Abstract
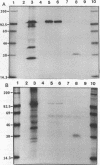
Defining conserved, protective epitopes is essential to the design of an effective vaccine against bovine anaplasmosis. MSP4, one of six initial body proteins recognized by a neutralizing serum, is conserved among Anaplasma marginale isolates at both the protein and the DNA levels. Sera from cattle immunized with an outer membrane fraction of A. marginale and protected from a virulent challenge bind MSP4. The gene for MSP4 has been cloned, and the recombinant protein has been expressed, isolated, and demonstrated to share epitopes with the native protein expressed on initial bodies. MSP4 may have a greater potential to protect cattle from a challenge by heterologous isolates than other A. marginale surface proteins, which vary widely in size and structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdala A. A., Pipano E., Aguirre D. H., Gaido A. B., Zurbriggen M. A., Mangold A. J., Guglielmone A. A. Frozen and fresh Anaplasma centrale vaccines in the protection of cattle against Anaplasma marginale infection. Rev Elev Med Vet Pays Trop. 1990;43(2):155–158. [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C. A., Adams L. G., Todorovic R. A. An antigenic and serologic comparison of two virulent strains and an attenuated strain of Anaplasma marginale. Am J Vet Res. 1970 Jun;31(6):1071–1078. [PubMed] [Google Scholar]
- Everett E. T., Kao K. J., Scornik J. C. Class I HLA molecules on human erythrocytes. Quantitation and transfusion effects. Transplantation. 1987 Jul;44(1):123–129. doi: 10.1097/00007890-198707000-00025. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Commins M. A., Wright I. G., Mirre G. B., Waltisbuhl D. J., White M. Babesia bovis: vaccination trial with a dominant immunodiffusion antigen in splenectomised calves. Z Parasitenkd. 1986;72(6):715–722. doi: 10.1007/BF00925093. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Plowe C. V., Woollett G. R., Beier J. C., Chulay J. D., Wirtz R. A., Hollingdale M. R., Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987 Aug 7;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- Hurley W. L., Finkelstein E., Holst B. D. Identification of surface proteins on bovine leukocytes by a biotin-avidin protein blotting technique. J Immunol Methods. 1985 Dec 17;85(1):195–202. doi: 10.1016/0022-1759(85)90287-x. [DOI] [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kao K. J., Klein P. A. A monoclonal antibody-based enzyme-linked immunosorbent assay for quantitation of plasma thrombospondin. Am J Clin Pathol. 1986 Sep;86(3):317–323. doi: 10.1093/ajcp/86.3.317. [DOI] [PubMed] [Google Scholar]
- Kieser S. T., Eriks I. S., Palmer G. H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990 Apr;58(4):1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., Venable J. H., Brock W. E. Ultrastructure of anaplasmal inclusions (Pawhuska isolate) and their appendages in intact and hemolyzed erythrocytes and in complement-fixation antigen. Am J Vet Res. 1978 Jul;39(7):1123–1130. [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Kuttler K. L., Winward L. D. Serologic comparisons of 4 Anaplasma isolates as measured by the complement-fixation test. Vet Microbiol. 1984 Apr;9(2):181–186. doi: 10.1016/0378-1135(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Love J. N. Cryogenic preservation of Anaplasma marginale with Dimethyl sulfoxide. Am J Vet Res. 1972 Dec;33(12):2557–2560. [PubMed] [Google Scholar]
- Luther D. G., Cox H. U., Nelson W. O. Comparisons of serotests with calf inoculations for detection of carriers in anaplasmosis- vaccinated cattle. Am J Vet Res. 1980 Dec;41(12):2085–2086. [PubMed] [Google Scholar]
- McGuire T. C., Davis W. C., Brassfield A. L., McElwain T. F., Palmer G. H. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J Clin Microbiol. 1991 Apr;29(4):788–793. doi: 10.1128/jcm.29.4.788-793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Kocan K. M., Barron S. J., Hair J. A., Barbet A. F., Davis W. C., McGuire T. C. Presence of common antigens, including major surface protein epitopes, between the cattle (intraerythrocytic) and tick stages of Anaplasma marginale. Infect Immun. 1985 Dec;50(3):881–886. doi: 10.1128/iai.50.3.881-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipano E., Krigel Y., Frank M., Markovics A., Mayer E. Frozen Anaplasma centrale vaccine against anaplasmosis in cattle. Br Vet J. 1986 Nov-Dec;142(6):553–556. doi: 10.1016/0007-1935(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Potgieter F. T., Van Rensburg L. Infectivity virulence and immunogenicity of Anaplasma centrale live blood vaccine. Onderstepoort J Vet Res. 1983 Mar;50(1):29–31. [PubMed] [Google Scholar]
- Reddy G. R., Chakrabarti D., Yowell C. A., Dame J. B. Sequence microheterogeneity of the three small subunit ribosomal RNA genes of Babesia bigemina: expression in erythrocyte culture. Nucleic Acids Res. 1991 Jul 11;19(13):3641–3645. doi: 10.1093/nar/19.13.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Rovis L., Barbet A. F., Williams R. O. Characterisation of the surface coat of Trypanosoma congolense. Nature. 1978 Feb 16;271(5646):654–656. doi: 10.1038/271654a0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Straus W. Imidazole increases the sensitivity of the cytochemical reaction for peroxidase with diaminobenzidine at a neutral pH. J Histochem Cytochem. 1982 May;30(5):491–493. doi: 10.1177/30.5.6176617. [DOI] [PubMed] [Google Scholar]
- Tebele N., McGuire T. C., Palmer G. H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991 Sep;59(9):3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser E. S., McGuire T. C., Palmer G. H., Davis W. C., Shkap V., Pipano E., Knowles D. P., Jr The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect Immun. 1992 Dec;60(12):5139–5144. doi: 10.1128/iai.60.12.5139-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. G. Towards a synthetic Babesia vaccine. Int J Parasitol. 1991 Apr;21(2):156–159. [PubMed] [Google Scholar]
- Wright I. G., White M., Tracey-Patte P. D., Donaldson R. A., Goodger B. V., Waltisbuhl D. J., Mahoney D. F. Babesia bovis: isolation of a protective antigen by using monoclonal antibodies. Infect Immun. 1983 Jul;41(1):244–250. doi: 10.1128/iai.41.1.244-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]