Abstract
The ultimate goal of this study is to regenerate lost dental pulp and dentin via stem/progenitor cell–based approaches and tissue engineering technologies. In this study, we tested the possibility of regenerating vascularized human dental pulp in emptied root canal space and producing new dentin on existing dentinal walls using a stem/progenitor cell–mediated approach with a human root fragment and an immunocompromised mouse model. Stem/progenitor cells from apical papilla and dental pulp stem cells were isolated, characterized, seeded onto synthetic scaffolds consisting of poly-D,L-lactide/glycolide, inserted into the tooth fragments, and transplanted into mice. Our results showed that the root canal space was filled entirely by a pulp-like tissue with well-established vascularity. In addition, a continuous layer of dentin-like tissue was deposited onto the canal dentinal wall. This dentin-like structure appeared to be produced by a layer of newly formed odontoblast-like cells expressing dentin sialophosphoprotein, bone sialoprotein, alkaline phosphatase, and CD105. The cells in regenerated pulp-like tissue reacted positively to anti-human mitochondria antibodies, indicating their human origin. This study provides the first evidence showing that pulp-like tissue can be regenerated de novo in emptied root canal space by stem cells from apical papilla and dental pulp stem cells that give rise to odontoblast-like cells producing dentin-like tissue on existing dentinal walls.
Introduction
Regeneration of dental pulp/dentin tissues in the pulp space of teeth serves the ultimate goal of preserving teeth via endodontic approaches. Attempts to induce tissue regeneration in the pulp space have been a longstanding quest. Pulp tissue regeneration has been explored using the biodegradable synthetic material polyglycolic acid seeded with pulp cells, and results showed pulp-like tissue formation in both in vitro and in vivo models.1–3 These previous approaches were the proof-of-principle studies that only tested the formation of a pulp-like soft tissue without dentin. From a clinical perspective, the following issues must be considered when attempting to regenerate functional pulp/dentin tissues in a root canal space: (i) regenerated pulp tissue must be vascularized although the blood supply is only available from the apical end; (ii) newly differentiated odontoblasts should form on the existing dentinal wall in the root canal space, and (iii) new dentin should be produced by the new odontoblasts onto the existing dentin.4,5
Using a tooth slice model (horizontal section, 1 mm thick), it was shown that the stem cells from human exfoliated deciduous teeth seeded onto the synthetic scaffolds that were fabri-cated in the pulp chamber space formed well-vascularized pulp-like tissue in an in vivo study model. In addition, odontoblast-like cells derived from the pulp-like tissue were localized against the existing dentin surface.6 Tooth slice model was used to ensure blood supply to the stem cell–seeded scaffolds. To date, there is a lack of evidence demonstrating that the human pulp tissue can be regenerated in an emptied root canal space with only one opening to the blood supply, and showing that the regenerated pulp tissue would form a continuous layer of newly deposited dentin onto the existing dentinal walls. Using human DPSCs from permanent teeth seeded onto a dentin surface, small amounts of discontinuous dentin-like mineralized tissue on the existing dentin surface have been observed in vivo.7 Previously, we reported that the developing organ at the apex of the tooth, the apical papilla, contained multipotent stem cells, named stem cells from apical papilla (SCAP), that were able to form pieces of pulp-dentin complex in an animal model.8,9 Moreover, SCAP are cells from a developing tissue and are more robust than DPSCs in terms of population doubling capacity, proliferation rate, telomerase activity, and cell migration ability.8 We hypothesized that vascularized pulp-like and dentin-like tissues can be regenerated in an emptied root canal space with DPSCs and SCAP. Using a model system that includes ex vivo–expanded human DPSCs and SCAP, synthetic scaffolds, human root segments, and immunocompromised mice, we show here for the first time the regeneration of vascularized pulp-like tissue and the formation of dentin-like mineral structures depositing onto the existing dentinal wall in the root canal space.
Materials and Methods
Sample collection
Normal human-impacted third molars (n = 12) with or without immature roots were collected from healthy consenting patients (eight donors aged 16–24 years) in the Dental Clinics at the University of Maryland and the University of Southern California. Freshly extracted teeth were stored in serum-free cell culture medium and transported to the laboratory for processing. Bone marrow was obtained from the long bone of healthy consenting patients (aged 20–63) undergoing orthopedic surgery at the Walter Reed Army Medical Center. Sample collection conformed to approved protocols by the respective Medical Institutional Review Boards and the National Institutes of Health.
Cell culture
Isolation of DPSCs and SCAP was described previously.8–11 In brief, pulp tissue and apical papilla were removed from teeth, minced, and digested in a solution of 3 mg/mL collagenase type I and 4 mg/mL dispase (Sigma-Aldrich, St. Louis, MO) for 30–60 min at 37°C. The digested mixtures were passed through a 70-μm cell strainer (Falcon; BD Labware, Franklin Lakes, NJ) to obtain single-cell suspensions. Cells were seeded onto six-well plates and cultured with α-minimum essential medium (Gibco–Invitrogen, Carlsbad, CA) supplemented with 15–20% fetal bovine serum (FBS; Gemini Bio-Products, Woodland, CA), 2 mM L-glutamine, 100 μM L-ascorbic acid-2-phospate, 100 U/mL penicillin-G, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone (Gemini Bio-Products) and maintained in 5% CO2 at 37°C. Colony formation units of fibroblastic cells were normally observed within 1–2 weeks after cell seeding and were passaged at 1:3 ratio when they reached ∼80% confluence. Heterogeneous populations of DPSCs and SCAP were frozen and stored in liquid nitrogen at passages 0–2. Cells were thawed and expanded for experimentation at passage 3. Human bone marrow mesenchymal stem cells (BMMSCs) were isolated and cultured as previously described11–13 and were cultured in expansion medium containing Dulbecco's modified Eagle's medium and 10% preselected FBS. Heterogeneous populations of isolated colony formation units of fibroblastic cells were used for all the assays and tissue regeneration experiments.
Flow cytometry
Cell aliquots of SCAP and DPSCs (>2 × 105 cells) were washed and resuspended in phosphate-buffered saline (PBS) + 0.1% FBS, containing saturating concentrations (1:100 dilution) of the following conjugated mouse IgG1,κ anti-human monoclonal antibodies (BD Biosciences, San Jose, CA): CD14-PE, CD34-PE, CD45-FITC, CD73-PE, CD90-FITC, and CD105-PE for 1 h at 4°C. Cell suspensions were washed twice and resuspended in 0.1% FBS/PBS for analysis on a flow cytometer (FACS Calibur; BD Biosciences) using the CellQuest Pro™ software (BD Biosciences).
Multiple lineage differentiation
Odonto/osteogenic differentiation
Cells were seeded onto 48-well plates, grown to ∼70% confluence, and incubated in the differentiation medium containing 10 nM dexamethasone, 10 mM β-glycerophosphate, 50 μg/mL ascorbate phosphate, 10 nM 1, 25 dihydroxyvitamin D3, and 10% FBS for 5 weeks. Cultures were fixed in 60% isopropanol, and mineralization of extracellular matrix stained with 1% Alizarin Red S.
Adipogenic differentiation
Cells were seeded onto 12-well plates, grown to subconfluence, and incubated in the adipogenic medium containing 1 μM dexamethasone, 1 μg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 10% FBS for 6 weeks. Cells were fixed in 10% formalin for 60 min, washed with 70% ethanol, and lipid droplets were stained with 2% (w/v) Oil Red O reagent for 5 min and washed with water.
Neurogenic differentiation
Cells at subconfluence in chamber slides were incubated in the neurogenic induction medium Neurobasal A (Gibco-Invitrogen) with B27 supplement (GIBCO-BRL), 20 ng/mL epidermal growth factor (EGF) (BD Biosciences), and 40 ng/mL fibroblast growth factor (FGF) (BD Biosciences). After 4 weeks, cells were analyzed by immunocytofluorescence for the expression of the neural cell marker, βIII-tubulin. Isotype-matched antibodies were used as negative controls. After fixation in 100% ice-cold methanol, cells were incubated in blocking buffer (32.5 mM NaCl, 3.3 mM Na2HPO4, 0.76 mM KH2PO4, 1.9 mM NaN3, 0.1% [w/v] bovine serum albumin, 0.2% [v/v] Triton-X 100, 0.05% [v/v] Tween 20, and 5% goat serum) for 30 min followed by the addition of monoclonal mouse anti-human βIII-tubulin antibodies (Promega, Madison, WI) for 1 h at room temperature. After washing, cultures were incubated with anti-mouse Alexa Fluor 594 for 1 h at room temperature and the cell nuclei stained with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen, Carlsbad, CA). Images were analyzed under a fluorescence microscope.
Myogenic differentiation
Cells were seeded onto 12-well plates, grown to subconfluence, and induced in the myogenic medium containing 0.1 μM dexamethasone, 50 μM hydrocortisone, and 5% horse serum for 6 weeks. Cells were then harvested for RNA isolation using RNeasy Mini Kit (Qiagen, Valenicia, CA). cDNA was synthesized with SuperScript reverse transcription (RT)-polymerase chain reaction kit (Invitrogen) followed by polymerase chain reaction using Taq DNA polymerase (Invitrogen) to detect the expression of myogenic genes. The following genes were examined with specific primers: MyoD1 (forward, 5′-AAG CGA CCT CTC TTG AGG TA-3′; reverse, 5′-GCG CCT TTA TTT TGA TCA CC-3′), myogenin (forward, 5′-TAA GGT GTG TAA GAG GAA GTC G-3′; reverse, 5′-CCA CAG ACA CAT CTT CCA CTG T-3′), myosin heavy polypeptide 1 (forward, 5′-TGT GAA TGC CAA ATG TGC TT-3′; reverse, 5′- GTG GAG CTG GGT ATC CTT GA-3′), and GAPDH (forward, 5′-CAA GGC TGA GAA CGG GAA GC-3′; reverse, 5′-AGG GGG CAG AGA TGA TGA CC-3′).
Scaffold fabrication
A copolymer of poly-D,L-lactide and glycolide (PLG) (75:25 molar ratio) (Boehringer Ingelheim, Ingelheim, Germany) was employed to create porous scaffolds using a gas foaming/particulate leaching process, and cells were seeded onto the scaffolds according to the previously described procedures.14 Briefly, porous polymer scaffolds were formed by mixing PLG microspheres and salt crystals (diameter 250–425 μm), which were then loaded into a cylindrical mold of 5 (diameter) × 2 (height) mm. The mixture was then compressed at 1500 psi, yielding solid disks and foamed in a pressure vessel using CO2 at 850 psi. Subsequent solvent leaching rendered the scaffolds porous with pore diameters of 250–425 μm.
In vitro analysis of dental stem cells grown in scaffolds
Each PLG scaffold disk was cut into small pieces of ∼1.5–2.0 mm3 to allow maximal stem-cell attachment. Cells (107/mL) were suspended in cell culture medium, and 5 μL of cells per scaffold piece were loaded into the polymer scaffold for a 5-min incubation period. For the in vitro studies, the cell-seeded PLG scaffolds were placed in wells of 12-well plates, and the culture medium was changed every 2–3 days. At different time points, the cell-seeded scaffolds were processed for standard histological and scanning electronic microscopy (SEM) studies to observe their attachment and morphology. The SEM procedures followed the protocol as described previously.10,15
Preparation of root fragments of human teeth
Radicular portions of freshly extracted human teeth were horizontally sectioned into segments of ∼6–7 mm in length using an Isomet saw (Buhler, Lake Bluff, IL). The root canal space was enlarged to ∼1–2.5 mm in diameter. One end of the canal was sealed with mineral trioxide aggregate (MTA) cement ∼1 mm into the canal space, leaving the depth of the canal space ∼5–6 mm (Fig. 1). Root fragments were soaked at room temperature in 17% ethylenediamine tetraacetic acid for 10 min and 19% citric acid for 1 min to remove the smear layer,10,15 followed by treatment with betadine for 30 min and 5.25% NaOCl for 10–15 min for sterilization. Fragments were then rinsed in sterile PBS, soaked in PBS, and then incubated at 37°C for 3–7 days to remove residual sterilization agents and to ensure that there is no microbial contamination.
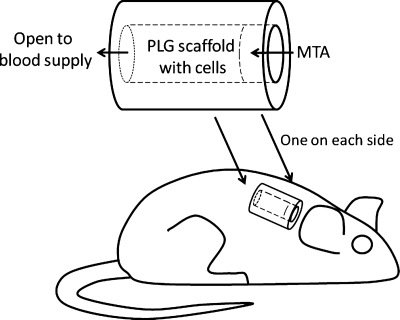
FIG. 1.
SCID mouse subcutaneous study model for pulp/dentin regeneration. The canal space of human tooth root fragments (∼6–7 mm long) was enlarged to ∼1–2.5 mm in diameter. One end of the canal opening was sealed with MTA cement. SCID, severe combined immunodeficient; MTA, mineral trioxide aggregate.
Insertion of cells/scaffolds into root fragments and implantation into mouse subcutaneous space
Cells (107/mL) were seeded onto the PLG scaffolds for 5 min as described previously for in vitro analysis. Immediately, the cells/PLG were inserted into the canal space of each root fragment and kept in the wells of 12-well plates with a minimal amount of cell culture medium. The tooth constructs were then implanted into the subcutaneous space on the back of 6- to 8-week-old female severe combined immunodeficient mice (NOD.CB17-Prkdc-scid/J; Jackson Laboratory, Bar Harbor, ME). Mice were anesthetized, and the dermal space was created by blunt lateral dissection from a single dorsal midline incision. Each mouse received two tooth fragments, one on each side. The wounds were sutured to obtain primary closure. In parallel, root fragments with empty canal space were implanted into severe combined immunodeficient mice as controls. Three to 4 months after the transplantation, the mice were euthanized and the tooth fragments removed for histological analysis. All animal procedures followed a protocol approved by the Institutional Animal Care and Use Committee at the University of Maryland.
Histological examination
Samples (normal teeth and resected transplants) were fixed in 4% phosphate-buffered paraformaldehyde, decalcified in 10–15% ethylenediaminetetraacetic acid for 1–2 months, paraffin embedded, longitudinally sectioned, and stained with hemotoxylin and eosin for histological analysis. Some of the sections were used for immunohistochemical analysis.
Immunohistochemistry
Deparaffinized sections were immersed in 3% H2O2/methanol for 15 min to quench endogenous peroxidase activity and incubated with primary antibodies (1:200 to 1:500 dilution) overnight at 4°C. Primary antibodies used were as follows: alkaline phosphatase (ALP; LF-47), dentin sialoprotein (DSP, LF-21), and bone sialoprotein (BSP; LF-120), provided by Dr. Larry Fisher (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD). Antibodies to CD105 were from BD Biosciences and to human mitochondria were from Chemicon (Temecula, CA). Isotype-matched control antibodies were used under the same conditions as the primary antibodies. For enzymatic immunohistochemical staining, Zymed SuperPicTure polymer detection kit (Zymed–Invitrogen, Carlsbad, CA) was used according to the manufacturer's protocol. Sections were counterstained with hematoxylin.
Results
In vitro characterization of dental stem cells
Cell marker analysis
Human DPSCs and SCAP consisting heterogeneous populations of stem and progenitor cells were reproducibly isolated using established protocols.8–11 Cells at passage 3 were stained with antibodies against a number of cell surface markers and analyzed by flow cytometry. CD14, CD34, and CD45 were not expressed, whereas CD73, CD90, and CD105 were expressed moderately to highly on these cells (Table 1). The surface marker expression profile suggests that these cells were of typical mesenchymal cell lineages similar to what normally is found in BMMSCs.16,17
Table 1.
Surface Epitope Phenotype of Dental Stem/Progenitor Cellsa
| Cell type | CD14 | CD34 | CD45 | CD73 | CD90 | CD105 |
|---|---|---|---|---|---|---|
| DPSC | − | − | − | ++ | ++ | + |
| SCAP | − | − | − | +++ | +++ | + |
Surface epitopes or CD markers identified on the basis of immunoreactivity analyzed by fluorescence-activated cell sorting (FACS). FACS analysis showed that more than 95% of these cells were negative (−) for the expression of CD14, CD34, and CD45 and were positive (+) for CD73, CD90, and CD105. Controls consisted of fluorescence observed with isotypic controls.
DPSC, dental pulp stem cells; SCAP, stem cells from apical papilla.
Multipotentiality of SCAP and DPSCs
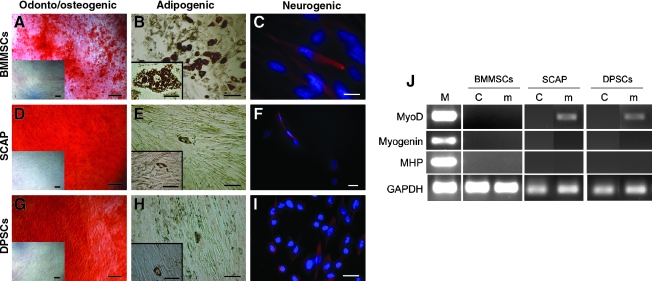
To verify that isolated dental cells qualified as stem/progenitor cells, we examined their odonto/osteogenic, adipogenic, neurogenic, and myogenic potential with BMMSCs included for comparison. Extracellular matrix production and mineralization by SCAP and DPSCs after odonto/osteogenic stimulation was comparable to or greater than those of BMMSCs (Fig. 2). SCAP and DPSCs were much weaker in adipogenicity because fewer cells developed intracellular lipid droplet accumulation and showed delayed adipogenesis compared with BMMSCs. Of those SCAP and DPSCs that did accumulate lipid droplets, they did not expand to the size of adipocyte-like cells that emerged from BMMSCs (Fig. 2B, E, H insets). Cells under neurogenic induction underwent morphological changes (not shown). In general, BMMSCs exhibited spherical cell bodies with long and multiple cellular extensions, whereas SCAP and DPSCs still possessed spindle-shaped fibroblastic cell bodies with mainly long cellular processes. We found a subpopulation of these cells (20–30%) expressing the neurogenic marker βIII-tubulin (Fig. 2C, F, I). Under myogenic stimulation, BMMSCs did not show any signs of myotube formation. SCAP and DPSCs had a slender morphology and became elongated, but without myotube formation. Myogenic gene expression analysis revealed that BMMSCs did not express any of the three genes examined (myoD1, myogenin, and myosin heavy polypeptide 1), while myoD1 was detected in SCAP and DPSCs after stimulation (Fig. 2J).
FIG. 2.
Multiple lineage differentiation properties of SCAP and DPSCs compared with BMMSCs. (A, D, G) Odonto/osteogenic induction and Alizarin Red staining of matrix mineralization. Insets: uninduced control. All scale bars including insets: 200 μm. (B, E, H) Adipogenic induction and Oil Red O staining of the accumulated lipid droplets in cells. Inset images: higher magnification views of the adipocyte-like cells from the same culture shown in the main images. Scale bars: main images, 100 μm; insets, 50 μm. (C, F, I) Neurogenic induction. βIII-tubulin: red fluorescence; 4',6-diamidino-2-phenylindole dihydrochloride (DAPI): blue fluorescence. Scale bars: (C) 20 μm; (F, I) 30 μm. (J) Myogenic induction and RT-polymerase chain reaction analysis of gene expression. M, RNA from cultured human myoblasts (obtained from Dr. Wesley M. Jackson, NIH/NIAMS, Bethesda, MD); C, uninduced control; m, induction in myogenic medium. SCAP, stem cells from apical papilla; DPSC, dental pulp stem cells; BMMSCs, bone marrow mesenchymal stem cells.
Growth of stem cells in PLG
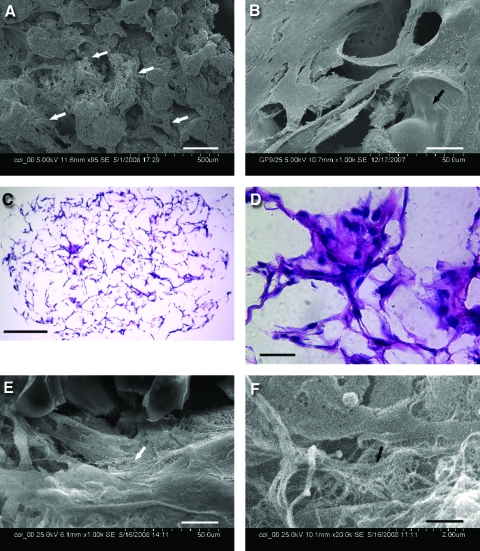
SCAP or DPSCs (5 × 105/50 μL) were seeded onto a PLG copolymer scaffold (75:25 molar ratio) cut into ∼1.5–2 mm3, cultured in vitro for 10 days or up to 8 weeks, and processed for histological or SEM analysis. Cells seeded onto PLG attached well as revealed by SEM analysis shown in Figure 3A, B, E, and F. The hemotoxylin and eosin staining showed that blank PLG scaffolds were completely colorless (not shown), suggesting that the eosin staining seen in the DPSC-seeded PLG represented the cytoplasm of cells and extracellular matrix (Fig. 3C, D). SEM analysis of the long-term cultures (8 weeks) revealed that cells laid down fiber-like matrix (Fig. 3E, F). Minimal or no contraction of cells/PLG constructs was noted after 7–8 weeks in cultures.
FIG. 3.
Cell attachment and growth in the PLG scaffold in vitro. Scanning electronic microscopy of DPSCs seeded onto PLG after (A) 10 days and (B)14 days in culture. (C, D) H&E staining of DPSCs seeded onto PLG after 7 weeks in culture. (E, F) Scanning electronic microscopy of SCAP seeded onto PLG after 8 weeks in culture. Scale bars: (A) 200 μm; (B, D, E) 20 μm; (C) 250 μm; and (F) 1 μm. Arrows in (A) indicate attached cells, in (B) scaffold surface, in (E and F) fiber-like matrix. PLG, poly-D,L-lactide and glycolide; H&E, hemotoxylin and eosin. Color images available online at www.liebertonline.com/ten.
In vivo pulp/dentin tissue regeneration
Ingrowth of subcutaneous tissue into emptied root canal space
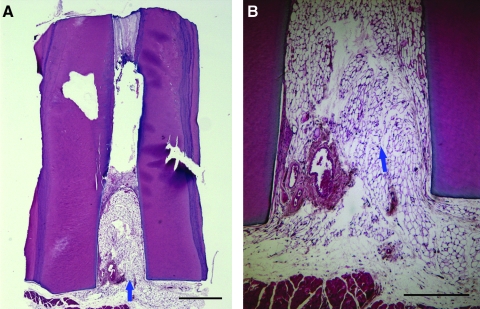
The control root fragment did not receive any cell-seeded PLG in the emptied root canal space. Three months after being transplanted into the mouse subcutaneous space, the canal space appeared to be filled with the subcutaneous connective tissue, mainly adipose tissue, which grew more than 2 mm into the canal (Fig. 4). The other half (to the MTA side) of the canal space appeared empty, or the tissue was lost during the sample processing.
FIG. 4.
Histological analysis of control group in the mouse subcutaneous model. (A) A root fragment with empty canal space was transplanted into a SCID mouse for 3 months before being removed and processed for H&E staining. Mouse subcutaneous tissue ingrown from the canal opening (arrow). (B) Higher magnification view showing the ingrown fatty tissue (arrow). Scale bars: (A) 1 mm; (B) 0.5 mm.
Vascularized pulp tissue regeneration in emptied root canal space
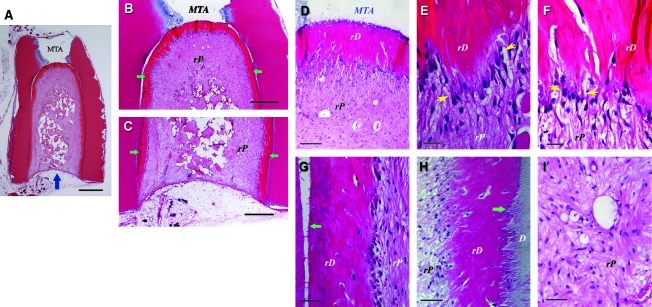
Longitudinal sections of the root constructs inserted with PLG seeded with SCAP or DPSCs revealed that the emptied canal was filled with regenerated pulp-like tissue as shown in Figures 5 and 6. Voids were scattered in the pulp-like tissue, which was most likely the result of unresorbed PLG scaffolds. There was a sharp distinction between the histological characteristics of the regenerated pulp-like tissue situated only within the canal space and those of the subcutaneous soft tissues in the mouse dermal space. A thin layer of fibrous capsule separated the two types of tissues. The entire pulp-like tissue was vascularized with a uniform cell density resembling the natural pulp (Fig. 5I).
FIG. 5.
Histological analysis of in vivo pulp/dentin regeneration using SCAP. A root fragment was prepared and the canal space inserted with SCAP/PLG and transplanted into a SCID mouse for 3 months. The sample was harvested and processed for H&E staining. D, original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Blue arrow in (A) indicates the blood supply entrance; green arrows in (B) and (C) indicate continuous layer of uniformed thickness of rD; yellow arrows in (E) and (F) indicate the region of well-aligned odontoblast-like cells with polarized cell bodies; green arrows in (G and H) indicate junctions between D and rD. Scale bars: (A) 1 mm; (B and C) 500 μm; (D) 100 μm; (E and F) 20 μm; and (G–I) 50 μm.
FIG. 6.
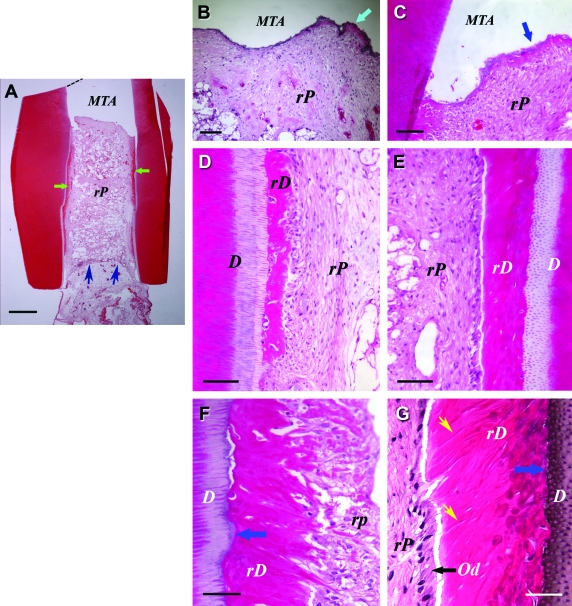
Histological analysis of in vivo pulp/dentin regeneration using DPSCs. Samples were prepared using the same procedures as described in Figure 5, except that the sample was harvested from the SCID mouse 4 months after implantation. D, original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Od, odontoblast-like cells. Green arrows in (A) indicate rD; blue arrows in (A) indicate the entrance of blood supply; blue arrows in (B and C) indicate the thin layer of rD under MTA cement; blue arrows in (F and G) indicate the junction of D and rD; black arrow in (G) indicates well-aligned odontoblast-like cells. Yellow arrows in (G) indicate dentinal tubule-like structures. Scale bars: (A) 1 mm; (B) 200 μm; (C–E) 100 μm; and (F and G) 50 μm. Color images available online at www.liebertonline.com/ten.
Formation of dentin-like tissue and odontoblast-like cells.
A representative sample using SCAP/PLG showed a continuous layer of mineralized tissue with uniform thickness (∼200 μm) deposited onto the existing dentinal walls and onto the MTA cement surface in the canal (Fig. 5); herein referred to as “regenerated dentin-like” tissue. When using DPSCs/PLG, a layer of regenerated dentin-like tissue was also deposited onto the canal dentinal walls (Fig. 6). In this sample, the regenerated dentin-like tissue had less continuity and thickness compared with the sample shown in Figure 5.
Higher magnification observations revealed that a layer of odontoblast-like cells were lined against the mineralized dentin-like tissue (Figs. 5 and 6). There were also scattered cells embedded within the dentin-like structure. Some located between the original dentin and the regenerated dentin. The newly deposited dentin-like tissue appeared to tightly adhere to the original dentin except where there were gaps that resulted from sample processing artifacts. The newly formed mineralized tissue also appeared to fill into the space of dentinal tubules (Fig. 5H). At higher magnification, unlike the natural dentin, the pulp side of the regenerated dentin-like tissue was not smooth; instead, there were projections of the structures into the pulp side (Figs. 5 and 6).
The odontoblast-like cells were not well organized, nor well aligned as the natural counterparts, and it was difficult to observe the typical characteristics that natural odontoblasts possess (e.g., polarized cell bodies). Nonetheless, some regions of the odontoblast-like cells showed somewhat typical odontoblast characteristics. Groups of odontoblast-like cells were well aligned, such that each cell was spatially juxtaposed to adjacent cells with overt polarized morphology (Fig. 5E, F, and Fig. 6G). The regenerated dentin-like tissue did not form observable well-organized dentinal tubules, except in a few regions where odontoblast-like cells were better aligned (Fig. 6G).
Cells in regenerated pulp-like tissue are of human origin.
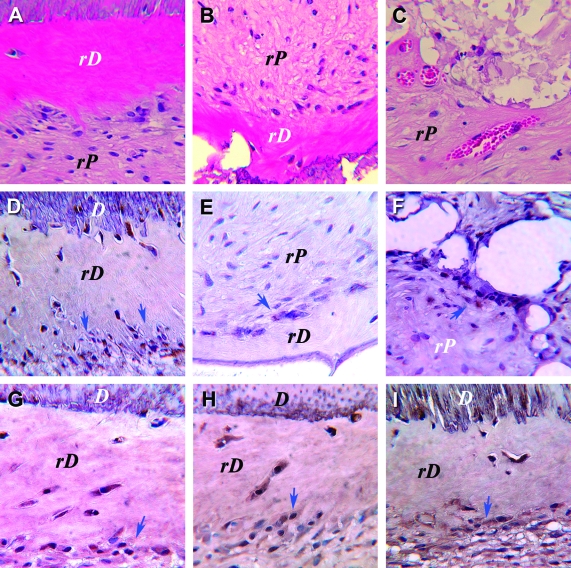
To verify that the cells responsible for generating the pulp/dentin-like tissues in the canal space were of human origin, antibodies against human mitochondria were used to detect human cells in the tissues. As shown in Figure 7D–F, a majority of the cells stained positively for the antibodies, confirming the human cell origin of these cells in the regenerated pulp-like tissue.
FIG. 7.
Immunohistochemical analysis of pulp tissue regenerated in vivo using SCAP. (A–C) H&E staining; immunostaining for (D–F) human mitochondria; (G) dentin sialoprotein; (H) alkaline phosphatase and (I) bone sialoprotein. D, original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Arrows indicate cells with positive staining. Color images available online at www.liebertonline.com/ten.
Odontoblast-like cells express odontogenic markers
To further verify the dentin-like tissue was generated by cells of odontoblast lineage, several odonto/osteogenic markers were examined immunohistochemically. Positive staining for DSP, BSP, and ALP was seen in cells lining against as well as those embedded in the newly deposited dentin-like tissues, suggesting that the differentiated odontoblast-like cells were responsible for the production of the calcified tissue (Fig. 7G–I).
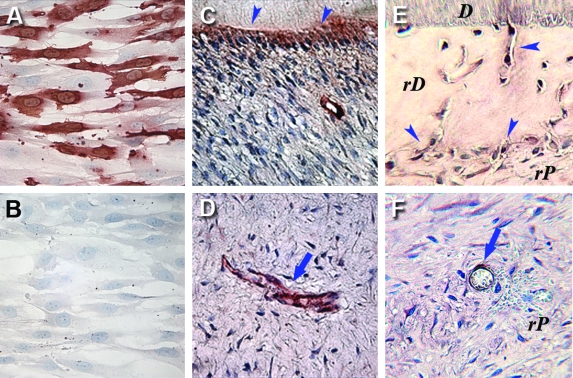
In addition, we compared CD105 expression in the regenerated and the natural pulp. CD105 was expressed by DPSCs and SCAP based on the flow cytometry analysis presented in Table 1. Immunocytochemical analysis of SCAP in cultures confirmed their CD105 expression (Fig. 8A). In the natural pulp, CD105 expression was detected in association with blood vessels and in odontoblasts (Fig. 8C, D). CD105 (Endoglin) is known to be expressed on human vascular endothelial cells. In comparison, CD105 was also expressed by odontoblast-like cells and paravascular cells in the regenerated pulp-like tissus (Fig. 8E, F), suggesting that the regenerated tissue closely resembles natural pulp.
FIG. 8.
Immunocyto/histochemical staining of CD105. Cultured SCAP immunostained with (A) anti-CD105 antibody or (B) nonimmune IgG as negative control. (C) Positive staining of odontoblasts (arrowheads) in a sample of natural human dental pulp/dentin tissue. (D) A blood vessel in the natural pulp is positive for CD105 (arrow). (E) Positive immunostaining is seen in odontoblast-like cells lining against rD or inside rD (arrowheads). (F) CD105-positive cells are seen in rP and around a blood vessel (arrow). rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Color images available online at www.liebertonline.com/ten.
Discussion
Our study is the first in vivo evidence demonstrating de novo synthesis of vascularized human pulp/dentin-like tissues in an emptied human root canal space 5–6 mm deep with an opening end of only ∼2.5 mm. The most striking phenomenon is the formation of a continuous layer of dentin-like tissue on the existing canal dentinal walls and on MTA cement surface. Expression analysis of several key genes, including DSP, BSP, ALP, and CD105, indicates that the regenerated pulp tissues closely resemble natural pulp tissue. Our findings constitute the first step toward stem cell–mediated de novo regeneration of pulp/dentin in clinical endodontic practice. Use of heterogeneous population of stem/progenitor cells appeared to be able to achieve this purpose. Both SCAP and DPSCs as mesenchymal-like stem cells presented a different profile of multipotency from BMMSCs. This may be because of their different developmental origin—derived from or associated with neural crest cells.
Previously, we discussed several issues regarding the establishment of a protocol for pulp/dentin regeneration, including the scaffolds suitable for this purpose, the extent of vascularization of the regenerated pulp, and the formation of odontoblasts and new dentin on the existing dentin surface.4,5,10,16 Our previous in vitro studies showed that although collagen gels are a convenient cell carrier for pulp regeneration in the root canal space, severe contraction may hamper the regeneration process.10 A recent in vivo study targeting root dentin perforation repair with the use of DPSCs and dentin matrix protein 1 carried by collagen matrix showed that soft connective pulp-like tissue was formed in the perforated site, but no hard tissue was generated.18 Regeneration of a small portion of pulp after pulpotomy using a collagen matrix appears to be successful as demonstrated in a dog study model.19 Biosynthetic scaffolds such as PLG or poly-L-lactic acid are much more resistant to contraction than collagen. They are generally made into porous form to provide inner spaces for cells to populate. Cordeiro et al. reported successful regeneration of well-vascularized pulp-like soft tissue utilizing poly-L-lactic acid as a scaffold fabricated in the pulp chamber space of a tooth slice that was seeded with stem cells from human exfoliated deciduous teeth.6 Together with our findings in this study, synthetic polymers of lactide and/or glacolide appeared to be suitable scaffold systems for de novo pulp regeneration. We speculated that the slow degradation of the PLG in our study negatively affected the quality of the regenerated pulp-like tissue. In future studies, modification of the ratio of lactide and glycolide or selection of other type of scaffold materials such as silk may improve this situation.20,21
Vascularization in the regenerated pulp tissue in canal space was a major concern because the blood supply for the space can only come from the apex.4 The above mentioned studies have tried to avoid this issue in testing pulp regeneration by using a thin tooth slice model.6,18 Utilizing factors such as vascular endothelial growth factor and/or platelet-derived growth factor to enhance angiogenesis or the addition of vascular cells or hematopoietic progenitors has been an effective approach to resolve to this issue for tissue regeneration.14,22–25 In this study, angiogenic enhancement was not employed. The tooth fragments contained a canal space 5–6 mm deep; therefore, it was likely that the blood vessel growth into the end of the canal space to provide nutrients for the stem cells could not occur shortly after the tooth fragment was transplanted into the mouse subcutaneous space. However, our results indicate that cells not only survived well but also were able to regenerate tissues. It is possible that the nutrients were able to diffuse into the canal space, allowing the stem cells to build new tissues; once the blood vessels were generated in the entire canal space, long-term stability of the tissue was established. The question arises as to whether a smaller opening of the canal, that is, <2 mm, will affect the efficacy of tissue regeneration in the coronal end of the canal. Further studies are needed to test the limit of the canal opening size that allows pulp regeneration and whether the addition of angiogenic factors can compensate the smaller canal opening.
It was unknown whether de novo regenerated pulp in the canal space can produce new dentin onto the existing dentin. To generate new dentin, stem cells must first differentiate into odontoblasts. It was not clear whether using a scaffold such as PLG, without osteo-inductive properties, will lead to stem-cell differentiation into odontoblasts. However, regenerating pulp/dentin in the root canal space is not the same as regenerating bone. Pulp and dentin in the canal space have their specific locations; therefore, any scaffold system that is osteoinductive, such as hydroxyapatite and tricalcium phosphate, is in fact not appropriate for pulp/dentin in the root canel space regeneration. This approach would likely generate scattered calcified tissue in the entire canal space.
In vivo studies have demonstrated that stem cells from human exfoliated deciduous teeth were able to differentiate into odontoblast-like cells lining against the existing dentin surface.6 These findings suggest that existing dentin is sufficient to guide stem cells in the canal space to differentiate into odontoblast-like cells. Even the chemical treatment of dentin did not appear to affect this capacity. From these observations, it appears that stem cells seeded in the scaffold are attracted to the dentinal wall, differentiate into odontoblast-like cells, and extend their cellular processes into the dentinal tubules. The mechanism underlying this phenomenon has been speculated to be the release of growth factors such as transforming growth factor-β that is known to be embedded in dentin,26,27 which attracts and induces odontoblast differentiation of the seeded stem cells.10 Chemical disinfection of the root canal space may damage these embedded growth factors. Further investigation is needed to seek ways to avoid this potential damage.
The most interesting and important finding of this study is the formation of a continuous layer of dentin-like tissue with uniform thickness on the existing canal dentin walls and the MTA cement surface, especially with SCAP-seeded samples. This fulfills, at least in part, a major requirement of functional tissue engineering/regeneration because dentin production is one of the major functions of pulp. However, the characteristics of the regenerated dentin-like tissue are quite different from the natural dentin. First, dentinal tubules are not obvious, possibly because of their convoluted paths. Second, it is quite cellular, and third, the formation is not synchronous. There were only some regions of the new dentin-like tissue that showed dentinal tubule-like structures (Fig. 6G). Our previous in vitro work demonstrated that DPSCs seeded onto a processed dentin surface morphologically transformed into odontoblast-like cells each with a cellular process extending into the dentinal tubule.10,15 Based on this observation along with the present in vivo findings, the following events were likely to have occurred subsequent to in vivo transplantation of the tooth fragments: (i) some SCAP or DPSCs on PLG in the canal space migrate toward the dentin surface, (ii) these cells receive signals from dentin and differentiate into odontoblast-like cells, (iii) a cellular process is extended from each cell into the dentinal tubules, (iv) cells initiate production of extracellular matrix in the dentinal tubule space as well as onto the dentin surface, and (v) the remaining steps of dentin-like tissue apposition are similar to the natural dentin production, except cells laid down dentin-like tissue at a different pace. The question remains as to whether every dentinal tubule is occupied by a newly differentiated odontoblast-like cell of equal potentiality. If not, it may explain the disorganized nature of the newly synthesized dentin-like tissue and the alignment of those odnotoblast-like cells as well as the entrapment of many of these cells in the dentin-like structure. Tertiary dentin produced by the natural pulp has less organization of dentinal tubules and may have cell entrapment in the dentin; therefore, the newly regenerated dentin-like tissue may be similar to tertiary dentin.
Taken together, our findings show that pulp/dentin regeneration can be accomplished with a stem/progenitor cell–mediated tissue engineering approach. This study is the first step toward reaching the goal of regenerating functional pulp/dentin that is very similar to its natural counterparts. To achieve this goal, future investigations must include the use of larger animals, such as minipigs, to regenerate these tissues in situ in the jaw bone. Besides gaining vascularity, the innervation of the regenerated pulp/dentin tissue by ingrowth of nerve fibers from the apical tissues requires extensive investigation. Further, if a higher quality of the regenerated dentin tissue is desired, that is, producing new dentin with well-organized dentinal tubules, the method of treating dentin surface may need modification to provide better signaling stimulation to guide stem cell differentiation toward odontoblast lineage in a more synchronous manner.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NIH) R01 DE019156-01 (G.T.-J.H.), RO1 DE17449 (S.S.), by the NIAMS/NIH Intramural Research Program Z01 AR41131 (R.S.T), and by an Endodontic Research Grant from American Association of Endodontists Foundation (G.T.-J.H.). We wish to thank Dr. Matthew Daniels (NIH/NHLBI, Electron Microscopy Core) for the assistance of SEM analysis, Dr. Larry Fisher (NIH/NIDCR) for providing antibodies, Dr. Wesley M. Jackson (NIH/NIAMS) for providing the RNA from cultured human myoblasts, and Dr. Yi Liu (USC) for the assistance of neurogenic studies.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mooney D.J. Powell C. Piana J. Rutherford B. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12:865. doi: 10.1021/bp960073f. [DOI] [PubMed] [Google Scholar]
- 2.Bohl K.S. Shon J. Rutherford B. Mooney D.J. Role of synthetic extracelluar matrix in development of engineered dental pulp. J Biomaterials Science. (Polymer Edition) 1998;9:749. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- 3.Buurma B. Gu K. Rutherford R.B. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999;107:282. doi: 10.1046/j.0909-8836.1999.eos107408.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang G.T.J. Sonoyama W. Liu Y. Liu H. Wang S. Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang G.T.-J. Apexification: the beginning of its end. Int Endod J. 2009;42:855. doi: 10.1111/j.1365-2591.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro M.M. Dong Z. Kaneko T. Zhang Z. Miyazawa M. Shi S. Smith A.J. Nor J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Batouli S. Miura M. Brahim J. Tsutsui T.W. Fisher L.W. Gronthos S. Robey P.G. Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- 8.Sonoyama W. Liu Y. Fang D. Yamaza T. Seo B.M. Zhang C. Liu H. Gronthos S. Wang C.Y. Shi S. Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonoyama W. Liu Y. Yamaza T. Tuan R.S. Wang S. Shi S. Huang G.T.J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G.T.J. Sonoyama W. Chen J. Park S. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S. Mankani M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronthos S. Brahim J. Li W. Fisher L.W. Cherman N. Boyde A. DenBesten P. Robey P.G. Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 13.Caterson E.J. Nesti L.J. Danielson K.G. Tuan R.S. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan M.H. Shea L.D. Peters M.C. Mooney D.J. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release. 2000;64:91. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 15.Huang G.T.J. Shagramanova K. Chan S.W. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Huang G.T. Gronthos S. Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. Dent Res. 2009;88:792. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuli R. Tuli S. Nandi S. Wang M.L. Alexander P.G. Haleem-Smith H. Hozack W.J. Manner P.A. Danielson K.G. Tuan R.S. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 18.Prescott R.S. Alsanea R. Fayad M.I. Johnson B.R. Wenckus C.S. Hao J. John A.S. George A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iohara K. Zheng L. Ito M. Ishizaka R. Nakamura H. Into T. Matsushita K. Nakashima M. Regeneration of dental pulp after pulpotomy by transplantation of CD31-/CD146- side population cells from a canine tooth. Reg Med. 2009;4:377. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 20.Wenk E. Meinel A.J. Wildy S. Merkle H.P. Meinel L. Microporous silk fibroin scaffolds embedding PLGA microparticles for controlled growth factor delivery in tissue engineering. Biomaterials. 2009;30:2571. doi: 10.1016/j.biomaterials.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 21.Chang G. Kim H.J. Vunjak-Novakovic G. Kaplan D.L. Kandel R. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32326. [Epub ahead of print] DOI: 10.1002/jbm.a.32326. [DOI] [PubMed] [Google Scholar]
- 22.Peters M.C. Polverini P.J. Mooney D.J. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q. Chen R.R. Shen Y. Mooney D.J. Rajagopalan S. Grossman P.M. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 24.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 25.Moioli E.K. Clark P.A. Chen M. Dennis J.E. Erickson H.P. Gerson S.L. Mao J.J. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PLoS ONE. 2008;3:e3922. doi: 10.1371/journal.pone.0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan A.J. Perry H. Matthews J.B. Smith A.J. Transforming growth factor-beta isoform expression in mature human healthy and carious molar teeth. Histochem J. 2000;32:247. doi: 10.1023/a:1004007202404. [DOI] [PubMed] [Google Scholar]
- 27.Cassidy N. Fahey M. Prime S.S. Smith A.J. Comparative analysis of transforming growth factor-beta isoforms 1–3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42:219. doi: 10.1016/S0003-9969(96)00115-X. [DOI] [PubMed] [Google Scholar]