Abstract
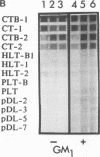
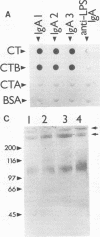
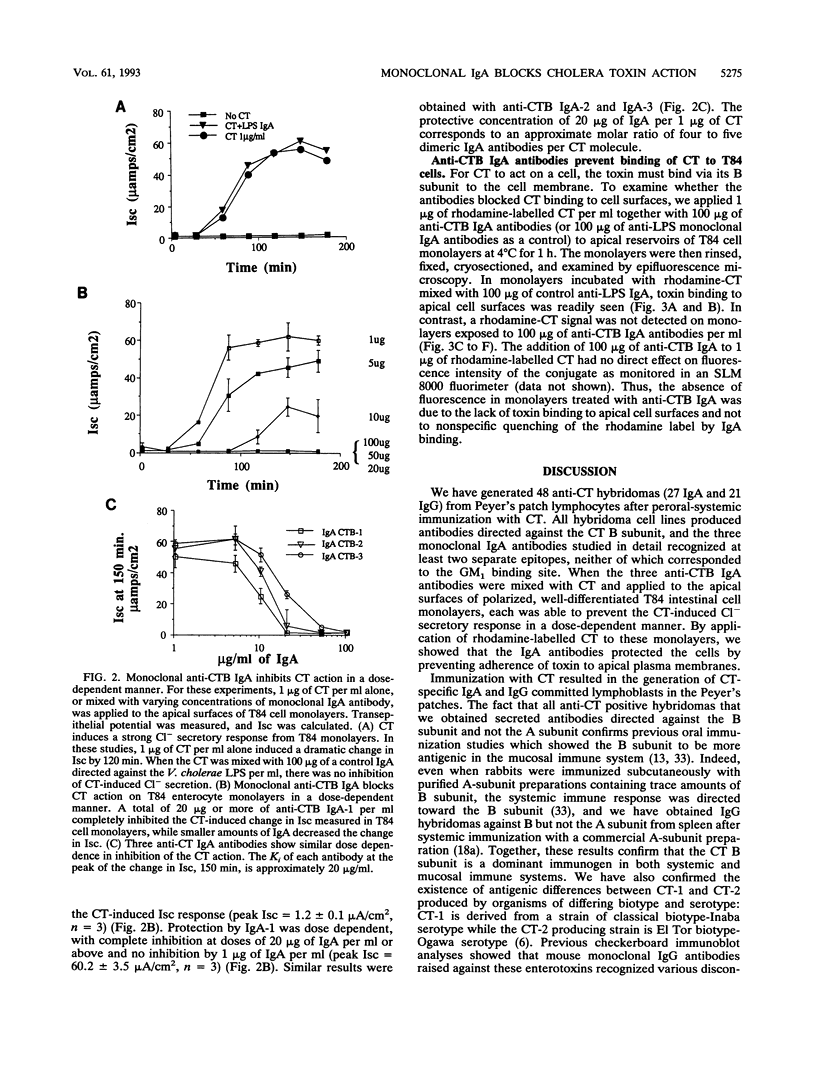
Secretory immunoglobulin A (IgA) antibodies directed against cholera toxin (CT) are thought to be important in resistance to oral challenge with virulent Vibrio cholerae, although alternative mechanisms for protection of intestinal epithelia against CT-induced fluid secretion have been proposed. The ability of anti-CT IgA to block the effects of CT on human enterocytes has not been directly tested because of the lack of a well-defined in vitro intestinal epithelial cell system to directly measure toxin action and the limited availability of purified anti-CT IgA antibodies. We have generated hybridomas that produce monoclonal IgA and IgG antibodies directed against CT by fusion of Peyer's patch cells with mouse myeloma cells after oral-systemic immunization of mice with CT and CT B-subunit protein. All of the anti-CT antibodies recognized the B subunit. Three clones (designated anti-CTB IgA-1, IgA-2, and IgA-3) which produced IgA antibodies in dimeric and polymeric forms were selected. Checkerboard immunoblotting demonstrated that IgA-1 recognized an epitope distinct from that recognized by IgA-2 and IgA-3 and that none of the antibodies were directed against the binding site of GM1, the intestinal cell membrane toxin receptor. The protective capacity of these IgAs was tested in vitro with human T84 colon carcinoma cells grown on permeable supports as confluent monolayers of polarized enterocytes. When each anti-CTB IgA was mixed with 10 nM CT and applied to the apical surfaces of T84 cell monolayers, all three IgAs blocked CT-induced Cl- secretion in a dose-dependent manner and completely inhibited binding of rhodamine-labelled CT to apical cell membranes. Thus, monoclonal anti-CTB IgA antibodies are sufficient to protect human enterocytes in vitro against CT binding and action.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apter F. M., Michetti P., Winner L. S., 3rd, Mack J. A., Mekalanos J. J., Neutra M. R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993 Dec;61(12):5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Madara J. L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. A., Lange S., Lönnroth I. Internalization in vivo of cholera toxin in the small intestinal epithelium of the rat. Acta Pathol Microbiol Immunol Scand A. 1984 Jan;92(1):15–21. doi: 10.1111/j.1699-0463.1984.tb04372.x. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Hörnqvist E., Goldschmidt T. J., Holmdahl R., Lycke N. Host defense against cholera toxin is strongly CD4+ T cell dependent. Infect Immun. 1991 Oct;59(10):3630–3638. doi: 10.1128/iai.59.10.3630-3638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986 Aug;24(2):203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Study of epitopes of cholera enterotoxin-related enterotoxins by checkerboard immunoblotting. Infect Immun. 1990 Jul;58(7):2352–2360. doi: 10.1128/iai.58.7.2352-2360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Lönnroth I., Nygren H. Protection against experimental cholera in the rat. A study on the formation of antibodies against cholera toxin and desensitization of adenylate cyclase after immunization with cholera toxin. Int Arch Allergy Appl Immunol. 1984;75(2):143–148. [PubMed] [Google Scholar]
- Lencer W. I., Delp C., Neutra M. R., Madara J. L. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992 Jun;117(6):1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Modern vaccines. Enteric infections. Lancet. 1990 Apr 21;335(8695):958–961. doi: 10.1016/0140-6736(90)91013-z. [DOI] [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Lycke N., Bromander A. K., Holmgren J. Role of local IgA antitoxin-producing cells for intestinal protection against cholera toxin challenge. Int Arch Allergy Appl Immunol. 1989;88(3):273–279. doi: 10.1159/000234806. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand J Immunol. 1987 Apr;25(4):407–412. doi: 10.1111/j.1365-3083.1987.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Lange S., Skadhauge E. The antisecretory factors: inducible proteins which modulate secretion in the small intestine. Comp Biochem Physiol A Comp Physiol. 1988;90(4):611–617. doi: 10.1016/0300-9629(88)90675-5. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P., Mahan M. J., Slauch J. M., Mekalanos J. J., Neutra M. R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992 May;60(5):1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Mekalanos J. J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988 Nov;56(11):2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinz H. Pathogenesis of intestinal infections. Arch Pathol. 1969 Jun;87(6):556–562. [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Tamaru T., Brown W. R. IgA antibodies in rat bile inhibit cholera toxin-induced secretion in ileal loops in situ. Immunology. 1985 Aug;55(4):579–583. [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin R., Lucia-Jandris P., Michetti P., Fields B. N., Kraehenbuhl J. P., Neutra M. R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989 May;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. L., Walker W. A. Immunological control mechanism against cholera toxin: interference with toxin binding to intestinal receptors. Infect Immun. 1976 Oct;14(4):1034–1042. doi: 10.1128/iai.14.4.1034-1042.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]