Abstract
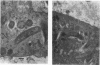
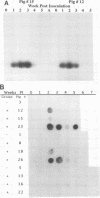
The relationship between Ileal symbiont (IS) intracellularis, formerly known as a Campylobacter-like organism, and porcine proliferative enteritis (PE) was studied by use of pigs with experimentally transmitted PE. Twenty one pigs were experimentally inoculated with homogenized ileal mucosa from a pig that died with PE, and 7 were maintained as uninoculated controls. Fecal samples were collected, and pigs were necropsied weekly postinoculation. Light microscopy and electron microscopy were used to examine tissues for lesions of PE and infectious agents. DNA was extracted from the fecal samples and assayed for the presence of sequences specific for IS intracellularis by dot blot hybridization and polymerase chain reaction amplification. IS intracellularis was detected by the polymerase chain reaction in the feces of 20 of 21 inoculated pigs but not in the feces of uninoculated pigs. Seven inoculated pigs but no uninoculated pigs were detected shedding IS intracellularis by dot blot hybridization. Shedding was detected 1 to 5 weeks after inoculation, and clinical signs were seen in the second to fifth weeks after inoculation. Few pigs without lesions of PE were found to shed IS intracellularis. There was a highly significant association between the presence of IS intracellularis in feces or tissue and the presence of microscopic proliferative lesions and between the severity of the lesions of PE and the percentage of IS intracellularis-infected intestinal crypts. Pigs that ceased shedding IS intracellularis were significantly less likely to have proliferative lesions. These and previous reports are consistent with the hypothesis that IS intracellularis is a necessary causative agent of PE.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton M. R., Borland R., Coloe P. J. Experimental reproduction of porcine proliferative enteritis. J Comp Pathol. 1992 Feb;106(2):159–167. doi: 10.1016/0021-9975(92)90045-v. [DOI] [PubMed] [Google Scholar]
- Anderson W. I., Parchman M. B., Cline J. M., Flanders J. A., Harvey H. J., King J. M. Nasal cavernous haemangioma in an American short haired cat. Vet Rec. 1989 Jan 14;124(2):41–41. doi: 10.1136/vr.124.2.41. [DOI] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boosinger T. R., Thacker H. L., Armstrong C. H. Campylobacter sputorum subsp mucosalis and Campylobacter hyointestinalis infections in the intestine of gnotobiotic pigs. Am J Vet Res. 1985 Oct;46(10):2152–2156. [PubMed] [Google Scholar]
- Chang K., Kurtz H. J., Ward G. E., Gebhart C. J. Immunofluorescent demonstration of Campylobacter hyointestinalis and Campylobacter sputorum subsp mucosalis in swine intestines with lesions of proliferative enteritis. Am J Vet Res. 1984 Apr;45(4):703–710. [PubMed] [Google Scholar]
- Chu R. M., Hong C. B. Haemorrhagic Bowel Syndrome in pigs in Taiwan. Vet Rec. 1973 Nov 24;93(21):562–563. doi: 10.1136/vr.93.21.562. [DOI] [PubMed] [Google Scholar]
- Dodd D. C. Adenomatous intestinal hyperplasia (proliferative ileitis) of swine. Pathol Vet. 1968;5(4):333–341. doi: 10.1177/030098586800500404. [DOI] [PubMed] [Google Scholar]
- Evans A. S. Causation and disease: a chronological journey. The Thomas Parran Lecture. Am J Epidemiol. 1978 Oct;108(4):249–258. doi: 10.1093/oxfordjournals.aje.a112617. [DOI] [PubMed] [Google Scholar]
- Gebhart C. J., Barns S. M., McOrist S., Lin G. F., Lawson G. H. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993 Jul;43(3):533–538. doi: 10.1099/00207713-43-3-533. [DOI] [PubMed] [Google Scholar]
- Gebhart C. J., Lin G. F., McOrist S. M., Lawson G. H., Murtaugh M. P. Cloned DNA probes specific for the intracellular Campylobacter-like organism of porcine proliferative enteritis. J Clin Microbiol. 1991 May;29(5):1011–1015. doi: 10.1128/jcm.29.5.1011-1015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne S. R., Howell J. M., Gawthorne J. M. Intravenous administration of thiomolybdate for the prevention and treatment of chronic copper poisoning in sheep. Br J Nutr. 1981 Nov;46(3):457–467. doi: 10.1079/bjn19810054. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Johnson E. A. Transmissible ileal hyperplasia. Adv Exp Med Biol. 1981;134:267–289. doi: 10.1007/978-1-4757-0495-2_25. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Jacoby R. O. Transmissible ileal hyperplasia of hamsters. II. Ultrastructure. Am J Pathol. 1978 Jun;91(3):451–468. [PMC free article] [PubMed] [Google Scholar]
- Jones G. F., Ward G. E., Collins J. E., Gebhart C. J. Transmission of proliferative enteritis to swine by use of embryonating chicken eggs. Am J Vet Res. 1993 Aug;54(8):1256–1261. [PubMed] [Google Scholar]
- Jones G. F., Ward G. E., Gebhart C. J., Murtaugh M. P., Collins J. E. Use of a DNA probe to detect the intracellular organism of proliferative enteritis in swine feces. Am J Vet Res. 1993 Oct;54(10):1585–1590. [PubMed] [Google Scholar]
- Jones G. F., Ward G. E., Murtaugh M. P., Lin G., Gebhart C. J. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993 Oct;31(10):2611–2615. doi: 10.1128/jcm.31.10.2611-2615.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson L., Martinsson K. Regional ileitis in pigs: morphological and pathogenetical aspects. Acta Vet Scand. 1976;17(2):223–232. doi: 10.1186/BF03547930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. H., McOrist S., Rowland A. C., McCartney E., Roberts L. Serological diagnosis of the porcine proliferative enteropathies: implications for aetiology and epidemiology. Vet Rec. 1988 Jun 4;122(23):554–557. doi: 10.1136/vr.122.23.554. [DOI] [PubMed] [Google Scholar]
- Lawson G. H., Roberts L., McCartney E., Rowland A. C., Luckins A. G. Presence of serum agglutinins to Campylobacter sputorum subspecies mucosalis in pigs. Res Vet Sci. 1982 Jan;32(1):89–94. [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C. Intestinal adenomatosis in the pig: a bacteriological study. Res Vet Sci. 1974 Nov;17(3):331–336. [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C., MacIntyre N. Demonstration of a new intracellular antigen in porcine intestinal adenomatosis and hamster proliferative ileitis. Vet Microbiol. 1985 Jun;10(4):303–313. doi: 10.1016/0378-1135(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Lomax L. G., Glock R. D., Harris D. L., Hogan J. E. Porcine proliferative enteritis: experimentally induced disease in cesarean-derived colostrum-deprived pigs. Am J Vet Res. 1982 Sep;43(9):1622–1630. [PubMed] [Google Scholar]
- Lomax L. G., Glock R. D. Naturally occurring porcine proliferative enteritis: pathologic and bacteriologic findings. Am J Vet Res. 1982 Sep;43(9):1608–1614. [PubMed] [Google Scholar]
- Love R. J., Love D. N., Edwards M. J. Proliferative haemorrhagic enteropathy in pigs. Vet Rec. 1977 Jan 22;100(4):65–68. doi: 10.1136/vr.100.4.65. [DOI] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A., Glock R. D. Investigations into the aetiology of porcine proliferative enteritis. Vet Rec. 1987 Jul 25;121(4):86–86. doi: 10.1136/vr.121.4.86. [DOI] [PubMed] [Google Scholar]
- McOrist S., Boid R., Lawson G. H. Antigenic analysis of Campylobacter species and an intracellular Campylobacter-like organism associated with porcine proliferative enteropathies. Infect Immun. 1989 Mar;57(3):957–962. doi: 10.1128/iai.57.3.957-962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOrist S., Lawson G. H. Possible relationship of proliferative enteritis in pigs and hamsters. Vet Microbiol. 1987 Dec;15(4):293–302. doi: 10.1016/0378-1135(87)90017-4. [DOI] [PubMed] [Google Scholar]
- McOrist S., Lawson G. H. Reproduction of proliferative enteritis in gnotobiotic pigs. Res Vet Sci. 1989 Jan;46(1):27–33. [PubMed] [Google Scholar]
- McOrist S., Lawson G. H., Rowland A. C., MacIntyre N. Early lesions of proliferative enteritis in pigs and hamsters. Vet Pathol. 1989 May;26(3):260–264. doi: 10.1177/030098588902600311. [DOI] [PubMed] [Google Scholar]
- McOrist S., Lawson G. H., Roy D. J., Boid R. DNA analysis of intracellular Campylobacter-like organisms associated with the porcine proliferative enteropathies: novel organism proposed. FEMS Microbiol Lett. 1990 Jun 1;57(3):189–193. doi: 10.1016/0378-1097(90)90063-v. [DOI] [PubMed] [Google Scholar]
- McOrist S., MacIntyre N., Stokes C. R., Lawson G. H. Immunocytological responses in porcine proliferative enteropathies. Infect Immun. 1992 Oct;60(10):4184–4191. doi: 10.1128/iai.60.10.4184-4191.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L., Lawson G. H., Rowland A. C., Laing A. H. Porcine intestinal adenomatosis and its detection in a closed pig herd. Vet Rec. 1979 Apr 21;104(16):366–368. doi: 10.1136/vr.104.16.366. [DOI] [PubMed] [Google Scholar]
- Roberts L., Rowland A. C., Lawson G. H. Experimental reproduction of porcine intestinal adenomatosis and necrotic enteritis. Vet Rec. 1977 Jan 1;100(1):12–13. doi: 10.1136/vr.100.1.12. [DOI] [PubMed] [Google Scholar]
- Rowland A. C., Lawson G. H. Intestinal adenomatosis in the pig: immunofluorescent and electron microscopic studies. Res Vet Sci. 1974 Nov;17(3):323–330. [PubMed] [Google Scholar]
- Rowland A. C., Lawson G. H., Maxwell A. Intestinal adenomatosis in the pig: occurrence of a bacterium in affected cells. Nature. 1973 Jun 15;243(5407):417–417. doi: 10.1038/243417a0. [DOI] [PubMed] [Google Scholar]
- Rowland A. C., Lawson G. H., Roberts L. Intestinal adenomatosis in the pig: histochemical and electron microscopic studies of the mucosa. Res Vet Sci. 1978 Mar;24(2):191–199. [PubMed] [Google Scholar]
- Rowland A. C., Rowntree P. G. A haemorrhagic bowel syndrome associated with intestinal adenomatosis in the pig. Vet Rec. 1972 Sep 2;91(10):235–241. doi: 10.1136/vr.91.10.235. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y., Bueno L., Fioramonti J. Constituants alimentaires et motricité digestive. Reprod Nutr Dev. 1981;21(5B):749–771. [PubMed] [Google Scholar]
- Stills H. F., Jr Isolation of an intracellular bacterium from hamsters (Mesocricetus auratus) with proliferative ileitis and reproduction of the disease with a pure culture. Infect Immun. 1991 Sep;59(9):3227–3236. doi: 10.1128/iai.59.9.3227-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]