Abstract
Abscisic acid (ABA) is a plant hormone involved in the response of plants to reduced water availability. Reduction of guard cell turgor by ABA diminishes the aperture of the stomatal pore and thereby contributes to the ability of the plant to conserve water during periods of drought. Previous work has demonstrated that cytosolic Ca2+ is involved in the signal transduction pathway that mediates the reduction in guard cell turgor elicited by ABA. Here we report that ABA uses a Ca2+-mobilization pathway that involves cyclic adenosine 5′-diphosphoribose (cADPR). Microinjection of cADPR into guard cells caused reductions in turgor that were preceded by increases in the concentration of free Ca2+ in the cytosol. Patch clamp measurements of isolated guard cell vacuoles revealed the presence of a cADPR-elicited Ca2+-selective current that was inhibited at cytosolic Ca2+ ≥ 600 nM. Furthermore, microinjection of the cADPR antagonist 8-NH2-cADPR caused a reduction in the rate of turgor loss in response to ABA in 54% of cells tested, and nicotinamide, an antagonist of cADPR production, elicited a dose-dependent block of ABA-induced stomatal closure. Our data provide definitive evidence for a physiological role for cADPR and illustrate one mechanism of stimulus-specific Ca2+ mobilization in higher plants. Taken together with other recent data [Wu, Y., Kuzma, J., Marechal, E., Graeff, R., Lee, H. C., Foster, R. & Chua, N.-H. (1997) Science 278, 2126–2130], these results establish cADPR as a key player in ABA signal transduction pathways in plants.
Abscisic acid (ABA) is a plant hormone that plays major roles in the control of development and in the response to various environmental stresses. During periods of reduced water availability ABA builds up in the leaves and promotes reductions in the aperture of the stomatal pore. This reduction in stomatal aperture is beneficial to the plant as it serves to reduce the extent of transpirational water loss (1). The reductions in pore width are caused by decreases in the turgor of the two guard cells that surround the stomatal pore. ABA brings about reductions in turgor by promoting the efflux of potassium salt from the guard cells (2). Although it has been known for some time that ABA can operate in guard cells via Ca2+-dependent signal transduction pathways (ref. 3 and for recent reviews, refs. 4 and 5) the mechanism by which ABA increases cytosolic free Ca2+ concentration ([Ca2+]cyt) has not been thoroughly investigated. Indeed, this is the case for the wide variety of other plant signaling pathways that involve elevation of [Ca2+]cyt. Thus, although plant cells possess a number of Ca2+-permeable channel types on both the plasma membrane and endomembranes, the roles played by these channels in specific signal transduction pathways are unclear (6, 7). Assignment of a particular class or classes of Ca2+ ion channel as response elements in a given stimulus–response coupling pathway is very important in the context of understanding how stimulus-specificity is encoded. This is especially true in the case of the Ca2+ signature because the dynamic properties of the Ca2+ release channels that participate in the response are likely to characterize its spatiotemporal relationships (8).
In many animal cells, intracellular Ca2+ mobilization during signaling is achieved by activation of inositol 1,4,5-trisphosphate (InsP3) receptors and/or ryanodine receptors (9). The Ca2+-mobilizing properties of InsP3 in guard cells are established (10), and a recent report has shown that ABA can induce rapid phosphoinositide turnover in guard cells (11). Ca2+ release with the hallmark characteristics of mediation by ryanodine receptors has not been reported in guard cells, and although such release has been reported in the microsomal membranes of storage root cells of red beet (12), the physiological significance remains to be elucidated. In animals, one ryanodine receptor isoform (RyR2) is activated by the NAD+ metabolite cyclic adenosine 5′-diphosphoribose (cADPR) (13), and the results of photoaffinity investigations using 8-azido-cADPR suggest that the cADPR receptor is either an unknown variant of the ryanodine receptor or its accessory protein (14). The established Ca2+-mobilization properties of cADPR in other animal (14) and plant (15) systems and the recent report of the involvement of this compound in ABA-nuclear signal transduction pathways (16) prompted us to investigate whether cADPR elevates [Ca2+]cyt in guard cells. Our results show not only that cADPR induces increases in [Ca2+]cyt with consequent decreases in stomatal aperture but also that this second messenger is intimately involved in ABA-turgor signaling in guard cells. These data firmly establish a physiological role for cADPR in plant cell signal transduction and provide an insight into the mechanistic basis of the control of specificity in plant Ca2+-based signal transduction systems.
MATERIALS AND METHODS
Plant Material.
Seedlings of Commelina communis L. were grown from seed at a minimum temperature of 20°C, 16-h day length, at a minimum photon flux density of 100 μmol⋅m−2⋅s−1, 400–700 nm. Plants were transferred to a controlled environment chamber (temperature 25°C ± 1°C, 16-h day length at a photon flux density of 100 μmol⋅m−2⋅s−1) several days before use and were kept free from water stress at all stages of development.
Guard Cell Microinjection.
Microinjection experiments using iontophoresis were performed on C. communis epidermis, prepared and mounted in a perfusion system as previously described (17). Guard cells were impaled first with a filamented glass microelectrode (0.25-μm tip diameter) containing 10 mM fura-2 pentapotassium salt (Calbiochem–Novabiochem) in the tip. Fura-2 was microinjected into the cytosol of cells iontophoretically [1.0-nA negative pulses, 2 Hz, 200-ms duration (3)], for < 30 s, following which the electrode was removed. Injected cells were perfused with CO2-free 10 mM Mes/50 mM KCl, pH 6.15, under continuous illumination (photon flux density of 1000 μmol⋅m−2⋅s−1, 400–700 nm) from a Schott KL 1500 halogen cold light source (Cologne, Germany) for 1 h to promote stomatal opening. Experiments were conducted only on guard cells of stomata that were judged to be viable. To be viable an injected cell had to have the same degree of turgor, and a similar organelle distribution and shape, as the uninjected cell from the same stoma. In addition the stomatal aperture had to be identical in magnitude to the apertures on the rest of the epidermal strip (6–12 μm). Fura-2-loaded guard cells that met these criteria were impaled with a second microelectrode containing water, 10 mM ADPR (Sigma), or 10 mM cADPR (Cambridge BioScience, Cambridge, U.K.) in the tip. ADPR and cADPR solutions were made in ultrahigh-quality water by using a Milli-Q purification system (Millipore). Impaled guard cells were left to recover for 15 min with the electrode in place, after which the compound in the tip of the electrode was microinjected into the cytosol of the cell iontophoretically by using negative current pulses of 10-s duration. Fluorescence ratio photometric measurements were performed as described previously (18).
Guard cells for pressure microinjection were prepared as for iontophoresis. Cells were impaled with filamented quartz pipettes containing 1 mM 8-NH2-cADPR or 1 mM ADPR, 50 mM KCl, and 1 mM Lucifer yellow CH (Sigma). Pressure injection of the solution into the guard cell cytosol was achieved with a modified pressure probe (19) using an average pressure of ≈1.2 MPa. Location of the injected solution was followed by using a Nikon Diaphot inverted epifluorescence microscope with a 40× oil-immersion objective. Excitation of Lucifer yellow CH at 440 nm was from a 75-W xenon lamp (Osram) and emission fluorescence was monitored at 515 nm. Injected cells were maintained under conditions promoting stomatal opening for 1 h. Measurements were made only on stomata in which both the injected and noninjected cells of a single stoma exhibited the same increase in turgor, and where the injected cell met the criteria for estimating viability described previously. Half stomatal apertures were examined at specified times after the addition of 1 μM ABA with a calibrated micrometer.
Patch Clamp Experiments.
Vicia faba plants were grown and vacuoles were isolated from guard cell protoplasts exactly as described (20). In most experiments the pipette (vacuolar) solution contained 200 mM KCl, 5 mM 2-(N-morpholino)ethanesulfonic acid (Mes)–Tris, pH 5.5, and sorbitol to an osmolarity of 485 mosmol/kg. The bath (cytosolic) solution contained 200 mM KCl, 25 mM Tris–Mes, pH 7.5, 5 mM EGTA, sorbitol (to 485 mosmol/kg), and the appropriate CaCl2 concentration to give a free Ca2+ activity (aCa) in defined intervals between 0 and 1 mM, calculated by using the program calcium (21). In experiments to determine the selectivity of cADPR-induced currents the pipette solution was supplemented with 20 mM CaCl2 (aCa = 4.9 mM). Double-distilled water treated with Chelex 100 (iminodiacetic acid chelating resin, Sigma), to remove trace Ca2+, was used for all solutions. Ionic activities (aion) were calculated by using the extended Debye–Hückel relationship. cADPR was made up as a 100 μM stock in water and frozen in aliquots at −20°C. Aliquots were diluted directly to bath medium to the desired final concentration. Patch pipettes were pulled from thin-walled borosilicate glass capillaries (Kimax), coated with Sylgard (Dow Corning), and fire polished. Pipette resistance in the experimental solutions was commonly 30–50 MΩ. After pipette-vacuolar membrane seals of ≥10 GΩ were formed following gentle suction on the pipette, the whole-vacuole configuration was achieved by rupturing the membrane with voltage pulses up to 1 V delivered by an A310 Accupulser (World Precision Instruments, Sarasota, FL). The pipette solution was allowed to equilibrate with the vacuole lumen for 5 min before recording commenced. Whole vacuole current–voltage (I–V) relationships were determined by clamping the membrane potential by using a bipolar pulse protocol of 20-mV steps for 6 s (from a holding potential of 0 mV) between +100 and −100 mV. Instantaneous currents were sampled 50 ms after initiation of a voltage pulse. To ensure constancy of seal resistance, the characteristics of the current flowing in response to a 5-mV, 100-ms voltage pulse were monitored before and after a voltage protocol was run and before and after solutions were exchanged in the bath. The major component of this current in the region of the reversal potential is seal-mediated, and vacuoles showing a significant change in seal resistance during the course of an experiment were not used for analysis. Solutions were changed with two low-noise peristaltic pumps (Gilson Medical Electronics), with the exchange (22 times bath volume) being complete after 7 min. Membrane potentials are referenced to the vacuolar lumen, in accord with the convention of Bertl et al. (22). Recording and analysis of patch clamp data were as described (23).
Epidermal Strip Experiments.
To investigate the effect of nicotinamide (Sigma) on ABA-induced stomatal closure, epidermal peels of C. communis were prepared and then incubated in CO2-free 50 mM KCl/10 mM Mes-KOH, pH 6.15, under conditions promoting stomatal opening (at 25°C ± 1°C under a photon flux density of 150 μmol⋅m−2⋅s−1) for 2 h (24) before transfer to inhibitor solutions. The inhibitor was diluted in the incubation buffer to give final concentrations of 20 mM and 50 mM. After 2 h in the presence of the inhibitor, or 1 h in the presence of inhibitor and a further 1 h in inhibitor and 1 μM ABA, strips were examined to determine the aperture of stomatal pores. The effect of nicotinamide on the inhibition of stomatal opening by ABA was investigated by incubation of epidermal strips for 3 h under conditions promoting stomatal opening in the presence and/or absence of 50 mM nicotinamide and 1 μM ABA. The data presented are from 120 and 160 aperture measurements per treatment, collated from three and four independent experiments, for ABA-induced closure and inhibition of opening respectively. Aperture data are cited in the form mean ± SEM.
RESULTS
Guard Cells Are Competent to Respond to cADPR.
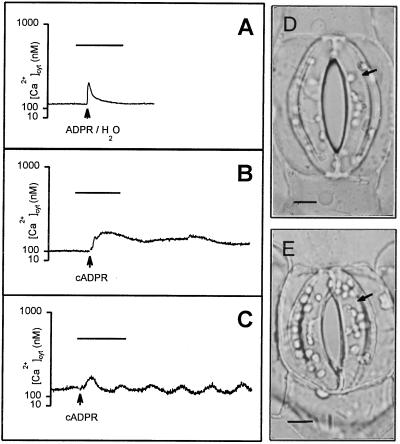
To determine whether guard cells were competent to respond to cADPR, single guard cells of C. communis were microinjected (first microinjection) with the ratiometric fluorescent Ca2+ indicator fura-2 (17). Cells exhibiting a stable [Ca2+]cyt signal (n = 60) were subsequently microinjected (second microinjection) with cADPR or, as controls, the noncyclic isomer ADPR (inactive in terms of Ca2+ agonist activity) or water. Representative results are shown in Fig. 1. Resting [Ca2+]cyt ranged from 80 to 250 nM (n = 60 cells), which is similar to values reported previously in guard cells (18). In the guard cells for which the second injection was performed successfully, microinjection of ADPR (five cells) or water (two cells) resulted in a transient increase in [Ca2+]cyt, typically amounting to around 100 nM above the resting levels (80–250 nM, as above) (Fig. 1A). However, this transient rise in [Ca2+]cyt was associated with the iontophoretic current, following which [Ca2+]cyt relaxed rapidly back to resting levels and was in no case associated with reductions in guard cell turgor (Fig. 1D). In contrast, cADPR induced a sustained increase in [Ca2+]cyt in five of the nine cells successfully microinjected (Fig. 1B). The increase in [Ca2+]cyt ranged between 50 and 400 nM above resting levels. In a further two cells, microinjection of cADPR induced oscillations in guard cell [Ca2+]cyt (Fig. 1C). The observed elevations in [Ca2+]cyt would be expected to have a physiological effect. Indeed, in all seven of these cells, microinjection of cADPR resulted in a decrease in cell turgor, whereas that of the uninjected guard cell of the same stoma was unaffected (Fig. 1E). The remaining two cells successfully microinjected with cADPR also showed a reduction in stomatal half-aperture, despite the absence of an observable increase in [Ca2+]cyt (data not shown). In all cells, injection of cADPR resulted in a reduction in stomatal half-aperture from 5.3 ± 2.8 μm to 0.3 ± 0.1 μm (n = 9) over a period of between 10 and 15 min. A reduction in stomatal aperture without a concomitant increase in [Ca2+]cyt in a minority of the cells may be explained by the operation of a Ca2+-independent closure signaling pathway (25). Alternatively, these results may simply be due to an inability to detect a genuine [Ca2+]cyt increase in these two cells (17).
Figure 1.
Microinjection of cADPR into guard cell cytosol elevates [Ca2+]cyt. (A) Typical results for the change in [Ca2+]cyt after microinjection of cells, by using pipettes filled with ADPR or water, into the cytosol of fura-2-loaded guard cells of open stomata of C. communis (n = 5 and 2 cells respectively). Data shown are from a cell injected with ADPR. A transient increase in [Ca2+]cyt was observed only during the 10-s current pulse. (B) Sustained increase in [Ca2+]cyt following microinjection of cADPR into the cytosol of fura-2-loaded guard cells of open stomata of C. communis (n = 5 cells). [Ca2+]cyt remained elevated for at least 10 min after the 10-s current pulse. (C) Oscillations in [Ca2+]cyt (amplitude, 200 nM; period, 3.75 min) occasionally observed following microinjection of cADPR into the cytosol of fura-2-loaded guard cells of open stomata of C. communis (n = 2 cells). For A–C bar = 5 min. (D) ADPR (data shown) or water injected into the cytosol caused no change in guard cell turgor (right-hand guard cell, arrowed). (E) In contrast, cADPR injected into the cytosol caused a reduction in guard cell turgor (right-hand guard cell, arrowed). (Bars in D and E = 5 μm.)
cADPR Induces Ca2+ Release from Isolated Guard Cell Vacuoles.
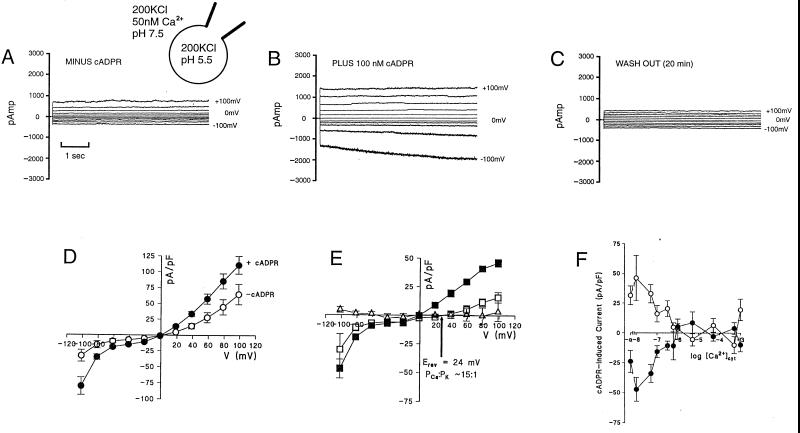
Vacuoles comprise the principal intracellular store of Ca2+ in guard cells and therefore might be anticipated a priori to be good candidates for the cADPR-labile Ca2+ pool. To determine whether cADPR can elicit Ca2+ release from this pool, guard cell vacuoles were tested for the presence of cADPR-induced currents in the whole-vacuole mode of patch clamp (20). In the absence of cADPR, and when K+ was used as a charge carrier, the membrane current–voltage (I–V) relationship was dominated by inward- and outward-rectifying currents (Fig. 2, A and D). This is typical of fast vacuolar (FV) channel activity (26–28). Addition of 100 nM cADPR to the bath elicited instantaneously activating inward and outward currents that were reversible on cADPR washout (Fig. 2 A–C). I–V relationships for cADPR-dependent currents, derived by subtraction, are nonohmic (Fig. 2E). The reversal potential (Erev) of 0 mV in symmetric K+ shifts to +24 ± 5 mV when the luminal solution is supplemented with 20 mM Ca2+ (aCa = 4.9 mM). Application of the Goldman–Hodgkin–Katz current equation yields an apparent cation permeability ratio PCa:PK = 15:1. Fig. 2E also shows that ADPR failed to evoke a current in 12 trials.
Figure 2.
Guard cell whole vacuole currents elicited by cADPR. (A) Whole vacuole currents from Vicia faba guard cell vacuole in the absence of cADPR in symmetric 200 mM KCl and other conditions as shown. (B and C) Currents from the same vacuole respectively in the presence of 100 nM cADPR on the cytosolic (bath) side of the membrane and then 20 min after onset of bath perfusion with cADPR-free solution. (D) I–V relationship for whole vacuole instantaneous currents before (○) and after (•) addition of 100 nM cADPR, conditions as in A and B. (E) Difference relationship for cADPR-induced currents, with conditions as in D (■) or with the addition of 20 mM CaCl2 (aCa = 4.9 mM) to the pipette solution (□). The I–V difference relationship for controls carried out with 100 nM noncyclic ADPR is also shown (▵). In D and E all data are replicates from 7 vacuoles. (F) Instantaneous currents induced by 100 nM cADPR (as in E, ■) at +100 mV (○) and −100 mV (•) over a range of [Ca2+]cyt concentrations. Each point is the mean ± SEM of n = 7 or 8.
In animal cells, cADPR augments the activation of ryanodine receptors by [Ca2+]cyt, thereby contributing to amplification of Ca2+ signaling by Ca2+-induced Ca2+ release (CICR) (9, 29). To test for the possibility that cADPR-gated cation currents at the vacuolar membrane are enhanced by [Ca2+]cyt, the cADPR-dependent current was measured in vacuoles over a range of [Ca2+]cyt. Surprisingly, the results (Fig. 2F) show that at both positive and negative potentials cADPR-gated currents down-regulate over the range of [Ca2+]cyt elevated by cADPR microinjection (Fig. 1).
cADPR Is Involved in ABA-Turgor Signaling in Guard Cells.
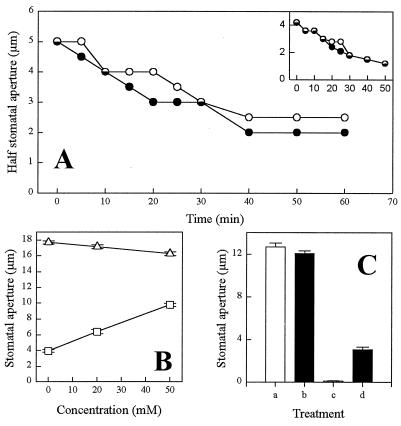
A direct investigation of the possible role of cADPR in ABA signaling in guard cells was tested by the introduction of a competitive antagonist of cADPR, 8-NH2-cADPR (30), into guard cells by pressure microinjection (19). In 7 of 13 guard cells examined, cells preloaded with 8-NH2-cADPR exhibited a slower rate of turgor loss (measured as decrease in half-aperture), in response to 1 μM ABA, than their uninjected counterparts (Fig. 3A). By contrast, all controls preloaded with ADPR (n = 5) failed to exhibit a marked difference in the rate of ABA-induced turgor loss in comparison with the uninjected cell (Fig. 3A Inset). In the remaining six 8-NH2-cADPR-injected cells we observed no effect in the response to ABA (data not shown). It is quite possible that some of the variation in the response of cells to the inhibitor is due to redundancy in the ABA signaling pathway, with a block in the transduction chain mediated by cADPR by-passed by the use of a different signaling pathway(s) (see also Discussion). Another likely source of variation is experimental in origin. With an average 1 h between the injection of the inhibitor and the application of ABA, significant hydrolysis of the inhibitor by endogenous enzymes may have occurred (31), resulting in suboptimal levels of inhibitor within the cytosol during ABA signaling.
Figure 3.
Effect of inhibitors of the cADPR signaling pathway on ABA-induced stomatal closure. (A) Representative plot showing half stomatal aperture measurements after challenge of a guard cell with 1 μM ABA, where 8-NH2-cADPR had previously been loaded into the cell cytosol by pressure microinjection (○). Also shown are similar measurements for the uninjected cell from the same stoma (•). (A Inset) Half stomatal aperture measurements of a cell previously loaded with ADPR and subsequently challenged with 1 μM ABA (○) and the uninjected cell from the same stoma (•). (B) Stomatal aperture measurements from isolated epidermal strips incubated under conditions that promote stomatal opening (50 mM KCl/10 mM Mes continuously perfused with CO2-free air under constant illumination) for 2 h and subsequently incubated for a further 2 h in the same buffer containing a range of nicotinamide concentrations in the absence (▵) or presence (□) of 1 μM ABA for the final 1 h of the experiment. (C) Stomatal aperture measurements from isolated epidermal strips incubated for 3 h under conditions promoting stomatal opening in the presence or absence of inhibitor or ABA. Bar a, buffer only; bar b, 50 mM nicotinamide; bar c, 1 μM ABA; and bar d, 1 μM ABA and 50 mM nicotinamide. For B and C the data are the means ± the SEMs. A total of 120 and 160 stomatal aperture measurements for each data point were obtained for B and C, respectively.
To provide further clarification of the role of cADPR in the control of guard cell turgor, we tested the efficacy of ABA both in stimulating stomatal closure and in inhibiting stomatal opening in the presence of nicotinamide, which, as a product of ADP-ribosyl cyclase activity, inhibits cADPR synthesis (32). Nicotinamide was studied over a concentration range similar to that shown to interfere with in vivo cADPR signaling in sea urchin oocytes (32). Nicotinamide had only a small effect on open stomata, decreasing the mean aperture from 17.7 ± 0.2 μm to 16.3 ± 0.3 μm at the highest concentration used (Fig. 3B). In the presence of 1 μM ABA, the mean stomatal aperture was reduced to only 3.9 ± 0.2 μm, but increasing concentrations of nicotinamide tended to restore the mean aperture, even in the presence of ABA, to a maximum value of 9.8 ± 0.2 μm (Fig. 3B). Epidermal strips incubated under opening conditions in the presence of 1 μM ABA for 3 h had mean apertures of 0.6 μm ± 0.1 μm, whereas the additional presence of 50 mM nicotinamide caused some reversal of this effect, with mean apertures of 3.1 ± 0.2 μm (Fig. 3C). Epidermal strips incubated in the presence of 50 mM nicotinamide without ABA had mean apertures similar to those incubated in buffer only (12.1 ± 0.3 μm and 12.7 ± 0.4 μm, respectively) (Fig. 3C). Taken together, these sets of data provide strong evidence that cADPR forms an important component in the ABA signal transduction pathway in stomatal guard cells.
DISCUSSION
The data presented in this paper have a direct bearing on issues that are currently attracting considerable interest in plant cell signal transduction. First, our data provide firm physiological evidence for the involvement of cADPR in an ABA-dependent response. It is notable that our results complement recent work on ABA nuclear signaling (16). Wu et al. (16) microinjected tomato hypocotyl subepidermal cells with chimeric gene constructs made from the promoters of the Arabidopsis ABA-regulated genes rd29A and kin2 fused to the GUS reporter gene. They showed that microinjection of cADPR resulted in a significant number of GUS (β-glucuronidase) expression events and, in common with our work, the process was also inhibited by 8-NH2-cADPR. These data provided convincing evidence that the ABA-regulated genes rd29A and kin2 were competent to respond to elevated cellular levels of cADPR.
By using a sea-urchin microsome bioassay, Wu et al. (16) also showed that levels of cADPR increased after the tissue had been treated with ABA. The present findings add to those results in tomato hypocotyl by providing the physiological dimension required to establish cADPR definitively as an important intracellular second messenger involved in ABA-mediated signal transduction. Thus, the present work demonstrates that addition of cADPR results in increases in guard cell [Ca2+]cyt (Fig. 1 B and C) and that this correlates with reductions in stomatal aperture (Fig. 1E). In common with Wu et al. (16), we found that microinjection of the cADPR antagonist 8-NH2-cADPR can interfere with ABA-induced reductions in stomatal aperture. To seek further confirmatory evidence that cADPR was involved in ABA signal transduction, we used another cADPR antagonist, nicotinamide, which also interfered with ABA–turgor signaling. Taken together with the data from Wu et al. (16), these data firmly establish cADPR as an intracellular second messenger involved in two different ABA signaling pathways.
Second, our data also have an important bearing on the question of the control of specificity in calcium-based signal transduction pathways in plants (8), as they describe a distinct calcium-mobilizing intracellular second messenger. The presence of cADPR in plant cells obviously increases the diversity of stimulus-accessible intracellular release pathways and as such will be an important element in the generation of the calcium signature. As previously discussed, some of the experimental variation observed in response to 8-NH2-cADPR may be explained by redundancy in ABA signaling, involving other Ca2+-mobilizing second messengers such as InsP3 and possibly nicotinic acid adenine dinucleotide phosphate (NAADP) (14) and the putative Ca2+-mobilizing second messenger myo-inositol hexakisphosphate (InsP6) (33) or Ca2+-independent pathways (25). The suggestion that differential access to different Ca2+-mobilizing pathways is likely to be important in the control of specificity is strongly supported by Wu et al (16), who found that although the expression of rd29A and kin2 promoters was regulated by cADPR, the promoter of another well characterized Ca2+-regulated gene, cab, was insensitive to cADPR. Such differential regulation could extend to downstream elements in Ca2+ signaling, such as Ca2+-dependent protein kinases and phosphatases, as well as Ca2+-independent signaling elements that are known to regulate the ABA response, such as cytoplasmic pH (34) and the protein phosphatases PP1, PP2A, and PP2C (5).
From the present investigation it is apparent that the elevations in [Ca2+]cyt after cADPR injection are similar in magnitude to those reported in guard cells after release of InsP3 from caged InsP3 (10). Intriguingly, however, the dynamics of the [Ca2+]cyt changes are quite different in the two instances; those subsequent to InsP3 release relax to the resting level over 5–10 min, whereas those induced by cADPR tend to remain elevated. Additionally, previous work has demonstrated that InsP3 and cADPR elicit discrete currents in plant vacuoles (15). It would thus appear that the operation of either an InsP3 or cADPR pathway has a direct bearing on the form of the Ca2+ signature. The cADPR-mediated pathway could therefore provide a novel mechanism for contributing to the generation of Ca2+ signatures, and thus stimulus specificity.
The dynamics of cADPR-mediated Ca2+ release indicate that cADPR is involved in the generation of prolonged [Ca2+]cyt signals in vivo, implicating cADPR in a possible guard cell CICR mechanism. However, as the present work has demonstrated, vacuolar currents induced by cADPR are inhibited by [Ca2+]cyt ≥ 600 nM. It therefore appears unlikely that cADPR-induced Ca2+ release alone accounts for large and prolonged Ca2+ signals in plants in which [Ca2+]cyt can attain values of 2 μM or more (35). Furthermore, the inhibition of cADPR-induced currents by [Ca2+]cyt ≥ 600 nM implies that CICR, if present in plants, is unlikely to be sustained solely by the activation of cADPR-gated channels. CICR may therefore require the activation of other channel types such as the slow vacuolar (SV) channel, which is both Ca2+-activated and Ca2+-permeable (26, 28, 36), although this possibility has recently been challenged (37). Prolonged Ca2+ elevations may also involve the activity of Ca2+-permeable ion channels in the plasma membrane (38). The likely role of cADPR-gated Ca2+ mobilization would be to provide a trigger for further Ca2+ release.
In summary, our results demonstrate that addition of cADPR to guard cell vacuoles causes Ca2+ release, which in turn leads to an elevation of [Ca2+]cyt and a consequent reduction in stomatal aperture. We also provide strong physiological evidence that cADPR is involved in ABA signal transduction. At present, the extent to which cADPR plays a role in other Ca2+-mediated transduction pathways in guard cells [including that in response to the well-known stomatal closing stimulus CO2 (24)] remains unknown. One possibility is that cADPR-facilitated Ca2+ mobilization constitutes a point of convergence of pathways that use different physiological stimuli to attain an identical physiological result (stomatal closure) (39). An alternative is that the point of convergence lies further downstream, and that other Ca2+-mobilization channels (e.g., those gated by InsP3) are used for other closing stimuli.
Acknowledgments
We thank A. Galione (Department of Pharmacology, University of Oxford, U.K.) for the kind gift of cADPR, and P. Crosby and S. Sparrow for their help with Fig. 2. M.R.M. is grateful to The Royal Society for the award of a University Research Fellowship. D.S. and A.M.H. thank the Biotechnology and Biological Sciences Research Council for support. A.M.H. acknowledges support from the European Community (Biotech Programme B104-CT96-0062).
ABBREVIATIONS
- ABA
abscisic acid
- [Ca2+]cyt
cytosolic free Ca2+ concentration
- InsP3
inositol 1,4,5-trisphosphate
- cADPR
cyclic adenosine 5′-diphosphoribose
- CICR
Ca2+-induced Ca2+ release.
References
- 1.Mittelheuser C J, Van Steveninck Nature (London) 1969;221:281–282. [Google Scholar]
- 2.MacRobbie E A C. J Exp Bot. 1981;32:563–572. [Google Scholar]
- 3.McAinsh M R, Brownlee C, Hetherington A M. Nature (London) 1990;343:186–188. [Google Scholar]
- 4.MacRobbie E A C. J Exp Bot. 1997;48:515–528. doi: 10.1093/jxb/48.Special_Issue.515. [DOI] [PubMed] [Google Scholar]
- 5.Leung J, Giraudat J. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Ward J M, Pei Z M, Schroeder J I. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen G J, Sanders D. Adv Bot Res. 1997;25:217–252. [Google Scholar]
- 8.McAinsh M R, Hetherington A M. Trends Plant Sci. 1998;3:32–35. [Google Scholar]
- 9.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 10.Gilroy S, Read N D, Trewavas A J. Nature (London) 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Choi Y B, Suh S, Lee J, Assmann S M, Joe C O, Kelleher J F, Crain R C. Plant Physiol. 1996;110:987–996. doi: 10.1104/pp.110.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muir S R, Sanders D. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- 13.Mészáros L G, Bak J, Chui A. Nature (London) 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- 14.Lee H C. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 15.Allen G J, Muir S R, Sanders D. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Kuzma J, Marechal E, Graeff R, Lee H C, Foster R, Chua N-H. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 17.McAinsh M R, Brownlee C, Hetherington A M. Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAinsh M R, Webb A A R, Taylor J E, Hetherington A M. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oparka K J, Murphy R, Derrick P M, Prior D A M, Smith J A C. J Cell Sci. 1991;98:539–544. [Google Scholar]
- 20.Allen G J, Sanders D. Plant Cell. 1994;6:685–694. doi: 10.1105/tpc.6.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fohr K J, Warchol W, Gratzl M. Methods Enzymol. 1993;221:149–157. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- 22.Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradmann D, Hille B, Köhler K, Kolb H A, MacRobbie E, et al. Science. 1992;258:873–874. doi: 10.1126/science.1439795. [DOI] [PubMed] [Google Scholar]
- 23.Allen G J, Sanders D. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 24.Webb A A R, McAinsh M R, Mansfield T A, Hetherington A M. Plant J. 1996;9:297–304. [Google Scholar]
- 25.Allan A C, Fricker M D, Ward J L, Beale M H, Trewavas A J. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedrich R, Neher E. Nature (London) 1987;329:833–836. [Google Scholar]
- 27.Allen G J, Sanders D. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 28.Tikhonova L I, Pottosin I I, Dietz K-J, Schönknecht G. Plant J. 1997;11:1059–1070. [Google Scholar]
- 29.Galione A, Lee H C, Busa W B. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 30.Walseth T F, Lee H C. Biochim Biophys Acta. 1993;1178:235–242. doi: 10.1016/0167-4889(93)90199-y. [DOI] [PubMed] [Google Scholar]
- 31.Sethi J K, Empson R M, Bailey V C, Potter B V L, Galione A. J Biol Chem. 1997;272:16358–16363. doi: 10.1074/jbc.272.26.16358. [DOI] [PubMed] [Google Scholar]
- 32.Sethi J K, Empson R M, Galione A. Biochem J. 1996;319:613–617. doi: 10.1042/bj3190613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brearley C A, Lemtiri-Chlieh F. In: 11th International Workshop on Plant Membrane Biology, Cambridge, UK. Tester M, Morris C, Davies J, editors. Berlin: Society for Experimental Biology and Springer; 1998. p. 259. [Google Scholar]
- 34.Blatt M R, Grabov A. J Exp Bot. 1997;48:529–537. doi: 10.1093/jxb/48.Special_Issue.529. [DOI] [PubMed] [Google Scholar]
- 35.Knight H, Trewavas A J, Knight M R. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward J M, Schroeder J I. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottosin I G, Tikhonova L I, Hedrich R, Schönknecht G. Plant J. 1997;12:1387–1398. [Google Scholar]
- 38.Schroeder J I, Hagiwara S. Proc Natl Acad Sci USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb A A R, Hetherington A M. Plant Physiol. 1997;114:1557–1560. doi: 10.1104/pp.114.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]