Abstract
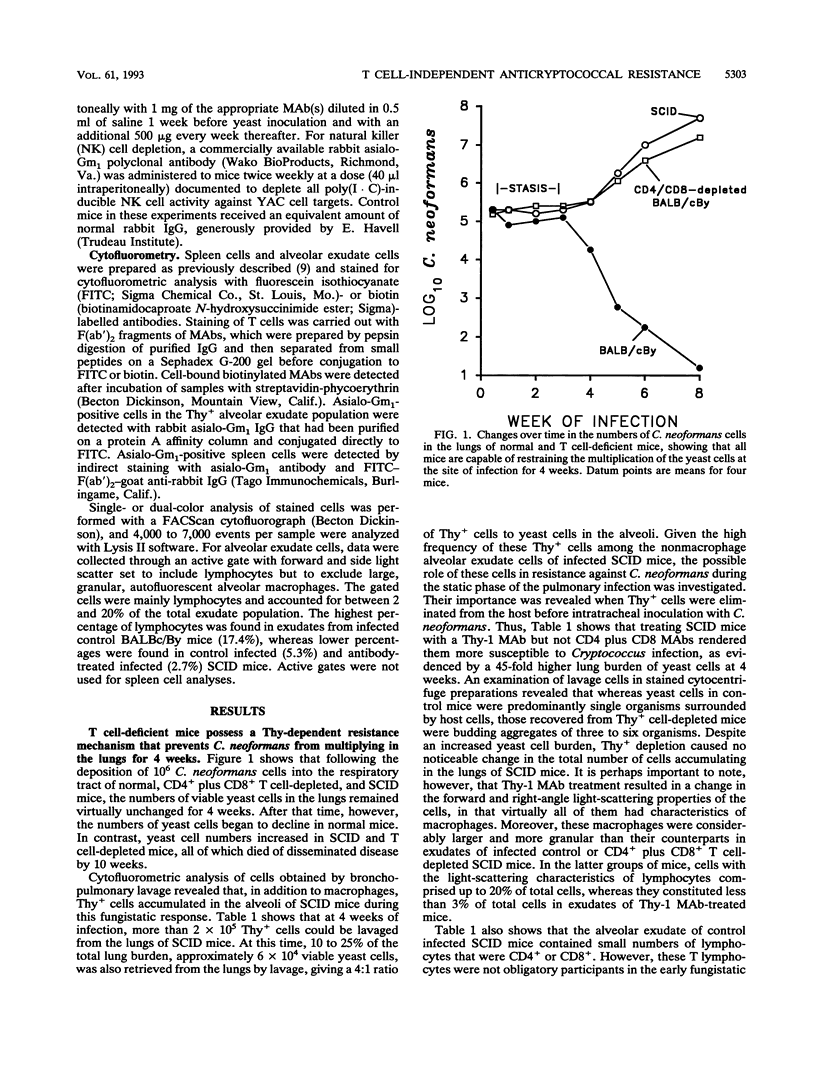
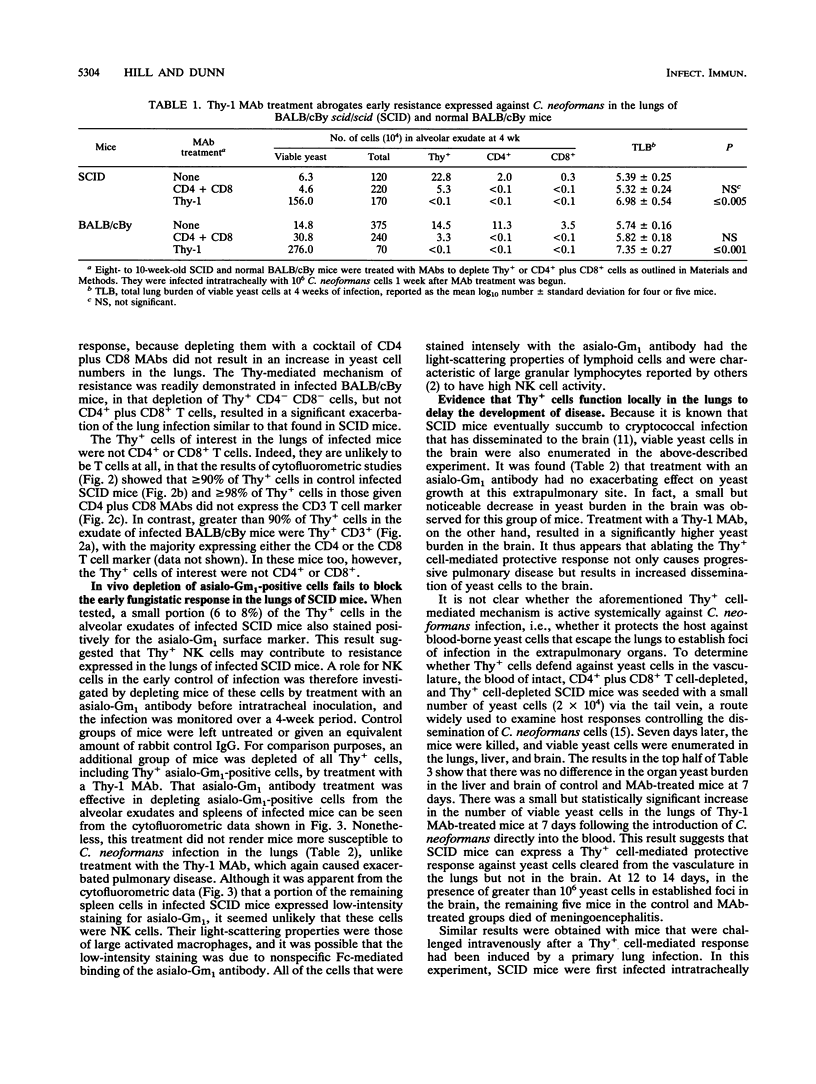
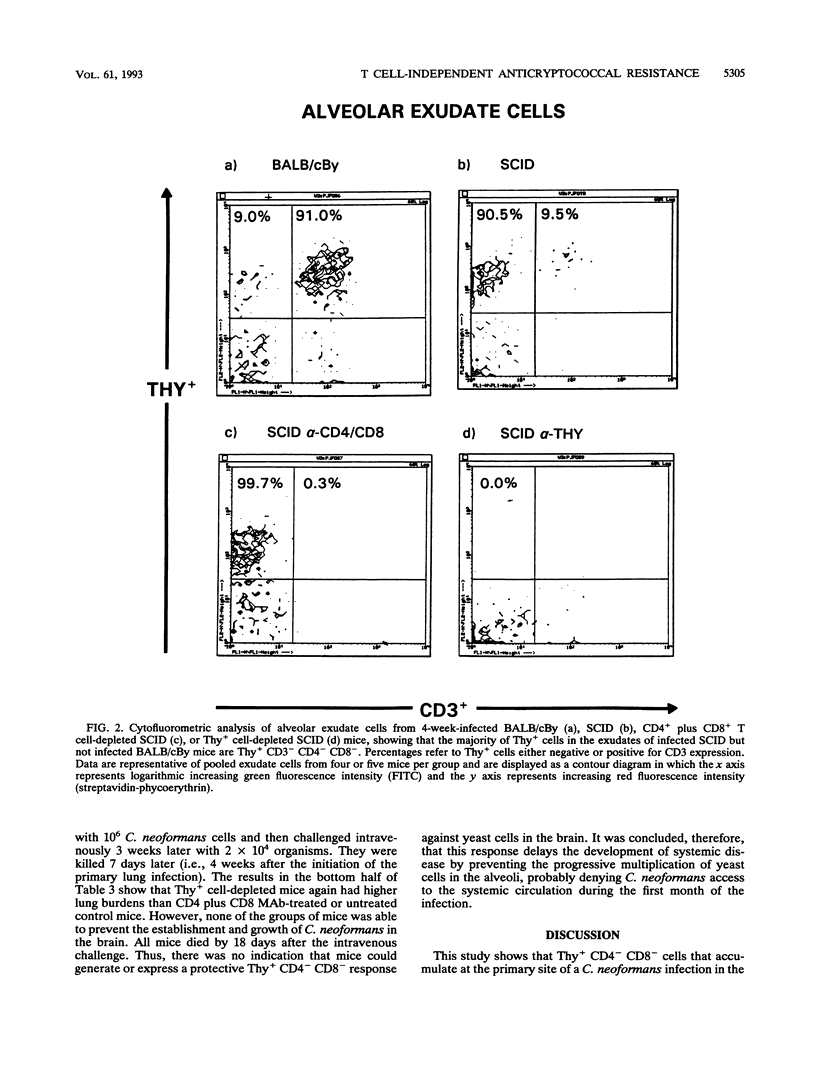
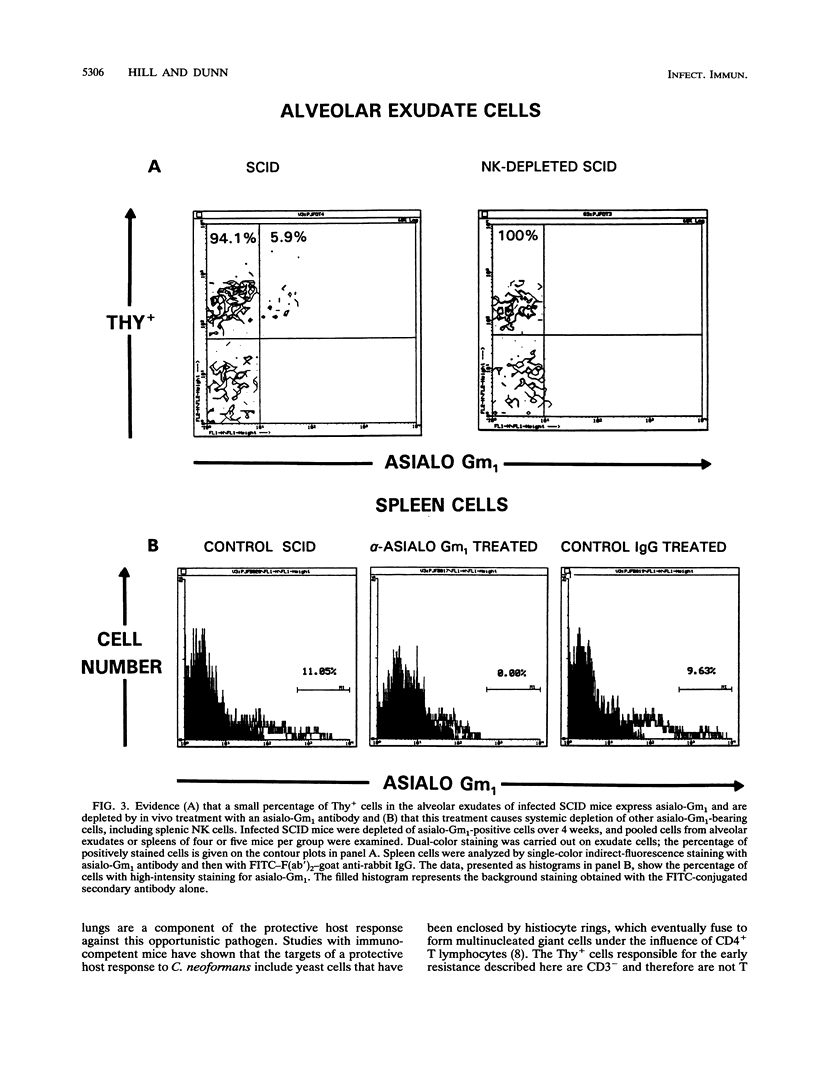
T cell-independent host resistance expressed against a primary lung infection with Cryptococcus neoformans was investigated. Following intratracheal inoculation of the yeast, BALB/cBy scid/scid mice or CD4+ plus CD8+ T cell-depleted BALB/cBy mice developed a primary lung infection that remained stable for several weeks before progressing and disseminating to kill the host. By contrast, normal BALB/cBy hosts resolved the infection after 4 to 8 weeks. Thy+ CD4- CD8- cells were found to accumulate in the pulmonary alveoli of infected scid/scid or normal mice. Depletion of these cells caused the infection to progress more rapidly and resulted 4 weeks later in a 30- to 70-fold increase in yeast numbers in the lungs and dissemination to extrapulmonary sites. Cytofluorometric studies revealed that the Thy+ CD4- CD8- cells responsible were negative for the CD3 T cell marker. A small percentage of these Thy+ CD3- cells expressed asialo-Gm1, but treatment with asialo-Gm1 antibody did not have the same infection-enhancing effect as Thy-1 monoclonal antibody treatment. Further experiments revealed that Thy-1 monoclonal antibody treatment had no effect on the establishment of infectious foci in the brain or liver following intravenous inoculation of the yeast. The data point to the existence of an early resistance mechanism for which Thy+ CD3- CD4- CD8- cells are essential. This mechanism of host defense, while insufficient for complete protection, may be capable of delaying the development of cryptococcal meningoencephalitis by restricting the growth of the yeast at primary sites of infection in the lungs, even in immunodeficient mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Resolution of primary murine listeriosis and acquired resistance to lethal secondary infection can be mediated predominantly by Thy-1+ CD4- CD8- cells. J Infect Dis. 1991 Nov;164(5):869–877. doi: 10.1093/infdis/164.5.869. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Greenfield R. A., Abrams V. L., Crawford D. L., Kuhls T. L. Effect of abrogation of natural killer cell activity on the course of candidiasis induced by intraperitoneal administration and gastrointestinal candidiasis in mice with severe combined immunodeficiency. Infect Immun. 1993 Jun;61(6):2520–2525. doi: 10.1128/iai.61.6.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991 May;59(5):1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992 Jun 1;175(6):1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Harmsen A. G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991 Mar 1;173(3):755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991 Apr 1;173(4):793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991 Apr;59(4):1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Vazquez-Torres A., Balish E. Poly(I.C)-induced interferons enhance susceptibility of SCID mice to systemic candidiasis. Infect Immun. 1992 Nov;60(11):4549–4557. doi: 10.1128/iai.60.11.4549-4557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Kovacs A. A., Polis M., Wright W. C., Gill V. J., Tuazon C. U., Gelmann E. P., Lane H. C., Longfield R., Overturf G. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Dupont M. P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J Clin Invest. 1993 Apr;91(4):1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Alvarellos T., Toews G. B., Tompkins R., Evans Z., Koo G., Kumar V. Role of natural killer cells in resistance to Cryptococcus neoformans infections in mice. Am J Pathol. 1987 Aug;128(2):354–361. [PMC free article] [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K., Mohri S., Watanabe T., Mori R. Latent infection of SCID mice with herpes simplex virus 1 and lethal cutaneous lesions in pregnancy. Microbiol Immunol. 1992;36(8):841–853. doi: 10.1111/j.1348-0421.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Hidore M. R., Wong S. C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Invest. 1993 Apr;91(4):1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford M. C., Ooi H. K., Oku Y., Kamiya M. Secondary Echinococcus multilocularis infection in severe combined immunodeficient (scid) mice: biphasic growth of the larval cyst mass. Int J Parasitol. 1992 Nov;22(7):975–982. doi: 10.1016/0020-7519(92)90056-q. [DOI] [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991 Feb;59(2):486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]


