Abstract
Mycobacterial fractions, some of which are associated with the cell envelope of Mycobacterium avium serovar 4, were assessed for their ability to affect various immunological functions of human peripheral blood mononuclear cells (PBM). Treatment of PBM with a total lipid fraction derived from M. avium serovar 4 resulted in a significant suppression of lymphoproliferative responsiveness to phytohemagglutinin stimulation at concentrations not affecting cell viability. Although a similar suppression was not observed when PBM were treated with purified serovar 4-specific glycopeptidolipids (GPL), treatment with the beta-lipid fragment derived from the GPL did result in a significant suppression of phytohemagglutinin responsiveness. Further studies revealed that the total lipid fraction and the beta-lipid fragment were effective at significantly reducing the ability of human macrophages to restrict the intracellular growth of mycobacteria and at stimulating PBM to secrete prostaglandin E2. These same effects were not observed when purified GPL or the reduced oligosaccharide fragment of the GPL was used. Other studies revealed that the total lipid and purified GPL fractions were effective at stimulating tumor necrosis factor alpha release from human PBM, whereas the beta-lipid fragment was not. These results indicate that mycobacterial lipids have various immunomodulatory capabilities, depending upon their chemical nature and ability to interact with certain host cells.
Full text
PDF




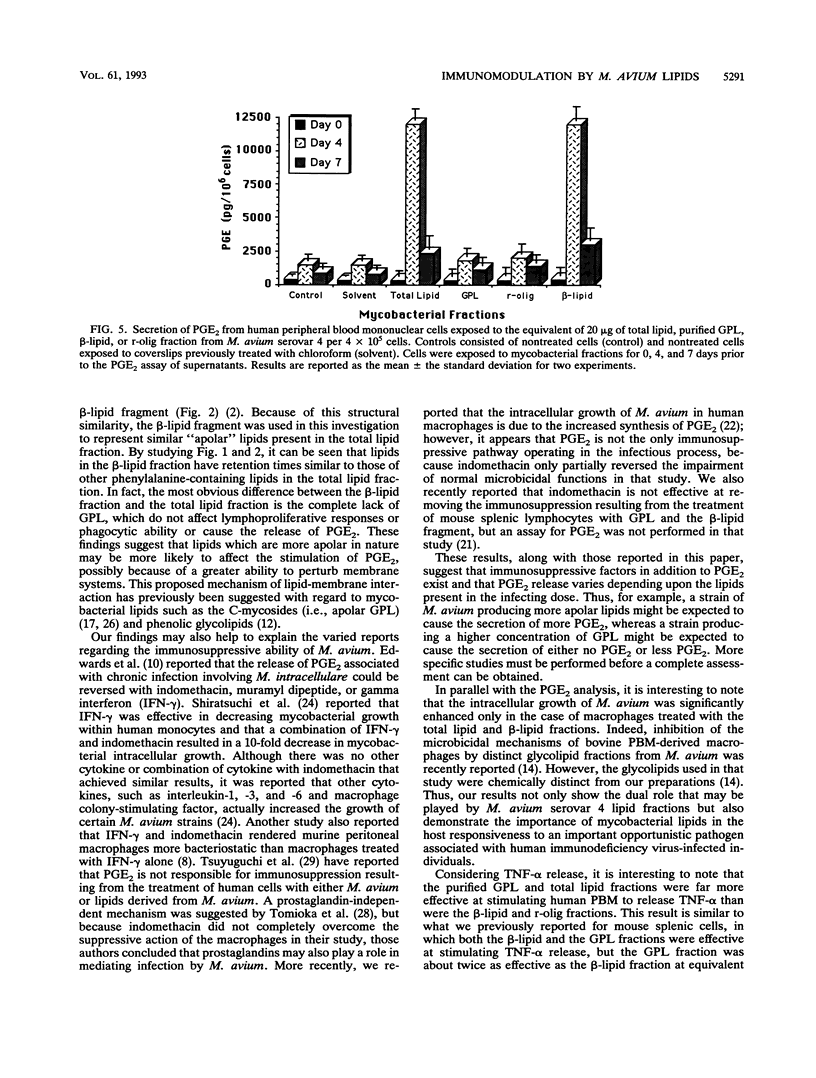
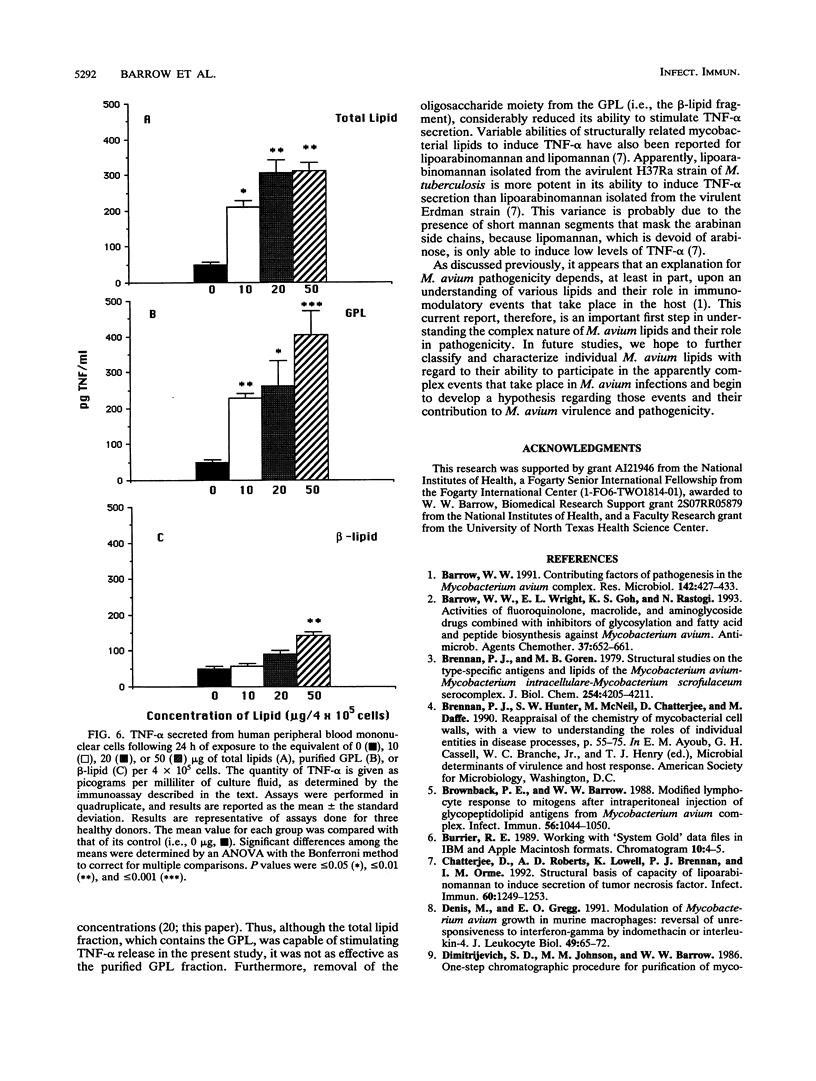

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W. Contributing factors of pathogenesis in the Mycobacterium avium complex. Res Microbiol. 1991 May;142(4):427–433. doi: 10.1016/0923-2508(91)90115-q. [DOI] [PubMed] [Google Scholar]
- Barrow W. W., Wright E. L., Goh K. S., Rastogi N. Activities of fluoroquinolone, macrolide, and aminoglycoside drugs combined with inhibitors of glycosylation and fatty acid and peptide biosynthesis against Mycobacterium avium. Antimicrob Agents Chemother. 1993 Apr;37(4):652–661. doi: 10.1128/aac.37.4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Modulation of Mycobacterium avium growth in murine macrophages: reversal of unresponsiveness to interferon-gamma by indomethacin or interleukin-4. J Leukoc Biol. 1991 Jan;49(1):65–72. doi: 10.1002/jlb.49.1.65. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. E., 2nd, Jaynes J. M., Barker S. A., Newton J. C., Enright F. M., Snider T. G., 3rd Isolation and partial characterization of glycolipid fractions from Mycobacterium avium serovar 2 (Mycobacterium paratuberculosis 18) that inhibit activated macrophages. Infect Immun. 1993 Jan;61(1):1–7. doi: 10.1128/iai.61.1.1-7.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L. C., Barrow W. W. Decreased mitogenic response of murine spleen cells following intraperitoneal injection of serovar-specific glycopeptidolipid antigens from the Mycobacterium avium complex. Adv Exp Med Biol. 1988;239:309–325. doi: 10.1007/978-1-4757-5421-6_31. [DOI] [PubMed] [Google Scholar]
- Lanéelle G., Daffé M. Mycobacterial cell wall and pathogenicity: a lipodologist's view. Res Microbiol. 1991 May;142(4):433–437. doi: 10.1016/0923-2508(91)90116-r. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Merino J., Casado J. A., Cid J., Sánchez-Ibarrola A., Subirá M. L. The measurement of transforming growth factor type beta (TGF beta) levels produced by peripheral blood mononuclear cells requires the efficient elimination of contaminating platelets. J Immunol Methods. 1992 Aug 30;153(1-2):151–159. doi: 10.1016/0022-1759(92)90317-m. [DOI] [PubMed] [Google Scholar]
- Pourshafie M., Ayub Q., Barrow W. W. Comparative effects of Mycobacterium avium glycopeptidolipid and lipopeptide fragment on the function and ultrastructure of mononuclear cells. Clin Exp Immunol. 1993 Jul;93(1):72–79. doi: 10.1111/j.1365-2249.1993.tb06499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Bachelet M., Carvalho de Sousa J. P. Intracellular growth of Mycobacterium avium in human macrophages is linked to the increased synthesis of prostaglandin E2 and inhibition of the phagosome-lysosome fusions. FEMS Microbiol Immunol. 1992 Jul;4(5):273–279. doi: 10.1111/j.1574-6968.1992.tb05006.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V., Goh K. S., De Sousa J. P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2473–2480. doi: 10.1128/aac.35.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Sors H., Pradelles P., Dray F., Rigaud M., Maclouf J., Bernard P. Analytical methods for thromboxane B2 measurement and validation of radioimmunoassay by gas liquid chromatography-mass spectrometry. Prostaglandins. 1978 Aug;16(2):277–290. doi: 10.1016/0090-6980(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Sut A., Sirugue S., Sixou S., Lakhdar-Ghazal F., Tocanne J. F., Lanéelle G. Mycobacteria glycolipids as potential pathogenicity effectors: alteration of model and natural membranes. Biochemistry. 1990 Sep 11;29(36):8498–8502. doi: 10.1021/bi00488a042. [DOI] [PubMed] [Google Scholar]
- Tassell S. K., Pourshafie M., Wright E. L., Richmond M. G., Barrow W. W. Modified lymphocyte response to mitogens induced by the lipopeptide fragment derived from Mycobacterium avium serovar-specific glycopeptidolipids. Infect Immun. 1992 Feb;60(2):706–711. doi: 10.1128/iai.60.2.706-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Saito H., Yamada Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J Gen Microbiol. 1990 May;136(5):965–973. doi: 10.1099/00221287-136-5-965. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Takashima T., Tsuyuguchi T., Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990 May;58(5):1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt R. F., Jr, Phillips D. L., Henderson L. O., Whitfield W., Spierto F. W. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J Immunol Methods. 1987 Jul 16;101(1):43–50. doi: 10.1016/0022-1759(87)90214-6. [DOI] [PubMed] [Google Scholar]
- Woodbury J. L., Barrow W. W. Radiolabelling of Mycobacterium avium oligosaccharide determinant and use in macrophage studies. J Gen Microbiol. 1989 Jul;135(7):1875–1884. doi: 10.1099/00221287-135-7-1875. [DOI] [PubMed] [Google Scholar]
- Wright E. L., Barrow W. W. Inhibition of glycopeptidolipid synthesis resulting from treatment of Mycobacterium avium with 2-deoxy-D-glucose. Res Microbiol. 1991 Jun;142(5):597–608. doi: 10.1016/0923-2508(91)90193-e. [DOI] [PubMed] [Google Scholar]