that may explain the decreased efficiency of the immune system in older individuals (Fletcher et al. 2005). Highly differentiated T cells are characterised by the loss of the cell surface co-stimulatory molecules CD27 and CD28, CD8+ T cells losing CD28 first followed by CD27 with the converse being true for CD4+ T cells (Appay et al. 2002; Amyes et al. 2003; Fletcher et al. 2005; Plunkett et al. 2005). Initially, it was thought that the loss of CD28 was a major factor in the reduced activation and function of these cells (Champagne et al. 2001; Effros et al. 2005). However, subsequent studies have suggested a greater plasticity with regard to co-stimulatory receptor expression and usage among T cells. For example, co-stimulation through ICOS, a CD28 family member, and CD137 and CD134, members of the TNF family, have all been shown to enhance the proliferation (Bukczynski et al. 2003; Serghides et al. 2005; Plunkett et al. 2007; Waller et al. 2007) and telomerase activity in CD8+CD28- T cells (Plunkett et al. 2007). This redundancy in co-stimulatory receptor usage suggests that changes in addition to the loss of co-stimulatory receptors are involved in T cell dysfunction during ageing. One such change may be a rise in co-inhibitory receptors, in particular the co-inhibitory receptor killer-cell lectin like receptor G1 (KLRG1).
Table 1.
Phenotypic and functional characteristics of human T cell subsetsa
| Phenotype | Naïve | Central memory | Effector memory | CD45RA memory | References |
|---|---|---|---|---|---|
| CD45RA | +++ | - | - | +++ | (Akbar et al. 1988; Sallusto et al. 2004) |
| CD45RO | - | +++ | +++ | - | (Akbar et al. 1988; Sallusto et al. 2004) |
| CD28 | +++ | ++ | +/- | +/- | (Hamann et al. 1997; Sallusto et al. 2004) |
| CD27 | +++ | ++ | +/- | +/- | (Akbar et al. 1988; Sallusto et al. 2004) |
| CCR7 | +++ | ++ | - | - | (Sallusto et al. 2004) |
| CD62L | +++ | +++ | + | + | (Sallusto et al. 2004) |
| CD11c | - | +++ | +++ | +++ | (Faint et al. 2001) |
| CD57 | - | + | ++ | +++ | (Appay et al. 2007; Koch et al. 2008) |
| KLRG1 | + | ++ | ++ | +++ | (Voehringer et al. 2001; Ouyang et al. 2003) |
| Telomere length | +++ | ++ | + | ++ | (Faint et al. 2001; Plunkett et al. 2005) |
aActivation of T cells results in phenotypic and functional changes. Using relative telomere length and cell surface phenotype as combined criteria, a scheme for identifying T cells at different stages of differentiation can be constructed. Following antigen stimulation, naïve T cells lose expression of CD45RA and become CD45RO+ memory cells. Upon differentiation to an effector memory population, T cells lose CCR7, CD62L, CD28 and CD27, while expression of CD11c, CD57 and KLRG1 increase. In general, similar phenotypic changes occur in both CD4+ and CD8+ T cells during differentiation; however, the rate at which these changes occur can vary within each subset (Appay et al. 2002). The balance of naïve and memory cells is altered during aging, with older adults showing significantly increased levels of highly differentiated effector memory and primed CD45RA+ T cells and a concomitant loss of naïve cells (Pawelec et al. 2004). These highly differentiated T cells have short telomeres and consequently function poorly, suggesting that, during the course of aging, these populations are eventually driven to end-stage differentiation
KLRG1—more than a marker for T cell memory
In both mice and humans, KLRG1 expression is found on NK cells and antigen-experienced T cells (Blaser et al. 1998; Hanke et al. 1998; Voehringer et al. 2002). Human KLRG1 is also found on a subset of γδ T cells (Eberl et al. 2005) and in a large proportion of CD4+ and CD8+ T cells found in cord blood (Marcolino et al. 2004). In young adults, the expression of KLRG1 is about 40% on CD8+ T cells and 20% on CD4+ T cells (Voehringer et al. 2002). The expression of KLRG1 rises dramatically with age, with greater than 90% expression of KLRG1 being seen on CD8+ T cells in individuals over 65 years of age (Ouyang et al. 2003; Ito et al. 2006; Henson et al. 2009). The expression of KLRG1 increases not only with age but also with differentiation, with the highest percentage of expression being seen on memory cells and highly differentiated end stage cells (Voehringer et al. 2002; Thimme et al. 2005). In mice, KLRG1 has been used to identify memory precursor cells from effector T cells. Through the use of acute viral infection models it has been shown that KLRG1 can be used to distinguish short-lived effector CD8+ T cells (KLRG1high) and memory precursor effector CD8+ T cells (KLRG1low) (Joshi et al. 2007; Mousavi et al. 2008).
Despite the extensive use of KLRG1 as a marker of differentiation, KLRG1 possesses an immune receptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain, suggesting that it may play a functional role in the immune system. An inhibitory role for KLRG1 has been demonstrated in mice, with antibody-mediated cross-linking of KLRG1 being shown to inhibit cytolytic activity and IFNγ production in NK cells (Robbins et al. 2002). However, this result is at odds with a number of reports that failed to observe an inhibitory cytolytic effect (Hanke et al. 1998; Grundemann et al. 2006); these differing outcomes may be the result of Robbins et al. using an NK clone over-expressing KLRG1. In murine T cells, the cross-linking of TCR and KLRG1 by plate-bound antibodies was shown to lower Ca2+ influx (Beyersdorf et al. 2001) and to decrease IL-2 production (Tessmer et al. 2007). The use of KLRG1-transgenic mice showed that antigen-stimulated T cells in the presence of KLRG1’s ligand, E-cadherin, inhibited the proliferative capacity of CD8+ T cells (Grundemann et al. 2006).
Data is now emerging suggesting that KLRG1 plays an inhibitory role in human NK cells and T cells. KLRG1-mediated inhibition of NK cell function revealed that KLRG1/ligand interactions inhibit the cytolytic activity of polyclonal human NK cells by interfering with both degranulation and IFNγ release (Schwartzkopff et al. 2007). Consistent with murine data, the authors also show the degree of inhibition to be modest, and to require high expression levels of KLRG1’s ligand, E-cadherin (Schwartzkopff et al. 2007).
A recent study has demonstrated a role for KLRG1 as an inhibitory receptor in T cells. The authors used a CD4+ T cell hybridoma transduced with KLRG1 and showed that KLRG1-ligation inhibited the NFAT-signaling pathway and down-regulated CD95 mediated lysis (Rosshart et al. 2008). Furthermore, they also demonstrated that both KLRG1 and CD3/TCR signals have to be provided in a spatially restricted manner in order to inhibit T-cell activation (Rosshart et al. 2008), suggesting that KLRG1 inhibits T cell function only when MHC/antigen and KLRG1 ligands are expressed on the same target cells. Data from our laboratory has also shown KLRG1 to have an inhibitory effect on primary CD8+ T cells; we have shown that blocking KLRG1 signaling during TCR activation using antibodies against its ligand, E-cadherin, enhanced proliferative activity that was linked directly to an Akt-mediated increase in synthesis of cyclin D and E and a decrease in the cyclin inhibitor p27 (Henson et al. 2009). Whist we observed a significant enhancement in proliferative capacity in CD8+ T cells isolated from young individuals the effect was not as great as that of CD8+ T cells isolated from old individuals, suggesting that other, as yet unidentified, age-related defects are contributing to the poor proliferative responses of CD8+ T cells from old donors and these remain to be clarified.
How KLRG1 exerts its inhibitory effects
KLRG1 has been shown to be a cadherin receptor, recognising E-, N- and R-cadherin (Grundemann et al. 2006; Ito et al. 2006; Schwartzkopff et al. 2007). The cadherins comprise a family of transmembrane glycoproteins that mediate Ca2+ dependent cell-cell adhesion (Gumbiner 2005). Classically, E-cadherin is expressed on epithelial cells and Langerhans cells, whereas N- and R-cadherin are expressed by the nervous system. We have shown E-cadherin to be expressed on peripheral blood cells, notably on myeloid DCs, with no expression of N-cadherin being found (Henson et al. 2009). Demonstrating that E-cadherin is found not only in the epithelium but on a wide range of antigen-presenting cells suggesting a broader range of scenarios for immune control by KLRG1/cadherin interactions. A recent report using a KLRG1-reporter cell assay with domain-deleted E-cadherin mutants (Δ1–Δ5) have shown that the first and the second extracellular domains of E-cadherin to be critical for interaction with KLRG1 (Rosshart et al. 2008).
KLRG1 contains one ITIM motif in its cytoplasmic domain, which mediates its effects through the recruitment of SHIP-1 and SHP-2 phosphatases and a tyrosine residue at position 7 in the ITIM (Xu et al. 2001; Tessmer et al. 2007). Murine KLRG1 also contains a PxxP motif in its cytoplasmic domain that could potentially interact with proteins containing SH3 domains (Abramson et al. 2002). KLRG1 forms both monomers and dimers, with a substantial fraction of KLRG1 being found on the cell surface as disulfide-linked trimeric and tetrameric complexes (Rosshart et al. 2008). The cysteine residues in human KLRG1 that are responsible for disulfide-linked multimer formation have not yet been defined; however, murine KLRG1 contains four cysteines proximal to the membrane in the extracellular domain that are probably not involved in intramolecular disulfide-bonding (Voehringer et al. 2001). It has been demonstrated that, in contrast to KLRG1-tetramers (Grundemann et al. 2006; Rosshart et al. 2008), monomeric KLRG1 shows little discernable binding to E-cadherin-expressing cells, suggesting that KLRG1 binds to E-cadherin with relatively low affinity. Multimerisation of KLRG1 increases the avidity and may thereby enhance the sensitivity for inhibition (Rosshart et al. 2008).
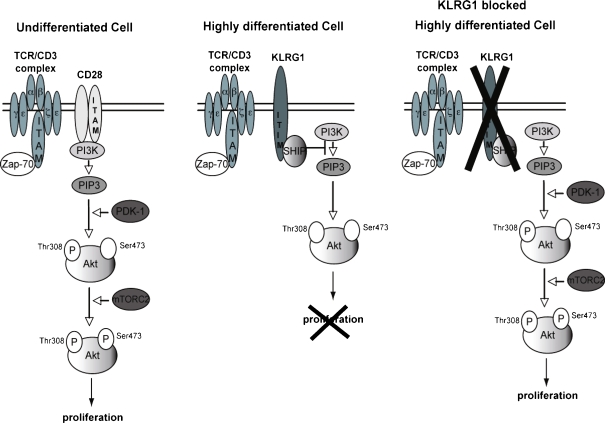
The effectors SHIP-1 and SHP-2 degrade PIP3 to PIP2, thus regulating the function of PI3K (Tessmer et al. 2007). PI3K plays a crucial role in a broad range of cellular functions in response to extracellular signals. A key downstream effector of PI3K is the serine-threonine kinase Akt, which, in response to PI3K activation, phosphorylates and regulates the activity of a number of targets, including kinases, transcription factors and other regulatory molecules (Donahue and Fruman 2004). The activation of Akt requires the binding of its pleckstrin homology (PH) domain to the phosphoinositide products of PI3K resulting in its recruitment to the plasma membrane. Once there, Akt activation is controlled by phosphorylation at two different sites, Thr308 and Ser473 (Alessi et al. 1996; Jacinto et al. 2006). Highly differentiated CD8+CD28-CD27- T cells are unable to phosphorylate Akt(ser473), with the Thr308 phosphorylation site being unaffected (Plunkett et al. 2007). We assessed whether signalling via KLRG1 contributes to any of the attenuated differentiation-related functional changes in CD8+ T cells. By blocking KLRG1 signalling during TCR activation using antibodies against its major ligand, E-cadherin, we showed a reversal of the defective Akt(ser473) phosphorylation in highly differentiated CD8+ T cells isolated from both young and old donors to the levels that are found after activation in the less differentiated CD8+ subsets (Fig. 1). This indicates that the defect in Akt phosphorylation is not a passive consequence of antigenic-driven differentiation of CD8+ T cells but that it is instead actively maintained by KLRG1 signalling (Henson et al. 2009).
Fig. 1.
Changes in Akt signaling with differentiation of CD8+ T cells and co-inhibitory receptor killer-cell lectin like receptor G1 (KLRG1)-blocked highly differentiated CD8+ T cells. An undifferentiated cell signals through CD28, initiating the Akt signalling pathway, which results in a broad range of cellular functions, including the initiation of proliferation. Upon differentiation, CD8+ T cells lose CD28 and gain the inhibitory molecule KLRG1, which acts through SHIP-1 and SHP-2 to degrade PIP3 to PIP2 (Tessmer et al. 2007), preventing phosphorylation of Akt(ser473), thus regulating the function of PI3K. Blocking KLRG1 signals in highly differentiated CD8+ T cells causes the conversion of PIP2 to PIP3 leading to a restoration of pAkt(Ser473) to the levels seen after activation in the less differentiated CD8+ subsets, enhancing proliferation in otherwise dysfunctional cells (Henson et al. 2009)
Conclusion
Highly differentiated T cells accumulate with age; these cells have numerous defects including a decreased capacity for proliferation, an inability to produce IL-2, defective Akt(ser473) phosphorylation after activation, short telomeres and low telomerase activity, indicating that they are close to replicative senescence. In addition, these cells express increased levels of the inhibitory receptor KLRG1. Despite the extensive use of KLRG1 as a marker of differentiation, KLRG1 possesses an ITIM in its cytoplasmic domain, suggesting that it may play a functional role in the immune system. KLRG1 signalling has been shown to inhibit the cytolytic activity of polyclonal human NK cells and T cell hybridomas, as well as interfering with proliferation via Akt-mediated changes in cyclins and cyclin inhibitors. Therefore, signalling through KLRG1 may be responsible in part for the defects observed in highly differentiated T cells. It is well recognised that older humans have decreased responses to vaccination (Hayward et al. 1994; Stepanova et al. 2002; Wick et al. 2000) and it is possible that modulating certain inhibitory receptors like KLRG1 that are preferentially expressed in highly differentiated T cells, which expand during ageing, could potentially boost immunotherapeutic regimes such as vaccination for the aged.
Acknowledgements
This work was supported by a Research into Ageing Fellowship to S.M.H. and funding from the British Biotechnology and Biological Research Council to A.N.A.
References
- Abramson J, Xu R, Pecht I. An unusual inhibitory receptor-the mast cell function-associated antigen (MAFA) Mol Immunol. 2002;38:1307–1313. doi: 10.1016/S0161-5890(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Ackermann RJ, Monroe PW. Bacteremic urinary tract infection in older people. J Am Geriatr Soc. 1996;44:927–933. doi: 10.1111/j.1532-5415.1996.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- Beyersdorf NB, Ding X, Karp K, Hanke T. Expression of inhibitory "killer cell lectin-like receptor G1" identifies unique subpopulations of effector and memory CD8 T cells. Eur J Immunol. 2001;31:3443–3452. doi: 10.1002/1521-4141(200112)31:12<3443::AID-IMMU3443>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Blaser C, Kaufmann M, Pircher H. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. J Immunol. 1998;161:6451–6454. [PubMed] [Google Scholar]
- Bukczynski J, Wen T, Watts TH. Costimulation of human CD28- T cells by 4-1BB ligand. Eur J Immunol. 2003;33:446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay B, Al-Zahawi M. Septicaemia and its unacceptably high mortality in the elderly. J Infect. 1983;7:134–138. doi: 10.1016/S0163-4453(83)90548-0. [DOI] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue AC, Fruman DA. PI3K signaling controls cell fate at many points in B lymphocyte development and activation. Semin Cell Dev Biol. 2004;15:183–197. doi: 10.1016/j.semcdb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Eberl M, Engel R, Aberle S, Fisch P, Jomaa H, Pircher H. Human Vgamma9/Vdelta2 effector memory T cells express the killer cell lectin-like receptor G1 (KLRG1) J Leukoc Biol. 2005;77:67–70. doi: 10.1189/jlb.0204096. [DOI] [PubMed] [Google Scholar]
- Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Faint JM, Annels NE, Curnow SJ, Shields P, Pilling D, Hislop AD, Wu L, Akbar AN, Buckley CD, Moss PA, Adams DH, Rickinson AB, Salmon M. Memory T cells constitute a subset of the human CD8+CD45RA+pool with distinct phenotypic and migratory characteristics. J Immunol. 2001;167:212–220. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse GJ, Thrupp LD, Nudleman KL, Wyle FA, Hawkins B, Cesario TC. Bacterial meningitis in the elderly. Arch Intern Med. 1984;144:1603–1607. doi: 10.1001/archinte.144.8.1603. [DOI] [PubMed] [Google Scholar]
- Grundemann C, Bauer M, Schweier O, Oppen N, Lassing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T, Corral L, Vance RE, Raulet DH. 2F1 antigen, the mouse homolog of the rat "mast cell function-associated antigen", is a lectin-like type II transmembrane receptor expressed by natural killer cells. Eur J Immunol. 1998;28:4409–4417. doi: 10.1002/(SICI)1521-4141(199812)28:12<4409::AID-IMMU4409>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunol. 1994;7:31–36. doi: 10.1089/vim.1994.7.31. [DOI] [PubMed] [Google Scholar]
- Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths S, Pircher HP, Soares MV, Akbar AN (2009) KLRG1 signaling induces defective Akt (Ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood (Apr):30. Epub ahead of print [DOI] [PubMed]
- Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Larbi A, Dehovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104:350–360. [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Marcolino I, Przybylski GK, Koschella M, Schmidt CA, Voehringer D, Schlesier M, Pircher H. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur J Immunol. 2004;34:2672–2680. doi: 10.1002/eji.200425282. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/S0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar AN, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Plunkett FJ, Franzese O, Belaramani LL, Fletcher JM, Gilmour KC, Sharifi R, Khan N, Hislop AD, Cara A, Salmon M, Gaspar HB, Rustin MH, Webster D, Akbar AN. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech Ageing Dev. 2005;126:855–865. doi: 10.1016/j.mad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson AD, Akbar AN. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- Rosshart S, Hofmann M, Schweier O, Pfaff AK, Yoshimoto K, Takeuchi T, Molnar E, Schamel WW, Pircher H. Interaction of KLRG1 with E-cadherin: new functional and structural insights. Eur J Immunol. 2008;38:3354–3364. doi: 10.1002/eji.200838690. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Schwartzkopff S, Grundemann C, Schweier O, Rosshart S, Karjalainen KE, Becker KF, Pircher H. Tumor-associated E-cadherin mutations affect binding to the killer cell lectin-like receptor G1 in humans. J Immunol. 2007;179:1022–1029. doi: 10.4049/jimmunol.179.2.1022. [DOI] [PubMed] [Google Scholar]
- Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- Sprenger MJ, Mulder PG, Beyer WE, Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol. 1993;22:334–340. doi: 10.1093/ije/22.2.334. [DOI] [PubMed] [Google Scholar]
- Stepanova L, Naykhin A, Kolmskog C, Jonson G, Barantceva I, Bichurina M, Kubar O, Linde A. The humoral response to live and inactivated influenza vaccines administered alone and in combination to young adults and elderly. J Clin Virol. 2002;24:193–201. doi: 10.1016/S1386-6532(01)00246-3. [DOI] [PubMed] [Google Scholar]
- Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int Immunol. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific "effector memory" (CD28(-) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- Wick G, Jansen-Durr P, Berger P, Blasko I, Grubeck-Loebenstein B. Diseases of aging. Vaccine. 2000;18:1567–1583. doi: 10.1016/S0264-410X(99)00489-2. [DOI] [PubMed] [Google Scholar]
- Xu R, Abramson J, Fridkin M, Pecht I. SH2 domain-containing inositol polyphosphate 5′-phosphatase is the main mediator of the inhibitory action of the mast cell function-associated antigen. J Immunol. 2001;167:6394–6402. doi: 10.4049/jimmunol.167.11.6394. [DOI] [PubMed] [Google Scholar]
- Yoshikawa TT. Perspective: aging and infectious diseases: past, present, and future. J Infect Dis. 1997;176:1053–1057. doi: 10.1086/516547. [DOI] [PubMed] [Google Scholar]