Abstract
Secretory immunoglobulin A (IgA) antibodies (sIgA) directed against cholera toxin (CT) and surface components of Vibrio cholerae are associated with protection against cholera, but the relative importance of specific sIgAs in protection is unknown. A monoclonal IgA directed against the V. cholerae lipopolysaccharide (LPS), secreted into the intestines of neonatal mice bearing hybridoma tumors, was previously shown to provide protection against a lethal oral dose of 10(7) V. cholerae cells. We show here that a single oral dose of 5 to 50 micrograms of the monoclonal anti-LPS IgA, given within 2 h before V. cholerae challenge, protected neonatal mice against challenge. In contrast, an oral dose of 80 micrograms of monoclonal IgA directed against CT B subunit (CTB) failed to protect against V. cholerae challenge. A total of 80 micrograms of monoclonal anti-CTB IgA given orally protected neonatal mice from a lethal (5-micrograms) oral dose of CT. Secretion of the same anti-CTB IgA antibodies into the intestines of mice bearing IgA hybridoma backpack tumors, however, failed to protect against lethal oral doses of either CT (5 micrograms) or V. cholerae (10(7) cells). Furthermore, monoclonal anti-CTB IgA, either delivered orally or secreted onto mucosal surfaces in mice bearing hybridoma tumors, did not significantly enhance protection over that provided by oral anti-LPS IgA alone. These results demonstrate that anti-LPS sIgA is much more effective than anti-CT IgA in prevention of V. cholerae-induced diarrheal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apter F. M., Lencer W. I., Finkelstein R. A., Mekalanos J. J., Neutra M. R. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993 Dec;61(12):5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buts J. P., Delacroix D. L. Ontogenic changes in secretory component expression by villous and crypt cells of rat small intestine. Immunology. 1985 Jan;54(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Harris J. R., Sack D. A., Chakraborty J., Ahmed F., Stanton B. F., Khan M. U., Kay B. A., Huda N., Khan M. R. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60–69. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Kay B. A., Khan M. U., Yunus M., Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986 Jul 19;2(8499):124–127. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Malburny G. N., Vaerman J. P. Hepatobiliary transport of plasma IgA in the mouse: contribution to clearance of intravascular IgA. Eur J Immunol. 1985 Sep;15(9):893–899. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Ito S. Form and function of the glycocalyx on free cell surfaces. Philos Trans R Soc Lond B Biol Sci. 1974 Jul 25;268(891):55–66. doi: 10.1098/rstb.1974.0015. [DOI] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986 Aug;24(2):203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Burwen S. J. Hepatic receptors and their ligands: problems of intracellular sorting and vectorial movement. Semin Liver Dis. 1985 May;5(2):136–146. doi: 10.1055/s-2008-1063918. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
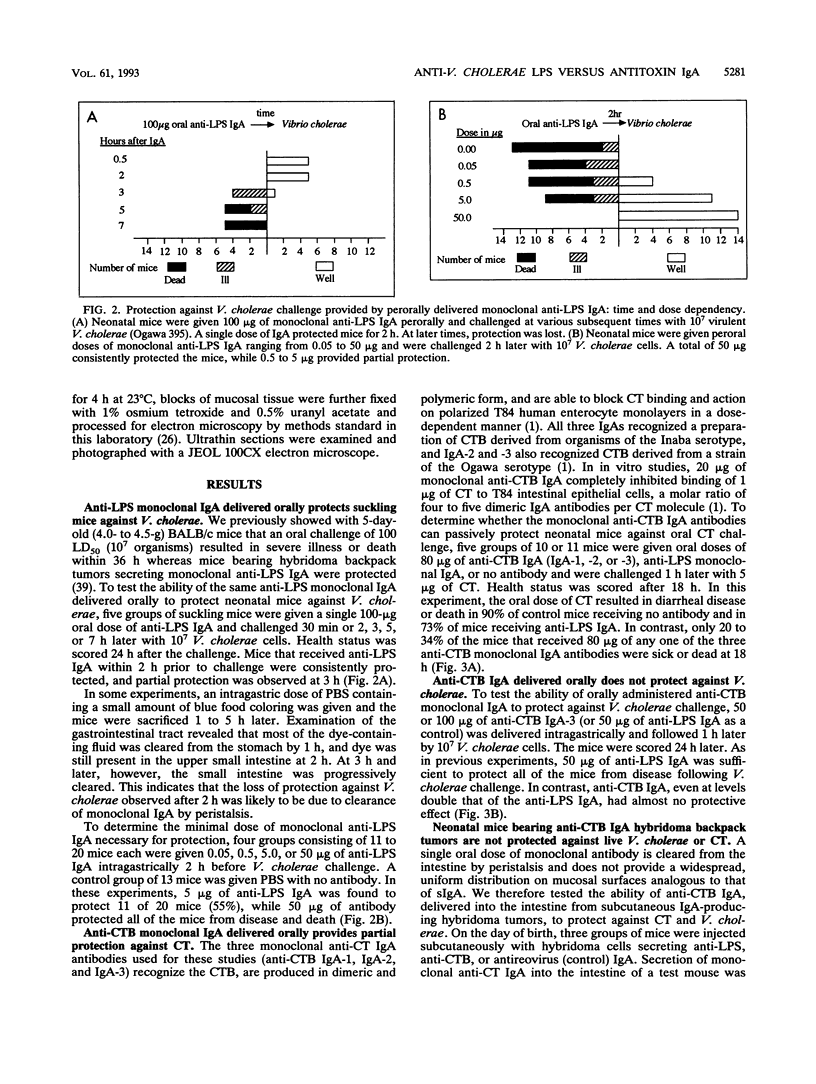
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Bromander A. K., Holmgren J. Role of local IgA antitoxin-producing cells for intestinal protection against cholera toxin challenge. Int Arch Allergy Appl Immunol. 1989;88(3):273–279. doi: 10.1159/000234806. [DOI] [PubMed] [Google Scholar]
- Lycke N., Eriksen L., Holmgren J. Protection against cholera toxin after oral immunization is thymus-dependent and associated with intestinal production of neutralizing IgA antitoxin. Scand J Immunol. 1987 Apr;25(4):413–419. doi: 10.1111/j.1365-3083.1987.tb02208.x. [DOI] [PubMed] [Google Scholar]
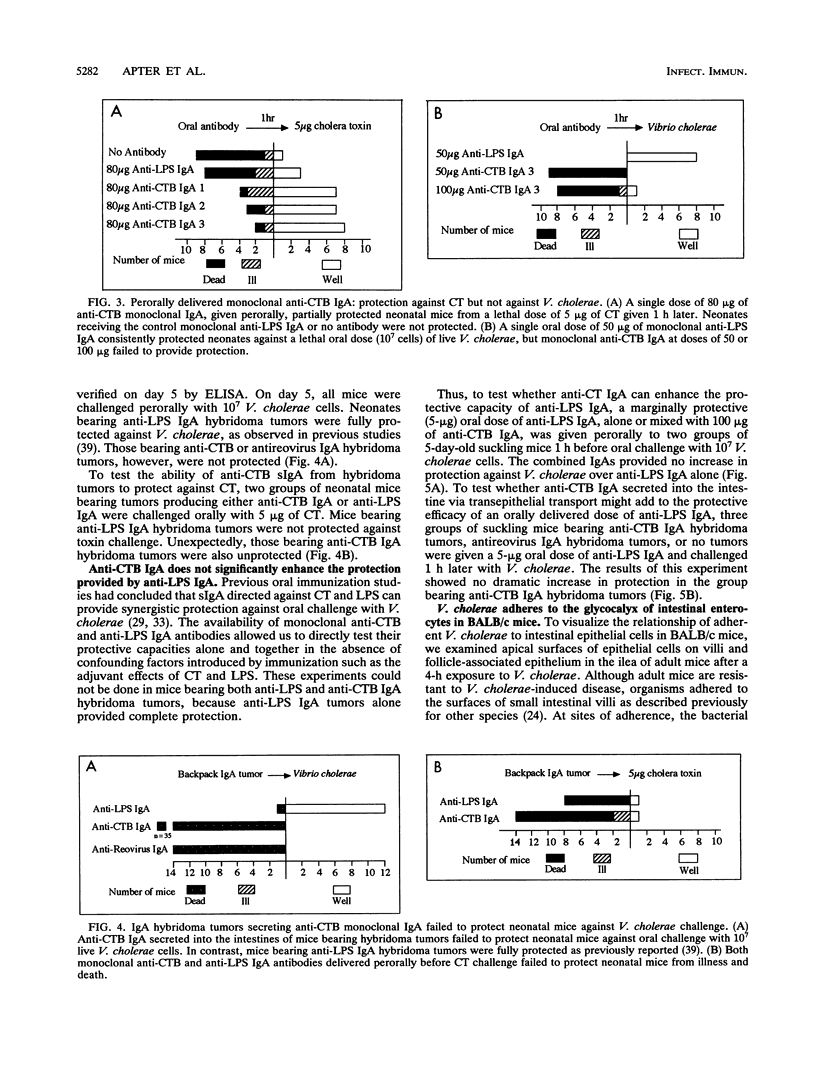
- Lycke N., Holmgren J. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand J Immunol. 1987 Apr;25(4):407–412. doi: 10.1111/j.1365-3083.1987.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A. S., Ghose A. C. Evaluation of the biological properties of different classes of human antibodies in relation to cholera. Infect Immun. 1981 Apr;32(1):9–14. doi: 10.1128/iai.32.1.9-14.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P., Mahan M. J., Slauch J. M., Mekalanos J. J., Neutra M. R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992 May;60(5):1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
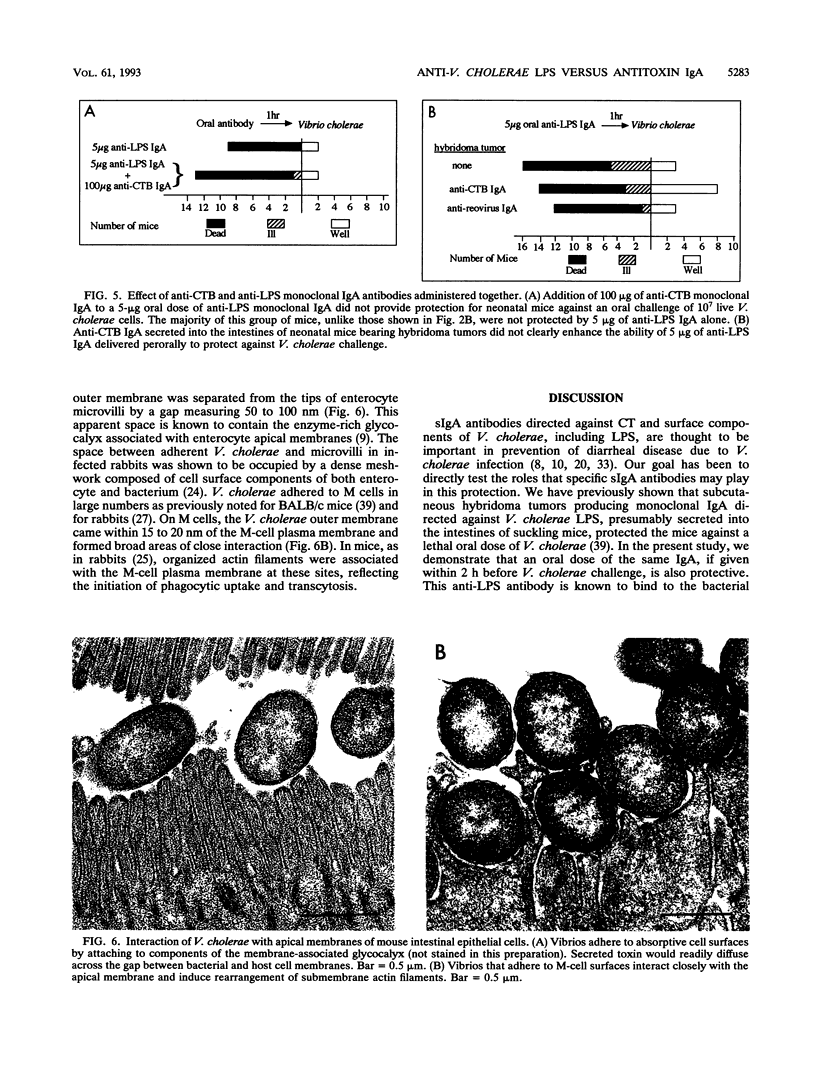
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M. R., Phillips T. L., Mayer E. L., Fishkind D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987 Mar;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Engel P. F. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect Immun. 1980 Feb;27(2):632–637. doi: 10.1128/iai.27.2.632-637.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P. G., Solbreux P., Vaerman J. P. Cholera toxin-induced fluid secretion in rat gut ligated loops: influence of bile from normal or cholera toxin-immunized rats. Immunology. 1989 Nov;68(3):319–324. [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Mestecky J. Preferential transport of IgA and IgA-immune complexes to bile compared with other external secretions. Mol Immunol. 1982 May;19(5):677–682. doi: 10.1016/0161-5890(82)90369-8. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Lake J. R., Renner E. L., Licko V., Van Dyke R. W. Fluid phase endocytosis by cultured rat hepatocytes and perfused rat liver: implications for plasma membrane turnover and vesicular trafficking of fluid phase markers. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9488–9492. doi: 10.1073/pnas.83.24.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerman J. P., Derijck-Langendries A., Rits M., Delacroix D. Neutralization of cholera toxin by rat bile secretory IgA antibodies. Immunology. 1985 Mar;54(3):601–603. [PMC free article] [PubMed] [Google Scholar]
- Weltzin R., Lucia-Jandris P., Michetti P., Fields B. N., Kraehenbuhl J. P., Neutra M. R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989 May;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]