Abstract
This study utilized a full-factorial design to investigate the effect of four factors: presence of whole bone marrow cells, presence of in vitro-generated mineralized extracellular matrix (ECM), presence of dexamethasone, and variations in culture duration, on the proliferation and osteogenic differentiation of mesenchymal stem cells (MSCs) cultured on a polymer scaffold. Electrospun poly(ɛ-caprolactone) (PCL) fiber mesh scaffolds were seeded with rat MSCs and cultured in complete osteogenic medium for 12 days to generate constructs containing mineralized ECM. MSCs or MSCs and whole bone marrow cells were seeded onto decellularized ECM constructs (PCL/ECM) or plain PCL scaffolds and cultured statically for 4, 8, and 16 days in medium either with or without dexamethasone. After each culture period, the cell number was determined by DNA analysis, and the osteogenic differentiation state of the cells was determined by alkaline phosphatase activity and calcium assays. MSCs seeded onto PCL/ECM constructs and cultured in medium either with or without dexamethasone demonstrated similar amounts of calcium deposition after 16 days. A significant increase in cell number over time compared with all other groups was observed when whole bone marrow cells were cocultured with MSCs on PCL scaffolds in medium without dexamethasone. This study establishes that the osteogenic differentiation of MSCs seeded onto ECM-containing constructs is maintained even in the absence of dexamethasone and that the coculture of MSCs and whole bone marrow cells without dexamethasone and ECM enhances the proliferation of a cell population (or populations) present in the whole bone marrow.
Introduction
Replacement of diseased or compromised bone tissue is often accomplished clinically with autologous bone grafts because of their osteogenic, osteoconductive, and osteo-inductive capabilities.1–5 However, drawbacks such as donor site morbidity, persistent severe pain, and limited availability are commonly associated with autograft bone.3,4,6,7 Alternatives to autologous bone grafts are being investigated, including polymers, collagen sponges, ceramics, and metals.8–11 To investigate the osteogenicity of these potential bone graft materials, osteoprogenitor cells are cultured in vitro on scaffolds generated from the graft material and are analyzed for their effect on the osteogenic differentiation and proliferation of the seeded osteoprogenitor cells.12–15 Research in our laboratory has previously demonstrated that a titanium mesh and extracellular matrix (ECM) composite scaffold was conducive to the osteogenic differentiation and proliferation of mesenchymal stem cells (MSCs), an osteoprogenitor cell population.16,17
In the bone marrow cavity, osteoprogenitor cells are in contact with other bone marrow cell populations, mineralized ECM, and biological factors.18–21 The coculture of MSCs with other bone marrow cell populations, including vascular endothelial cells, hematopoietic stem cells, hematopoietic progenitor cells, and osteoblasts, has been investigated for its effect on the osteogenic differentiation of MSCs.22–25 As well, culturing of MSCs on mineralized ECM16,26,27 and with a variety of growth factors including transforming growth factor-β1, fibroblast growth factor-2, and bone morphogenetic protein-21,28–30 has been explored for their effect on the proliferation and osteogenic differentiation of MSCs. Further, the supplementation of the culture medium with the corticosteroid dexamethasone has been investigated for its necessity in promoting the osteogenic differentiation of osteoprogenitor cells cultured in vitro.31,32 However, the coculture of other bone marrow cell populations with MSCs in a three-dimensional mineralized ECM and with dexamethasone medium supplementation has not been explored.
To address this issue, we utilized a full-factorial study design by culturing MSCs in a three-dimensional poly(ɛ-caprolactone) (PCL) scaffold in the presence of four factors: (i) whole bone marrow cells, (ii) in vitro-generated mineralized ECM, (iii) dexamethasone, and (iv) variations in culture duration. The focus of this study was to elucidate the effects and potential interactions of the four factors on the proliferation and osteogenic differentiation of MSCs in a three-dimensional environment.
Methods and Materials
Fabrication of PCL scaffolds
PCL (MW = 80,000; Sigma, St. Louis, MO) was dissolved in a 5:1 (vol/vol) chloroform:methanol solution at 12 wt% (wt/wt). The PCL solution was electrospun as previously described to fabricate fiber mesh mats that were approximately 1 mm thick, with approximately 5 μm average fiber diameters.33 PCL scaffolds were prepared by die-punching 8-mm-diameter discs from the electrospun mats. The scaffolds were then sterilized by exposure to ethylene oxide (Andersen Sterilizers, Haw River, NC) for 14 h, followed by aseptic aeration in a laminar flow cell culture hood, prewetted 1 day before cell seeding using an ethanol gradient series, and stored in sterile-filtered (0.22 μm) Millipore water until use.
MSC isolation
MSCs were harvested and pooled from the marrow of tibiae and femora of five male Fischer 344 rats (126–150 g; Charles River Laboratories, Wilmington, MA) as previously described.32 Care of the rats in this study was given in accordance with the Rice University Institutional Animal Care and Use Committee. Briefly, rats were anesthetized using 4% isofluorane (Baxter Healthcare, Deerfield, IL) in O2 and subsequently euthanized by CO2 asphyxiation followed by a bilateral thoracotomy to ensure death. The tibiae and femora were aseptically removed and placed in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Cambrex BioScience, Walkersville, MD) and 3% penicillin–streptomycin–fungizone (Invitrogen). The epiphyses were cut, the diaphyses were pierced with a sterile 16-gauge needle, and the marrow was flushed out with 5 mL of complete osteogenic medium containing minimum essential medium Eagle-alpha modification (Invitrogen), 10% FBS (Cambrex BioScience), 10 mM β-glycerol-2-phosphate, 10 nM dexamethasone, 50 μg/mL ascorbic acid, 50 μg/mL gentamicin, 100 μg/mL ampicillin, and 0.5 μg/mL fungizone (Sigma). The collected marrow pellets were broken up by trituration, and the cell suspension was plated in 75-cm2 tissue culture flasks and cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 until confluence. The medium was aspirated after 1 day to remove the nonadherent cell population and was replaced with complete osteogenic medium. Subsequent medium exchanges were performed every 2 days. In this study, we designated the adherent cell population as “MSCs,” given the established osteogenic potential of these cells under proper culture conditions.34 When the cells reached confluence, they were lifted using 2 mL of 0.25% (vol/vol) trypsin solution (Sigma), centrifuged at 1000 rpm for 10 min, and resuspended in complete osteogenic medium at a concentration of 1.25 million cells/mL.
Generation of PCL/ECM scaffolds
Before cell seeding, prewetted PCL scaffolds were transferred into complete osteogenic medium for 2 h, press-fit into poly(methyl methacrylate) (PMMA) cassettes, and maintained in the incubator. A quarter million of the isolated MSCs in 200 μL of complete osteogenic medium were seeded onto each PCL scaffold, and the MSCs were allowed to adhere to the scaffold for 2 h in the incubator. The scaffolds were then removed from their cassettes and placed in individual wells of a 12-well tissue culture polystyrene plate with 3 mL of complete osteogenic medium per well, which was exchanged every 2 days. At day 12, the scaffolds were collected and individually placed into 1.5 mL of sterile-filtered Millipore water. The MSCs that produced the in vitro-generated ECM in the PCL scaffolds were then removed by a previously described decellularization process, which involved three cycles of freezing in liquid N2 and thawing in a 37°C water bath, followed by 10 min of ultrasonication.16,17 The resulting acellular PCL/ECM scaffolds were then aseptically dried in a laminar air flow cell culture hood and sterilized by exposure to ethylene oxide (14 h) for later use.
Four-factor experimental design
The groups investigated in this study are given in Table 1. For the acellular scaffold group, six acellular PCL/ECM scaffolds per culture duration were press-fit into PMMA cassettes, incubated with 200 μL of complete osteogenic medium (+media) for 2 h, then removed to a fresh 12-well tissue culture polystyrene plate with 2 mL of +media per well. For the groups seeded with MSCs alone, six acellular PCL and six acellular PCL/ECM scaffolds per culture duration were press-fit into PMMA cassettes and seeded with 250,000 MSCs in 200 μL of either +media or complete osteogenic medium without dexamethasone (−media). The cells were allowed to adhere for 2 h in the incubator, and the scaffolds were removed to 12-well tissue culture polystyrene plates with 2 mL of either +media or −media per well. For the groups seeded with both MSCs and whole bone marrow, six acellular PCL and six acellular PCL/ECM scaffolds per culture duration were seeded with MSCs as stated above, but were left in the PMMA cassettes for 1 day in a 12-well tissue culture polystyrene plate with 2 mL of either +media or −media per well and were incubated in a humidified incubator. The following day, whole bone marrow was isolated from rat tibiae and femora using +media or –media and triturated, and then 250,000 cells from the whole bone marrow suspension in 200 μL of +media or −media were seeded onto the MSC-seeded PCL and PCL/ECM scaffolds. The cells were allowed to attach for 2 h, and the scaffolds were removed to fresh 12-well tissue culture polystyrene plates with 2 mL of either +media or −media per well. The approximate number of MSCs, defined as the adherent cell population in whole bone marrow, present within the whole bone marrow suspension was determined by counting the number of cells attached to one tissue culture flask after 4 h of incubation from a pool of 10,000,000 seeded whole bone marrow cells. After averaging the results from six flasks, approximately one MSC was observed to be attached for every 1000 plated whole bone marrow cells (data not shown), thus approximately 250 MSCs were present in the 250,000 marrow cells that were seeded onto the respective scaffolds.
Table 1.
Names of the Groups Investigated in This Study
| Group names | Cells | Scaffolds | Media | Duration |
|---|---|---|---|---|
| Acellular | No cells | PCL/ECM | With dexamethasone (+media) | 4 days 8 days 16 days |
| PCL MSCs+ | 250,000 MSCs | PCL | With dexamethasone (+media) | 4 days 8 days 16 days |
| PCL coculture+ | 250,000 MSCs 250,000 marrow cells | PCL | With dexamethasone (+media) | 4 days 8 days 16 days |
| PCL/ECM MSCs+ | 250,000 MSCs | PCL/ECM | With dexamethasone (+media) | 4 days 8 days 16 days |
| PCL/ECM coculture+ | 250,000 MSCs 250,000 marrow cells | PCL/ECM | With dexamethasone (−media) | 4 days 8 days 16 days |
| PCL MSCs− | 250,000 MSCs | PCL | Without dexamethasone (−media) | 4 days 8 days 16 days |
| PCL coculture− | 250,000 MSCs 250,000 marrow cells | PCL | Without dexamethasone (−media) | 4 days 8 days 16 days |
| PCL/ECM MSCs− | 250,000 MSCs | PCL/ECM | Without dexamethasone (−media) | 4 days 8 days 16 days |
| PCL/ECM coculture− | 250,000 MSCs 250,000 marrow cells | PCL/ECM | Without dexamethasone (−media) | 4 days 8 days 16 days |
The acellular group was taken as the control. Each of the other groups represents one combination of the four factors: cells seeded, scaffold type, media composition, and duration.
PCL, poly(ɛ-caprolactone); MSC, mesenchymal stem cell; ECM, extracellular matrix.
Osteogenic differentiation assays
At the 4, 8, and 16-day culture durations, four scaffolds from each group were individually placed into 1.5 mL of sterile-filtered Millipore water and frozen at −20°C for later analysis. Each scaffold underwent three cycles of freezing and thawing followed by ultrasonication to lyse the cells and was assayed for cellularity, alkaline phosphatase (ALP) activity, and calcium content. The cellularity of the seeded scaffolds was determined with the PicoGreen assay kit (Molecular Probes, Eugene, OR). Briefly, 50 μL of cell lysate solution, 100 μL of Tris-ethylenediaminetetraacetic acid buffer, and 150 μL of PicoGreen dye buffer were pipetted into an opaque 96-well plate, with each sample performed in triplicate, and allowed to incubate at room temperature for 10 min in the dark. The excitation of the solution at 485 nm and fluorescence measurement at 528 nm was performed using a FL X800 microplate spectrophotometer (Bio-Tek Instruments, Winooski, VT). A conversion factor of 6.4 pg of DNA per cell was used to calculate cellularity and was based on DNA extracted from known numbers of MSCs (data not shown). The ALP activity was measured using 1.5 M alkaline buffer solution and phosphatase substrate capsules (Sigma) and compared with dilutions of a 10 mM p-nitrophenol standard solution (Sigma). Briefly, 80 μL of the cell lysate solution, 100 μL of the substrate solution, and 20 μL of the buffer solution were added to a transparent 96-well plate, with each sample performed in triplicate, and allowed to incubate at 37°C for 1 h. The reaction was then stopped with 100 μL of 0.3 NaOH, and the absorbance was measured at 405 nm on a PowerWave X340 microplate spectrophotometer (Bio-Tek Instruments). After determining the cellularity and ALP activity, a volume of 1 N acetic acid equal to the volume remaining in each sample tube was added to each cell lysate solution and scaffold. The resulting 0.5 N acetic acid/cell lysate solution was placed on a shaker table for 1 day at 100 rpm to dissolve calcium present in the scaffold. The calcium content of the scaffolds was then determined by adding 20 μL of the acetic acid/cell lysate solution and 300 μL of calcium assay reagent containing Arsenazo III (Diagnostics Chemicals Limited, Prince Edward Island, Canada) to a transparent 96-well plate, with each sample performed in triplicate. The absorbance was then measured at 650 nm on a PowerWave X340 microplate spectrophotometer (Bio-Tek Instruments).
Scanning electron microscopy
One acellular PCL scaffold and one acellular PCL/ECM construct were fixed with 2.5% glutaraldehyde solution (Sigma) at room temperature for 2 h and rinsed three times in phosphate-buffered saline (Invitrogen). The scaffolds were then dehydrated in an ethanol gradient series, air dried in a laminar air flow cell culture hood, lyophilized, and sputter coated with gold before imaging. Each scaffold was imaged using a FEI Quanta 400 ESEM FEG (FEI Company, Hillsboro, OR) at 500× magnification in the center of each scaffold.
Statistical analysis
Results are presented as means ± standard deviations. Statistical significance was determined using Tukey's Honestly Significant Differences test with a 95% confidence interval and JMP IN 5.1 software (SAS Institute, Cary, NC); global effects were determined using four-factor analysis of variance with SAS system software (SAS Institute).
Results
Global factor effects
Table 2 demonstrates the significance of each of the global factors. The seeding of whole bone marrow cells with MSCs onto the scaffolds was found to have a significant effect (p < 0.05) only in the case of the overall cellularity of the scaffolds. The presence of an ECM in the PCL scaffolds and the presence of dexamethasone each resulted in a significant effect (p < 0.05) upon ALP activity and calcium deposition onto the scaffolds, although no significance was observed for cellularity in either case. The culture duration had a significant effect (p < 0.05) upon calcium deposition onto the scaffolds.
Table 2.
Global Effect of the Four Factors Investigated in This Study
| Factor comparison | Cellularity | ALP/DNA | Calcium deposition |
|---|---|---|---|
| No cells versus MSCs | p < 0.05 | p < 0.05 | p < 0.05 |
| No cells versus coculture | p < 0.05 | p < 0.05 | p < 0.05 |
| MSCs versus coculture | p < 0.05 | NS | NS |
| PCL versus PCL/ECM | NS | p < 0.05 | p < 0.05 |
| +Media versus −media | NS | p < 0.05 | p < 0.05 |
| Day 4 versus day 8 | NS | p < 0.05 | p < 0.05 |
| Day 4 versus day 16 | NS | NS | p < 0.05 |
| Day 8 versus day 16 | p < 0.05 | p < 0.05 | p < 0.05 |
Significance levels were determined by using four-factor analysis of variance and Turkey's Honestly Significant Differences with the SAS system software. Not significant is abbreviated as NS.
Cellularity of the scaffolds
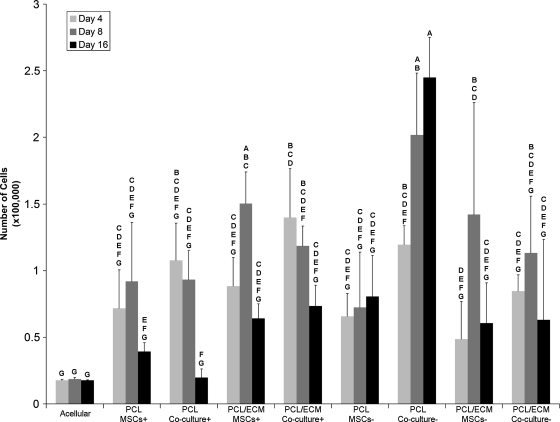
Table 1 clarifies the abbreviations for the groups investigated in this study. As can be seen in Figure 1, there was a trend for the cellularity of the scaffolds to increase at day 8 and then to decrease by day 16 for four groups: PCL MSCs+, PCL/ECM MSCs+, PCL/ECM MSCs−, and PCL/ECM coculture−. In addition, there was a trend for the cellularity to decrease with time for the PCL coculture+ and PCL/ECM coculture+ groups. However, the PCL coculture− group demonstrated a significant increase (p < 0.05) in cellularity at day 16 when compared with day 4, unlike any other group investigated.
FIG. 1.
Cellularity of scaffolds seeded with MSCs or whole bone marrow cells and MSCs. Cell numbers were determined with a PicoGreen assay kit and are represented as mean ± standard deviation with n = 4. Groups not connected by the same letter are significantly different (p < 0.05). MSC, mesenchymal stem cell; PCL, poly(ɛ-caprolactone); ECM, extracellular matrix.
ALP activity
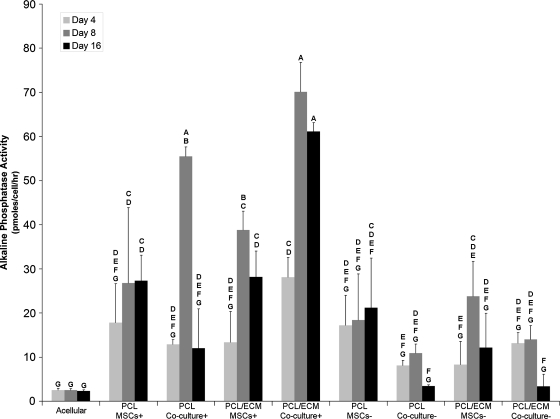
As illustrated in Figure 2, a significantly greater (p < 0.05) ALP activity was observed at day 8 for scaffolds cultured in +media when compared with the corresponding groups cultured in −media. The PCL coculture+ and PCL/ECM coculture+ groups demonstrated a significantly greater (p < 0.05) ALP activity than the corresponding groups seeded with MSCs alone at day 8. Additionally, there was a trend for a peak in ALP activity at day 8 for all groups except for the acellular, PCL MSCs+, PCL MSCs−, and PCL/ECM coculture− groups.
FIG. 2.
Alkaline phosphatase activity of scaffolds seeded with MSCs or whole bone marrow cells and MSCs. Alkaline phosphatase activity was determined by absorbance spectroscopy and is represented as mean ± standard deviation with n = 4. Groups not connected by the same letter are significantly different (p < 0.05).
Calcium deposition
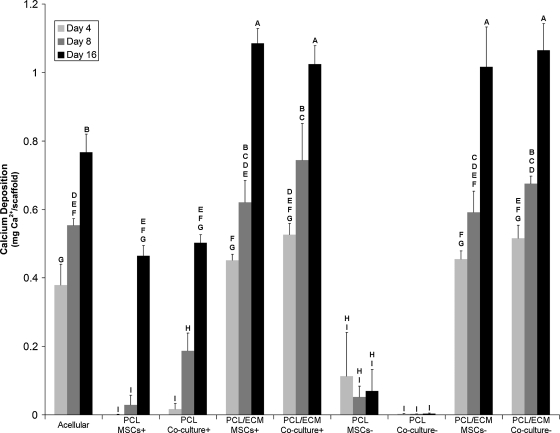
Figure 3 illustrates that the amount of calcium deposited significantly increased (p < 0.05) over time for all groups, excluding the PCL MSCs− and PCL coculture− groups. Both the PCL MSCs− and PCL coculture− groups demonstrated minimal calcium deposition. The calcium deposition at day 16 for the PCL/ECM coculture+ and PCL/ECM coculture− groups were similar to the corresponding groups seeded with MSCs alone. Interestingly, significantly increasing (p < 0.05) calcium deposition was observed over time for the acellular group.
FIG. 3.
Calcium ion amount present on scaffolds seeded with MSCs or whole bone marrow cells and MSCs. Calcium amount was determined by absorbance spectroscopy and is represented as mean ± standard deviation with n = 4. Groups not connected by the same letter are significantly different (p < 0.05).
Scanning electron microscopy
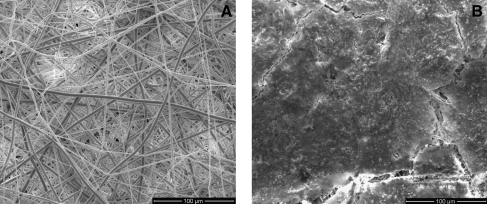
Figure 4 illustrates the in vitro-generated ECM contained within the electrospun PCL after 12 days of culture when compared with plain electrospun PCL scaffolds. Cracks in the surface layer of the dried ECM reveal the electrospun PCL fibers underneath.
FIG. 4.
Scanning electron micrographs of (A) PCL scaffolds and (B) PCL/ECM constructs at a magnification of × 500. Scale bar represents 100 μm.
Discussion
The goal of this study was to determine the effect and interactions of four culture factors: (i) the presence of whole bone marrow cells, (ii) the presence of in vitro-generated mineralized ECM, (iii) the presence of dexamethasone, and (iv) variations in culture duration on the proliferation and osteogenic differentiation of MSCs cultured on an electrospun PCL scaffold. The MSCs used in this study have been characterized in a previous study and adherent marrow cell populations (MSCs) have been widely studied in bone tissue engineering.35–38 Although cell markers were not utilized to directly measure osteogenic differentiation of the MSCs in this study, two well-established and accepted biochemical markers, ALP activity and calcium deposition, were assessed.39,40
Each of the four factors had an effect on cellularity, ALP activity, and calcium deposition of the MSC-seeded scaffolds. Coculture of whole bone marrow cells with the MSCs resulted in a significant global effect on the cellularity of the scaffolds and is attributable to the increased total number of cells seeded onto these scaffolds. The other three factors were found to globally affect the ALP activity of the seeded cells and the calcium deposition onto the scaffolds and are consistent with the results from our previous studies using sintered titanium mesh scaffolds.16,17,32,41
The results for the PCL MSCs+ and PCL/ECM MSCs+ groups are also similar to the results from our previous studies using sintered titanium mesh scaffolds.16,17,32,41 Both groups demonstrated an increase in cellularity at day 8 with a decrease at day 16, a peak in ALP activity at day 8, a trend for greater ALP activity in +media when compared with −media, and a significant increase in calcium deposition over time. The presence of dexamethasone is well known to initiate differentiation of MSCs toward the osteogenic lineage.32,42–45 An early-stage marker of the osteogenic differentiation of MSCs is an increase in ALP activity, whereas later stages are marked by the deposition of calcium phosphate.46 The peak in ALP activity observed at day 8 for the majority of groups investigated reflects that the MSCs have begun to differentiate along the osteogenic lineage, whereas the drop in ALP activity and increase in calcium deposition at day 16 indicate that the MSCs have reached late stages of osteogenic differentiation. Together, the data demonstrate that an in vitro-generated mineralized ECM, medium supplementation with dexamethasone, and longer culture durations encourage osteogenic differentiation of MSCs cultured on electrospun PCL fiber mesh scaffolds, a biodegradable and hydrophobic material, and this result is consistent with MSCs cultured on sintered titanium mesh scaffolds, a nondegradable and hydrophilic material.
An increase in cellularity over time was observed for the PCL coculture− group. This increase in scaffold cellularity can be partially explained by the greater total number of cells seeded than in groups with MSCs alone. However, the increased number of cells over time cannot be fully explained by the higher seeding density. The PCL coculture+ group demonstrated a decrease in the number of cells present, although there was a similar amount of cells present in the scaffold in either media condition at day 4. It is known that dexamethasone reduces the proliferation of MSCs, while increasing their osteogenic differentiation.47 Thus, the absence of dexamethasone in the culture medium for the PCL coculture− group may have allowed the MSCs to proliferate. Another explanation for the observed proliferation of the cells in this group was the low amount of calcium present in the scaffolds from this group at any time point. Low amounts of calcium in the scaffold indicates that the matrix has not mineralized, and thus after cell lysis, the cellular DNA was not trapped within a mineralized matrix surrounding the cell and was likely released in its entirety into the solution. As a result, the complete cellular DNA could be detected during the DNA assay, potentially resulting in a higher measured cell number than the PCL coculture+ group. However, the PCL MSCs− group with its similarly low amount of mineralization did not demonstrate a significant increase in cellularity over time when compared with the PCL coculture− group. These results imply that the absence of dexamethasone in the culture medium allowed for a cell population (or populations) present in the whole bone marrow to proliferate. Indeed, in a paper by Dexter et al., in vitro culture of hematopoietic tissue was accomplished by culturing whole bone marrow cells on a layer of bone marrow-derived adherent cells in Fischer's medium containing only FBS and antibiotics.48 As well, several studies have demonstrated that hematopoietic progenitor cells derived from bone marrow cocultured with a marrow stromal cell line were able to differentiate into T cells and B cells.49–51
Significantly greater ALP activity was observed at day 8 for the PCL coculture+ group versus the PCL MSCs+ group as well as for the PCL/ECM coculture+ group versus the PCL/ECM MSCs+ group. However, this result may be an artifact of the decreasing number of cells over time for the coculture groups, as only the differentiating MSCs are expected to produce ALP. When ALP activity is normalized to μmoles/hour/scaffold, there is no significant difference in ALP activity at day 8 between the PCL MSCs+ and PCL coculture+ groups or between the PCL/ECM MSCs+ and PCL/ECM coculture+ groups (data not shown). This implies that the whole bone marrow cells had no observable effect on the osteogenic differentiation of MSCs seeded onto either type of scaffolds, as was suggested by the global effects. The absence of any observable effect of coculture with whole bone marrow on the osteogenic differentiation of MSCs may be due to the supporting role that MSCs provide. It is known from in vitro experiments that MSCs produce an ECM that is supportive of hematopoeisis.48–51 Thus, instead of whole bone marrow cells affecting the osteogenic differentiation of the MSCs, the MSCs may be promoting hematopoietic engraftment of any hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs) present within the whole bone marrow cell population.
Significantly lower ALP activity was observed for the PCL coculture− and PCL/ECM coculture− groups when compared with the PCL coculture+ and PCL/ECM coculture+ groups. Because of the absence of the osteogenic supplement dexamethasone in the culture medium for the −groups, the MSCs present on the scaffold were not able to differentiate down the osteogenic lineage as efficiently as the +groups.
By day 16, there were no significant differences between the amount of calcium deposition on PCL/ECM constructs seeded with either MSCs alone or MSCs with whole bone marrow cells and cultured in either media. The similar calcium deposition levels suggest that the whole bone marrow cells had an insignificant effect on the late-stage differentiation of the MSCs, as was suggested by the global effects. In addition, the minimal calcium deposition observed for the PCL MSCs− and PCL coculture− groups was expected, as neither dexamethasone nor in vitro-generated ECM was present to induce the cells to differentiate down the osteogenic lineage.
Low cell numbers and a low ALP activity with no change over time were observed for the acellular group. This indicates that no living cells were present on the scaffold, but residual DNA and ALP were retained within the scaffold. Because of the absence of living cells on the scaffold, the increase in calcium deposition over time for acellular PCL/ECM constructs was unanticipated. An increase in calcium deposition was not observed in a previous study with acellular titanium/ECM constructs.16 In this study, it is feasible that any remaining DNA, phospholipid cell fragments, and ALP present on the scaffold could present nucleation sites for calcium deposition, resulting in the increase in calcium observed in this study. However, it is also plausible that a component of the ECM present in the construct induced calcium deposition. A limitation of the calcium assay was the inability to distinguish between calcium resulting from spontaneous deposition from the medium and calcium arising from late-stage osteogenic differentiation of cells. Accordingly, an acellular control group was included in the study and cultured under the same conditions as the groups with cells to provide a baseline to account for any spontaneous calcium deposition that may occur. Specifically, studies with acellular PCL/ECM constructs washed thrice with phosphate-buffered saline, ultrasonicated for 10 min, and subsequently cultured in +media demonstrated a linear increase in calcium deposition over time, whereas plain PCL scaffolds treated similarly showed no calcium deposition (data not shown). The increasing calcium deposition on the acellular PCL/ECM constructs implies that the ECM construct itself is conducive to calcium deposition and may mineralize over time in vivo without cells. Nevertheless, the calcium deposition observed at day 16 on cell-seeded PCL/ECM constructs was significantly higher when compared with acellular constructs. Thus, seeding MSCs onto the ECM constructs will be an integral part of future investigations of bone formation in vivo.
Significantly lower calcium deposition at all time points was observed for the PCL MSCs+ and PCL coculture+ groups when compared with the PCL/ECM MSCs+, PCL/ECM coculture+, PCL/ECM MSCs−, and PCL/ECM coculture− groups. This was a result of the PCL/ECM constructs being generated by osteogenically differentiated MSCs with 12 days of culture before decellularization. After 12 days, the deposited matrix on the construct had already started to mineralize, and thus each PCL/ECM construct contained approximately 0.4 mg of Ca2+ (data not shown) at the beginning of the second culture period, whereas the PCL scaffolds alone had none.
Calcium deposition at each of the culture durations was found to be similar when seeded PCL/ECM constructs were cultured in either +media or −media. However, previous results from our laboratory have shown that when MSCs were seeded onto titanium/ECM constructs in −media, there was little calcium deposited onto the constructs.16 Before seeding the MSCs onto the PCL/ECM constructs, isolation of the MSCs was performed by plating the whole bone marrow cells onto a tissue culture flask in +media and culturing the adherent cells until confluence, approximately 6 to 7 days, whereas the MSCs seeded onto the titanium/ECM constructs were isolated and expanded in −media for 6 days. This implies that the expansion of MSCs in medium containing dexamethasone may be sufficient to direct the MSCs toward the osteogenic lineage. However, to maintain the osteogenic differentiation of the MSCs, either continued exposure to dexamethasone or an in vitro-generated ECM is necessary. This is supported by the significant increase in calcium deposition observed over time for the PCL MSCs+ and PCL/ECM MSCs− groups, whereas no significant difference in calcium deposition over time was observed for the PCL MSCs− group. The sustained osteogenic differentiation of MSCs may be due to the retention of growth factors, secreted during the generation of the ECM construct, within the ECM present on the construct.
The osteogenic differentiation of the MSCs observed in the PCL/ECM MSCs− group has implications for future in vivo experiments. Continuous in vivo delivery of the corticosteroid dexamethasone, which is not naturally present in the body, is known to result in delayed wound healing, increased risk for infection, and diabetes mellitus, along with other side effects.52,53 Thus, it is desirable to avoid the in vivo delivery of dexamethasone to cell-seeded scaffold implants. The results from this study imply that when an in vitro-generated ECM is present in the scaffold, it may not be necessary to deliver an osteogenic medium supplement such as dexamethasone in vivo to maintain MSC differentiation down the osteogenic lineage.
The results of this study demonstrate that the presence of an ECM and dexamethasone are significant factors for the enhancement of the osteogenic differentiation of MSCs cultured in vitro in a three-dimensional environment. Indeed, the in vitro-generated ECM presents a level of biological activity sufficient to sustain the osteogenic differentiation of cells in the absence of exogenous osteogenic molecules, such as dexamethasone, and marks an innovative strategy for delivery of bioactive factors in the form of an ECM produced by cells under engineered conditions. The characterization of the protein and mineral components present within the in vitro-generated ECM is important for understanding the proteins necessary to maintain the osteogenic differentiation of MSCs and will be the subject of future studies. Although gene expression analysis was beyond the scope of this study, it may be applied in future investigations of focused groups to complement the insights gained from this study.
Conclusion
The goal of this study was to determine the effect of four factors on the osteogenic differentiation and proliferation of MSCs. Three factors, the presence of an in vitro-generated ECM contained within the electrospun PCL scaffolds, the presence of dexamethasone in the culture medium, and variations in the duration of culture, were significant with respect to the osteogenic differentiation of MSCs. However, the coculture of whole bone marrow cells with MSCs did not significantly influence the osteogenic differentiation of the MSCs under the conditions tested. This study establishes that the isolation and expansion of MSCs in medium containing the osteogenic supplement dexamethasone initiates the osteogenic differentiation of the MSCs, and subsequent culture upon constructs containing an in vitro-generated ECM, even without dexamethasone in the culture medium, sustains the osteogenic differentiation of cells. Additionally, this study suggests that the coculture of MSCs and whole bone marrow cells without dexamethasone or in vitro-generated ECM enhanced the proliferation of either MSCs or another cell population (or populations) present within the whole bone marrow. The elucidation of the effects and interactions of the four factors illuminates the necessary conditions for maintaining the osteogenic differentiation and proliferation of MSCs within a three-dimensional environment. Overall, the results demonstrate that engineered culture conditions can be applied to produce an ECM construct with sufficient biological activity to sustain the osteogenic differentiation of cells, even in the absence of dexamethasone, which may enable maintenance of osteogenic differentiation of MSCs transplanted with the constructs and/or osteogenically stimulated host cells without a need for exogenous drug delivery.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 AR057083), a grant from the Bioengineering Research Partnership with the Baylor College of Medicine through the National Institute of Biomedical Imaging and Bioengineering (R01 EB005173), and a Ruth L. Kirschstein National Research Service Award (F31 AR055874) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Disclosure Statement
No competing financial interests exist. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
References
- 1.Bauer T.W. Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10. [PubMed] [Google Scholar]
- 2.Mulliken J.B. Glowacki J. Kaban L.B. Folkman J. Murray J.E. Use of demineralized allogeneic bone implants for the correction of maxillocraniofacial deformities. Ann Surg. 1981;194:366. doi: 10.1097/00000658-198109000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Long W.G., Jr. Einhorn T.A. Koval K. McKee M. Smith W. Sanders R. Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89:649. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 4.Huffer W.E. Benedict J.J. Turner A.S. Briest A. Rettenmaier R. Springer M. Walboomers X.F. Repair of sheep long bone cortical defects filled with COLLOSS, COLLOSS E, OSSAPLAST, and fresh iliac crest autograft. J Biomed Mater Res B Appl Biomater. 2007;82:460. doi: 10.1002/jbm.b.30751. [DOI] [PubMed] [Google Scholar]
- 5.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Ahlmann E. Patzakis M. Roidis N. Shepherd L. Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A:716. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Sasso R.C. Williams J.I. Dimasi N. Meyer P.R., Jr. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J Bone Joint Surg Am. 1998;80:631. doi: 10.2106/00004623-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Giannoudis P.V. Dinopoulos H. Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Hak D.J. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg. 2007;15:525. doi: 10.5435/00124635-200709000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Rah D.K. Art of replacing craniofacial bone defects. Yonsei Med J. 2000;41:756. doi: 10.3349/ymj.2000.41.6.756. [DOI] [PubMed] [Google Scholar]
- 11.Artico M. Ferrante L. Pastore F.S. Ramundo E.O. Cantarelli D. Scopelliti D. Iannetti G. Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol. 2003;60:71. doi: 10.1016/s0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 12.Arpornmaeklong P. Pripatnanont P. Suwatwirote N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int J Oral Maxillofac Surg. 2008;37:357. doi: 10.1016/j.ijom.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Leong N.L. Jiang J. Lu H.H. Polymer-ceramic composite scaffold induces osteogenic differentiation of human mesenchymal stem cells. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2651. doi: 10.1109/IEMBS.2006.259459. [DOI] [PubMed] [Google Scholar]
- 14.Takamoto T. Hiraoka Y. Tabata Y. Enhanced proliferation and osteogenic differentiation of rat mesenchymal stem cells in collagen sponge reinforced with different poly(ethylene terephthalate) fibers. J Biomater Sci Polym Ed. 2007;18:865. doi: 10.1163/156856207781367738. [DOI] [PubMed] [Google Scholar]
- 15.Wall I. Donos N. Carlqvist K. Jones F. Brett P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009;45:17. doi: 10.1016/j.bone.2009.03.662. [DOI] [PubMed] [Google Scholar]
- 16.Datta N. Holtorf H.L. Sikavitsas V.I. Jansen J.A. Mikos A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Datta N. Pham Q.P. Sharma U. Sikavitsas V.I. Jansen J.A. Mikos A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103:2488. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai F. Hirao A. Ohmura M. Sato H. Matsuoka S. Takubo K. Ito K. Koh G.Y. Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Calvi L.M. Adams G.B. Weibrecht K.W. Weber J.M. Olson D.P. Knight M.C. Martin R.P. Schipani E. Divieti P. Bringhurst F.R. Milner L.A. Kronenberg H.M. Scadden D.T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 20.Kolf C.M. Cho E. Tuan R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs E. Tumbar T. Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 22.Sun H. Qu Z. Guo Y. Zang G. Yang B. In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online. 2007;6:41. doi: 10.1186/1475-925X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner W. Wein F. Roderburg C. Saffrich R. Faber A. Krause U. Schubert M. Benes V. Eckstein V. Maul H. Ho A.D. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp Hematol. 2007;35:314. doi: 10.1016/j.exphem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Ball S.G. Shuttleworth A.C. Kielty C.M. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36:714. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Csaki C. Matis U. Mobasheri A. Shakibaei M. Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol. 2009;131:251. doi: 10.1007/s00418-008-0524-6. [DOI] [PubMed] [Google Scholar]
- 26.Syed-Picard F.N. Larkin L.M. Shaw C.M. Arruda E.M. Three-dimensional engineered bone from bone marrow stromal cells and their autogenous extracellular matrix. Tissue Eng Part A. 2009;15:187. doi: 10.1089/ten.tea.2007.0140. [DOI] [PubMed] [Google Scholar]
- 27.Pham Q.P. Kasper F.K. Scott Baggett L. Raphael R.M. Jansen J.A. Mikos A.G. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 28.De Ranieri A. Virdi A.S. Kuroda S. Shott S. Dai Y. Sumner D.R. Local application of rhTGF-beta2 modulates dynamic gene expression in a rat implant model. Bone. 2005;36:931. doi: 10.1016/j.bone.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Jansen J.A. Vehof J.W. Ruhe P.Q. Kroeze-Deutman H. Kuboki Y. Takita H. Hedberg E.L. Mikos A.G. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101:127. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Reddi A.H. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 31.Jager M. Feser T. Denck H. Krauspe R. Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Ann Biomed Eng. 2005;33:1319. doi: 10.1007/s10439-005-5889-2. [DOI] [PubMed] [Google Scholar]
- 32.Holtorf H.L. Jansen J.A. Mikos A.G. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72:326. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 33.Pham Q.P. Sharma U. Mikos A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 34.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 35.Peter S.J. Liang C.R. Kim D.J. Widmer M.S. Mikos A.G. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Larsen K.H. Frederiksen C.M. Burns J.S. Abdallah B.M. Kassem M. Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone forming capacity. J Bone Miner Res. doi: 10.1359/jbmr.091018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Janssen F.W. van Dijkhuizen-Radersma R. Van Oorschot A. Oostra J. de Bruijn J.D. Van Blitterswijk C.A. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med. doi: 10.1002/term.197. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Prins H.J. Rozemuller H. Vonk-Griffioen S. Verweij V.G. Dhert W. Slaper-Cortenbach I. Martens A.C. Bone forming capacity of mesenchymal stromal cells when cultured in the presence of human platelet lysate as substitute for fetal bovine serum. Tissue Eng Part A. doi: 10.1089/ten.tea.2008.0666. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Lian J.B. Stein G.S. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids) J Oral Implantol. 1993;19:95. [PubMed] [Google Scholar]
- 40.Li I.W. Cheifetz S. McCulloch C.A. Sampath K.T. Sodek J. Effects of osteogenic protein-1 (OP-1, BMP-7) on bone matrix protein expression by fetal rat calvarial cells are differentiation stage specific. J Cell Physiol. 1996;169:115. doi: 10.1002/(SICI)1097-4652(199610)169:1<115::AID-JCP12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 41.Holtorf H.L. Datta N. Jansen J.A. Mikos A.G. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res A. 2005;74:171. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 42.Castano-Izquierdo H. Alvarez-Barreto J. van den Dolder J. Jansen J.A. Mikos A.G. Sikavitsas V.I. Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A. 2007;82:129. doi: 10.1002/jbm.a.31082. [DOI] [PubMed] [Google Scholar]
- 43.Fromigue O. Hamidouche Z. Chateauvieux S. Charbord P. Marie P.J. Distinct osteoblastic differentiation potential of murine fetal liver and bone marrow stroma-derived mesenchymal stem cells. J Cell Biochem. 2008;104:620. doi: 10.1002/jcb.21648. [DOI] [PubMed] [Google Scholar]
- 44.Maegawa N. Kawamura K. Hirose M. Yajima H. Takakura Y. Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1:306. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]
- 45.Maniatopoulos C. Sodek J. Melcher A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 46.Bilezikian J.P. Raisz L.G. Rodan G.A. Principles of Bone Biology. 2nd. Vol. 2. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 47.Taira M. Nakao H. Takahashi J. Araki Y. Effects of two vitamins, two growth factors and dexamethasone on the proliferation of rat bone marrow stromal cells and osteoblastic MC3T3-E1 cells. J Oral Rehabil. 2003;30:697. doi: 10.1046/j.1365-2842.2003.01118.x. [DOI] [PubMed] [Google Scholar]
- 48.Dexter T.M. Allen T.D. Lajtha L.G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 49.Lehar S.M. Bevan M.J. T cell development in culture. Immunity. 2002;17:689. doi: 10.1016/s1074-7613(02)00477-6. [DOI] [PubMed] [Google Scholar]
- 50.Dai B. Wang P. In vitro differentiation of adult bone marrow progenitors into antigen-specific CD4 helper T cells using engineered stromal cells expressing a notch ligand and a major histocompatibility complex class II protein. Stem Cells Dev. 2009;18:235. doi: 10.1089/scd.2008.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balciunaite G. Ceredig R. Massa S. Rolink A.G. A B220+ CD117+ CD19− hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 52.Schacke H. Docke W.D. Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 53.Stanbury R.M. Graham E.M. Systemic corticosteroid therapy—side effects and their management. Br J Ophthalmol. 1998;82:704. doi: 10.1136/bjo.82.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]