Abstract
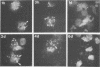
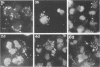
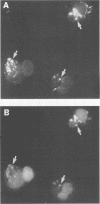
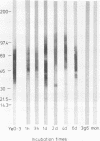
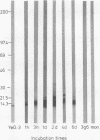
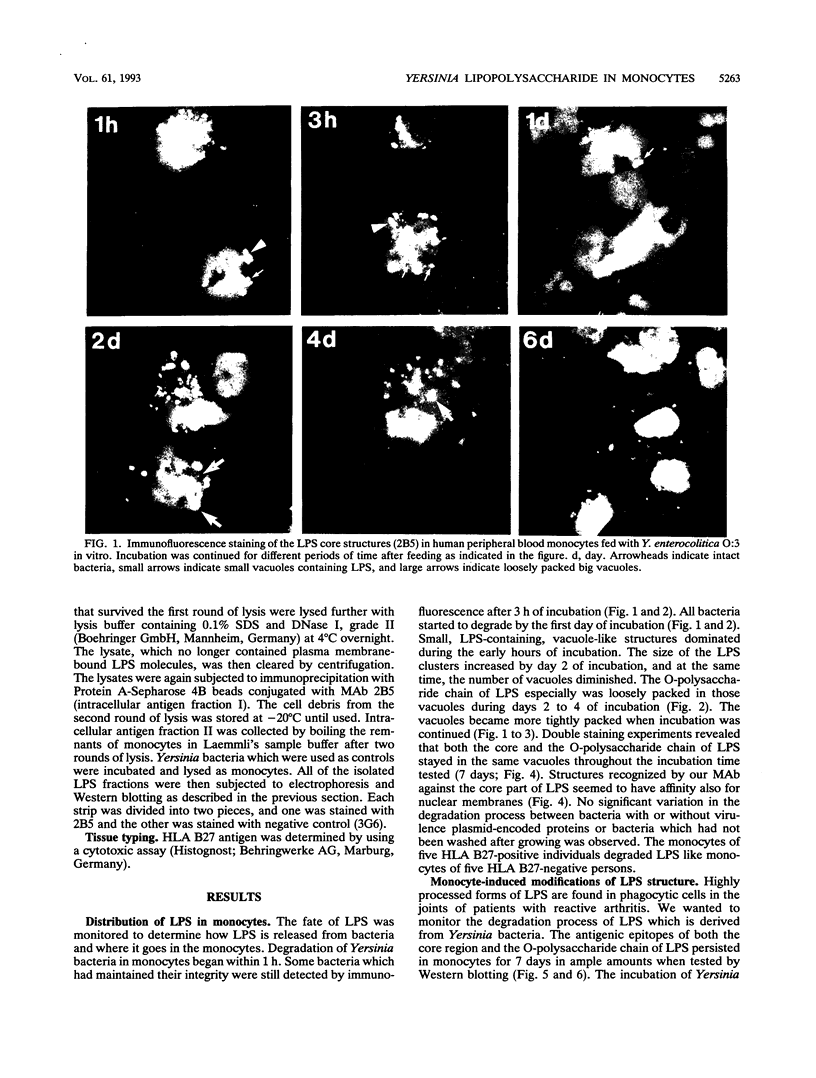
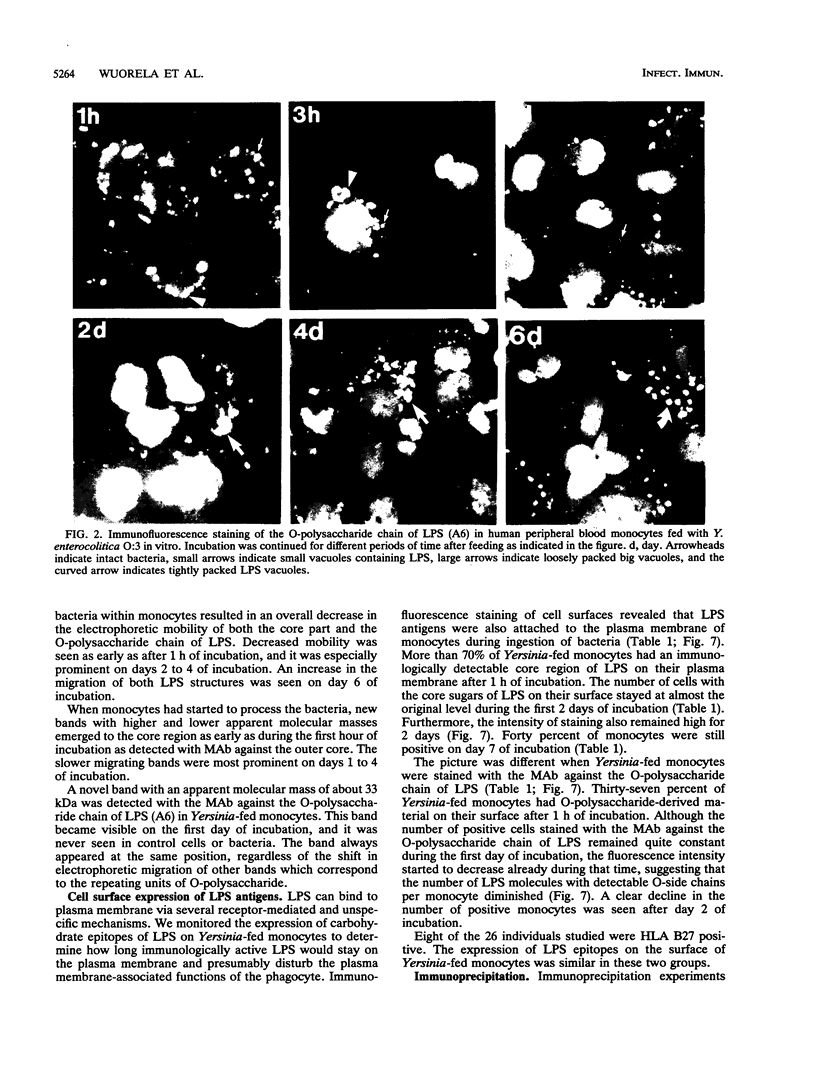
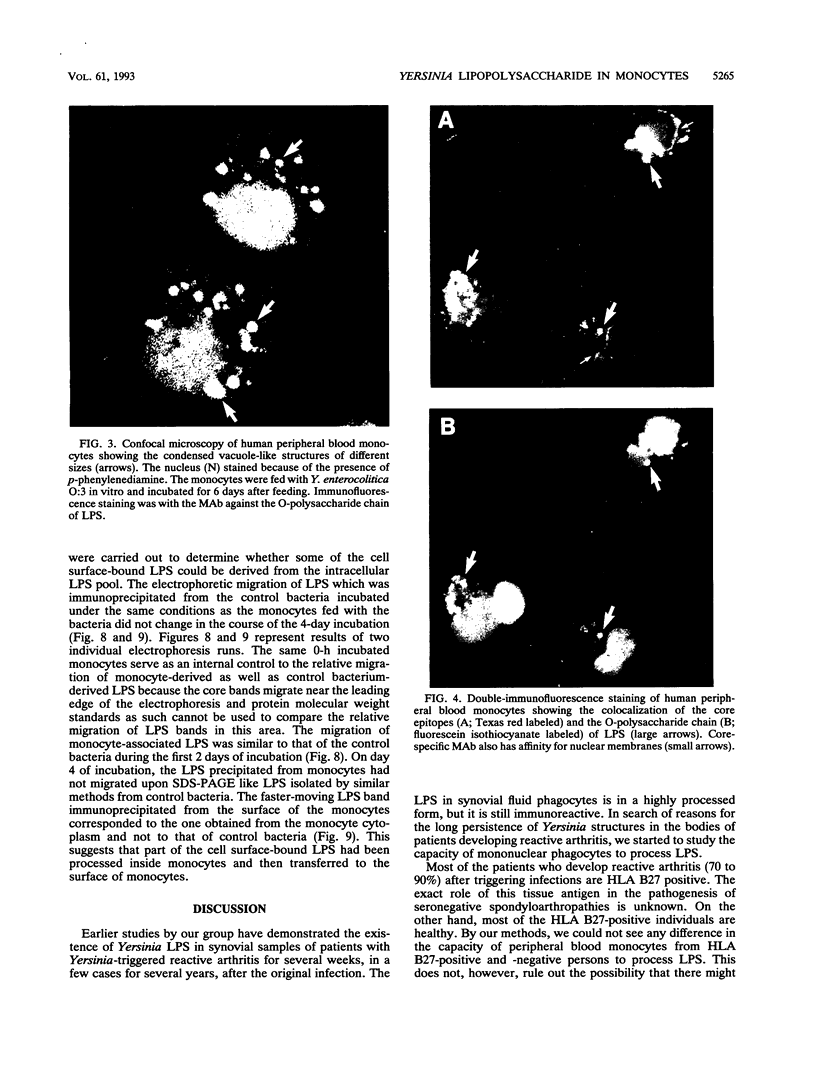
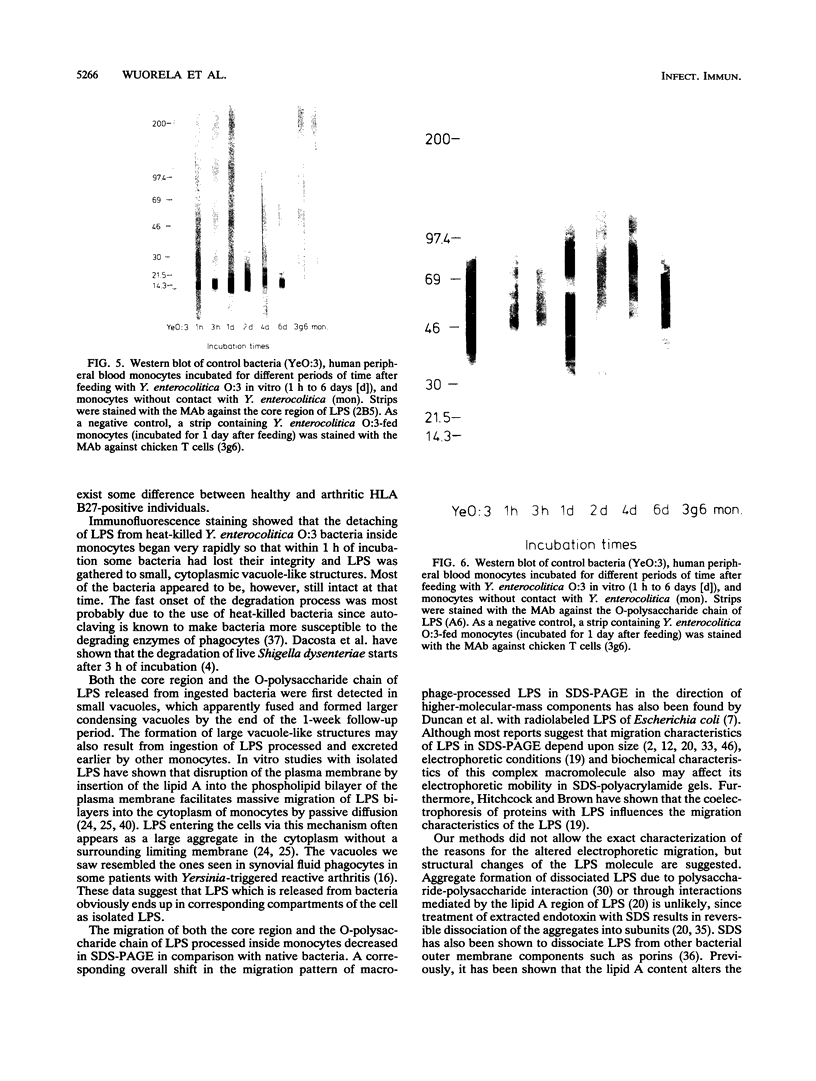
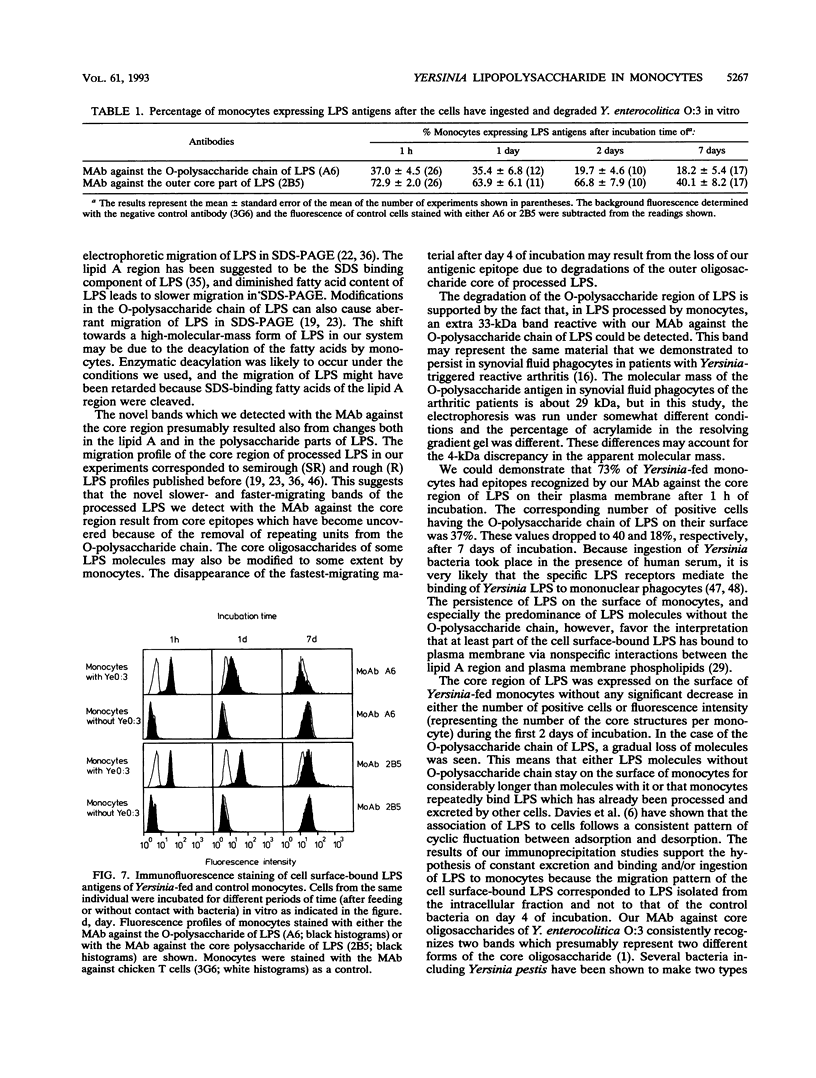
Reactive arthritis is usually self-limiting polyarthritis, which develops after certain gastrointestinal or urogenital tract infections, mostly in susceptible HLA B27-positive individuals. In the pathogenesis of this arthritis, it is probably important that structures of the causative bacteria are found in the affected joints. The structure found in the synovial fluid phagocytes of the patients with reactive arthritis after Yersinia, Salmonella, and Shigella infections has always been lipopolysaccharide (LPS) of the causative bacteria. It has been in a highly processed form but still immunoreactive. To follow the degradation process of LPS, we fed peripheral blood monocytes of healthy blood donors with heat-killed Yersinia enterocolitica O:3 bacteria in vitro and monitored the fate of LPS by immunofluorescence and immunoblotting methods. Heat-killed bacteria were used since Y. enterocolitica O:3 bacteria are able to live inside monocytes in vitro and dividing intracellular bacteria would have made it impossible to monitor the degradation process of LPS with these methods. Both the core region and the O-polysaccharide chain of LPS persisted in cytoplasmic vacuoles and on plasma membrane of monocytes through the 7-day follow-up time. Migration properties of processed LPS in sodium dodecyl sulfate-polyacrylamide gel electrophoresis suggested structural modifications of LPS. We also demonstrated that core epitopes appearing on the surface of Yersinia-fed monocytes on day 4 of incubation were processed intracellularly, suggesting that LPS-containing phagocytes are a constant source of membrane-active LPS in their microenvironment as well as in the joints of arthritic patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Dacosta B., Ryter A., Mounier J., Sansonetti P. Immunodetection of lipopolysaccharide in macrophages during the processing of non invasive Shigella dysenteriae. Biol Cell. 1990;69(3):171–178. doi: 10.1016/0248-4900(90)90343-2. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Charnetzky W. T., Hurlbert R. F., Hancock R. E. Effects of growth temperature, 47-megadalton plasmid, and calcium deficiency on the outer membrane protein porin and lipopolysaccharide composition of Yersinia pestis EV76. Infect Immun. 1983 Dec;42(3):1092–1101. doi: 10.1128/iai.42.3.1092-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M., Stewart-Tull D. E., Jackson D. M. The binding of lipopolysaccharide from Escherichia coli to mammalian cell membranes and its effect on liposomes. Biochim Biophys Acta. 1978 Apr 4;508(2):260–276. doi: 10.1016/0005-2736(78)90329-2. [DOI] [PubMed] [Google Scholar]
- Duncan R. L., Jr, Hoffman J., Tesh V. L., Morrison D. C. Immunologic activity of lipopolysaccharides released from macrophages after the uptake of intact E. coli in vitro. J Immunol. 1986 Apr 15;136(8):2924–2929. [PubMed] [Google Scholar]
- Fox E. S., Thomas P., Broitman S. A. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989 Feb;96(2 Pt 1):456–461. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Kleine B., Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984 Jul-Aug;6(4):483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Granfors K. Do bacterial antigens cause reactive arthritis? Rheum Dis Clin North Am. 1992 Feb;18(1):37–48. [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., Lindberg A. A., Mäki-Ikola O., von Essen R., Lahesmaa-Rantala R., Isomäki H., Saario R., Arnold W. J., Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990 Mar 24;335(8691):685–688. doi: 10.1016/0140-6736(90)90804-e. [DOI] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., Toivanen P., Koski J., Lindberg A. A. Bacterial lipopolysaccharide in synovial fluid cells in Shigella triggered reactive arthritis. J Rheumatol. 1992 Mar;19(3):500–500. [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., von Essen R., Lahesmaa-Rantala R., Isomäki O., Pekkola-Heino K., Merilahti-Palo R., Saario R., Isomäki H., Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989 Jan 26;320(4):216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander I. M., Hurme R., Haikara A., Moran A. P. Separation and characterization of two chemically distinct lipopolysaccharides in two Pectinatus species. J Bacteriol. 1992 May;174(10):3348–3354. doi: 10.1128/jb.174.10.3348-3354.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. D., Chiu B., Johnston M. E., Vas S., Falk J. HLA class I-related impairment in IL-2 production and lymphocyte response to microbial antigens in reactive arthritis. J Immunol. 1989 Jun 15;142(12):4256–4260. [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Kang Y. H., Dwivedi R. S., Lee C. H. Ultrastructural and immunocytochemical study of the uptake and distribution of bacterial lipopolysaccharide in human monocytes. J Leukoc Biol. 1990 Oct;48(4):316–332. doi: 10.1002/jlb.48.4.316. [DOI] [PubMed] [Google Scholar]
- Kang Y. H., Lee C. H., Monroy R. L., Dwivedi R. S., Odeyale C., Newball H. H. Uptake, distribution and fate of bacterial lipopolysaccharides in monocytes and macrophages: an ultrastructural and functional correlation. Electron Microsc Rev. 1992;5(2):381–419. doi: 10.1016/0892-0354(92)90016-j. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird W. J., Cavanaugh D. C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980 Apr;11(4):430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T., Tassin M. T., Ryter A. Bacterial antigen immunolabeling in macrophages after phagocytosis and degradation of Bacillus subtilis. Infect Immun. 1988 Feb;56(2):468–478. doi: 10.1128/iai.56.2.468-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Warner R. C. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967 Nov 10;242(21):4994–5001. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Beckerdite S., Pettis P., Elsbach P. Phospholipid metabolism by phagocytic cells. VII. The degradation and utilization of phospholipids of various microbial species by rabbit granulocytes. Biochim Biophys Acta. 1972 Sep 7;280(1):45–56. [PubMed] [Google Scholar]
- Pekkola-Heino K., Viljanen M. K., Ståhlberg T. H., Granfors K., Toivanen A. Monoclonal antibodies reacting selectively with core and O-polysaccharide of Yersinia enterocolitica O:3 lipopolysaccharide. Acta Pathol Microbiol Immunol Scand C. 1987 Feb;95(1):27–34. doi: 10.1111/j.1699-0463.1987.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., Munford R. S. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect Immun. 1987 Apr;55(4):974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. M., Jacobs D. M. Fluorescent detection of lipopolysaccharide interactions with model membranes. Biochim Biophys Acta. 1986 Jul 10;859(1):26–32. doi: 10.1016/0005-2736(86)90314-7. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992 Sep 4;257(5075):1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Salomonsen J., Skjødt K., Crone M., Simonsen M. The chicken erythrocyte-specific MHC antigen. Characterization and purification of the B-G antigen by monoclonal antibodies. Immunogenetics. 1987;25(6):373–382. doi: 10.1007/BF00396103. [DOI] [PubMed] [Google Scholar]
- Ståhlberg T. H., Granfors K., Toivanen A. Immunoblot analysis of human IgM, IgG and IgA responses to plasmid-encoded antigens of Yersinia enterocolitica serovar O3. J Med Microbiol. 1987 Sep;24(2):157–163. doi: 10.1099/00222615-24-2-157. [DOI] [PubMed] [Google Scholar]
- Tassin M. T., Lang T., Antoine J. C., Hellio R., Ryter A. Modified lysosomal compartment as carrier of slowly and non-degradable tracers in macrophages. Eur J Cell Biol. 1990 Aug;52(2):219–228. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Patel M., Miller D. S. Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992 Sep 1;176(3):719–727. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Hendy A., Toivanen P., Skurnik M. Lipopolysaccharide O side chain of Yersinia enterocolitica O:3 is an essential virulence factor in an orally infected murine model. Infect Immun. 1992 Mar;60(3):870–875. doi: 10.1128/iai.60.3.870-875.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]