Abstract
Rapid determination of glucose-6-phosphate dehydrogenase (G6PD) status is desirable when it is necessary to use a drug contraindicated in G6PD-deficient persons, such as use of primaquine for malaria prevention or treatment. The purpose of this study was to compare a new, rapid, qualitative enzyme chromatographic test for deficiency of G6PD to a standard reference method. Samples from 196 G6PD-normal persons and 50 G6PD-deficient persons were evaluated. The sensitivity of the experimental rapid test was 0.98 and the specificity was 0.98 using specimens preserved in heparin, and 0.98 and 0.97, respectively, for specimens preserved in EDTA. Positive and negative predictive values were 0.72 and 1.00, respectively, for the test for heparinized specimens and 0.65 and 1.00, respectively, for the EDTA-preserved samples. This rapid test for G6PD deficiency is a sensitive method for screening of G6PD deficiency that requires minimal training and equipment and enables rapid identification of G6PD-deficient persons.
INTRODUCTION
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an enzymatic disorder of erythrocytes affecting approximately 400 million persons worldwide.1,2 It is an X-linked disorder with more than 400 variant enzymes identified.3 The G6PD enzyme is involved in protection of erythrocytes from oxidative injury, and deficiency can result in hemolysis of erythrocytes. Depending on the G6PD variant present, hemolysis may be mild, moderate, or severe. Expression in females is dependent on the degree of lyonization and of expression of the abnormal G6PD variant. Most persons with G6PD deficiency are asymptomatic but exposure to oxidant drugs, such as the anti-malarial drug primaquine, may induce hemolysis.4
Rapidly ascertaining the G6PD status of a person is desirable when one is considering use of a drug contraindicated in patients with G6PD deficiency. Current approved methods of G6PD testing in the United States require specialized reagents and equipment and entail performing several steps, including timed incubations.5,6 We compared a new, rapid diagnostic test for G6PD deficiency with a standard approved method.
MATERIALS AND METHODS
Subjects.
A volunteer, convenient sample of Boston Medical Center (BMC) patients with G6PD deficiency were recruited for the study. Persons with unknown or normal G6PD activity were enrolled as controls in a ratio of 4 to 5 control subjects per G6PD-deficient subject. Subjects were enrolled between June 2007 and January 2008 according to a protocol approved by the Institutional Review Board at BMC. Consent was obtained from all adult subjects and from parents or guardians of minors.
It was anticipated that some subjects enrolled as controls could be G6PD deficient and some patients previously diagnosed with a G6PD deficiency could have a normal enzyme level when tested with the reference assay. If a subject enrolled as a control was deficient according to the reference method, they were notified of the result and received further information on G6PD deficiency. Similarly, if a G6PD-deficient subject had a normal G6PD level according to the reference method, they were notified of the result and advised to follow-up with their own physician.
Sample collection.
Blood was collected from each subject and placed into two tubes containing EDTA and heparin, respectively. Samples were stored at 4°C and tested within one week of collection. Subject test panels comprised of samples from at least 4 subjects were prepared. The investigator and laboratory technicians running the test assay were masked to the subjects’ G6PD status.
Trinity Biotech quantitative G6PD assay (reference assay).
Quantitative determination of G6PD activity was performed on the heparinized blood using a commercial kit (quantitative, ultraviolet, kinetic determination of G-6-PDH in blood at 340 nm, kit no. 345-UV; Trinity Biotech USA, St. Louis, MO) according to manufacturer's instructions. Quality control was performed each day of testing using G-6-PDH controls manufactured by Trinity Biotech. Three control levels, deficient level (catalog no. G5888), intermediate level (catalog no. G5029), and normal level (catalog no. G6888) were analyzed before the test panels and were considered valid if control values fell within the given range. For this assay, the cut-off for G6PD deficiency was set at 4.0 U/g hemoglobin, a priori, for our laboratory. Determination of hemoglobin content of the heparin sample was performed by the Department of Clinical Hematology at BMC.
BinaxNOW® G6PD test (test assay).
The BinaxNOW® G6PD test is a qualitative enzyme chromatographic test (ECT) for G6PD activity. The test device consists of a lateral flow test strip comprised of a white sample pad and a reaction pad. The reaction pad contains the reagents necessary for the G6PD enzymatic reaction and the subsequent reduction of a nitro blue tetrazolium dye into its concomitant blue formazan product. The resulting color change on the strip indicates that enough G6PD activity is present to presume the sample is not deficient. Test results are read visually. If no change in the red color of the sample front is observed at the test read time, the sample is presumed to be deficient in G6PD enzyme activity. Samples with normal G6PD activity produce a distinct color change: the red sample color changes to a brown/black color on the upper half of the reaction pad. To perform the test, a whole blood sample is mixed with an erythrocyte-lysing reagent in a sample preparation vial and then transferred to the test device sample pad. The lysed blood sample migrates up the test strip, reconstituting reagents in the reaction pad. When the sample front (or liquid migration) covers the entire reaction pad, the device is closed.
The test format relies on a temperature sensitive kinetic enzymatic reaction and should therefore only be performed when the temperature in the testing area is between 18°C and 25°C (64–77°F). Performance characteristics summarized in the manufacturer's product insert cannot be guaranteed if the test is performed outside of the stated temperature range. Performing the test outside of the range could lead to erroneous results (especially the possibility of a false-normal result if the temperature is above 25°C). The test devices may be stored at 15–30°C but the manufacturer cautions that performance cannot be guaranteed if stored at temperatures above 30°C for prolonged periods.
The heparin- and EDTA-preserved samples from each subject were tested. Results from samples preserved in heparin were read after 5 minutes and those from samples preserved in EDTA were read after 7 minutes. A sample was read as G6PD deficient if it failed to generate a distinct color change at the top of the test pad and G6PD normal if a color change appeared at the top of the test pad (Figure 1). A test was invalid if the sample failed to saturate the sample pad. The first valid result was reported.
Figure 1.
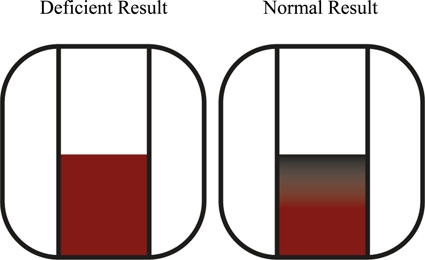
Example of the color change observed in the test device for glucose-6-phosphate dehydrogenase (G6PD)–normal samples and lack of a color change for G6PD-deficient samples. This figure appears in color at www.ajtmh.org.
Quality control was performed on each box of BinaxNOW® G6PD test devices using controls prepared at the BMC Maxwell Finland Laboratory. Normal controls were made from equal volumes of heparinized blood from 3 subjects with normal G6PD levels. These controls were stored at 4°C and used for no longer than 1 week from the draw date of the oldest sample pooled. G6PD-deficient controls were prepared according to a protocol designed at Inverness Medical. The calculated G6PD activity in the G6PD-deficient control was −3.0 U/dL of blood when tested by the quantitative Trinity Biotech method. This value was reported as no G6PD activity. Deficient controls were stored at −80°C and used for the duration of the study.
Data analysis.
For evaluation of the validity of the BinaxNOW® G6PD rapid test, subjects were grouped as G6PD deficient or G6PD normal by the result of the Trinity quantitative test. Those with a G6PD level ≤ 4.0 U/g of hemoglobin were considered G6PD deficient and those with levels > 4.0 U/g of hemoglobin were considered G6PD normal. Differences across comparison groups were evaluated by t-tests used to compare means, and chi-square tests were used to compare frequency within subgroups. Sensitivity, specificity, and positive and negative predictive values were calculated. Positive and negative predictive values of the BinaxNOW® G6PD test were calculated based on the prevalence of G6PD deficiency among BMC patients (5%).
RESULTS
Subjects.
Two hundred forty-eight subjects were enrolled. Of the 46 subjects believed to be deficient at enrollment, 3 had normal G6PD activity according to the Trinity quantitative assay. Six of the 202 subjects enrolled as controls were G6PD deficient by this method; four of these subjects had borderline deficient results (3.9, 3.9, 3.9, and 3.7 U/g of hemoglobin). There were 3 test devices that gave invalid BinaxNOW® G6PD test results; these had to be repeated. All three samples were preserved in heparin. Two of these samples had normal G6PD activity according to the reference method (7.48 and 7.67 U/g of hemoglobin) and one was deficient (3.71 U/g of hemoglobin). When testing was repeated on new devices, the control samples read as normal and the deficient sample read as deficient. Samples from two control subjects were not tested because a complete test panel could not be assembled within a week after the samples were obtained. Thus, results are available for 196 G6PD-normal and 50 G6PD-deficient samples. Demographic information for this cohort is shown in Table 1.
Table 1.
Demographics of 246 persons tested according to glucose-6-phosphate dehydrogenase level
| Characteristic | Total | Glucose-6-phosphate dehydrogenase level (Trinity Biotech quantitative assay) | P* | |
|---|---|---|---|---|
| ≤ 4 U/g hemoglobin | > 4 U/g hemoglobin | |||
| Number | 246 | 50 | 196 | |
| Sex, no. (%) | ||||
| Men | 83 (34) | 24 (48) | 59 (30) | 0.017 |
| Women | 163 (66) | 26 (52) | 137 (70) | |
| Age, years | ||||
| Mean ± SD† | 31.9 ± 12.2 | 33.5 ± 16.4 | 31.5 ± 11.1 | |
| Range | 3–72 | 3–72 | 4–62 | |
| Race/ethnicity, no. (%) | ||||
| White | 89 (36) | 0 (0) | 89 (45) | < 0.0001 |
| Black | 110 (45) | 43 (86) | 67 (34) | |
| Hispanic | 19 (8) | 2 (4) | 17 (9) | |
| Asian | 5 (2) | 0 (0) | 5 (3) | |
| Native American/Pacific Islander | 7 (3) | 3 (6) | 4 (2) | |
| Indian subcontinent | 10 (4) | 0 (0) | 10 (5) | |
| Multiracial | 6 (2) | 2 (4) | 4 (2) | |
For chi-square test of independence.
Mean differences in age were not significantly different.
G6PD results.
The mean ± SD G6PD activity of the deficient samples was 2.0 ± 2.3 U/g of hemoglobin. The mean ± SD hemoglobin level for that group was 13.2 ± 1.5 g/dL. In comparison, the mean ± SD G6PD activity for the control samples was 7.9 ± 2.0 U/g of hemoglobin with a mean ± SD hemoglobin level of 13.3 ± 1.5 g/dL (P > 0.05), respectively. Distribution of G6PD values by BinaxNOW® test result is shown in Figure 2.
Figure 2.
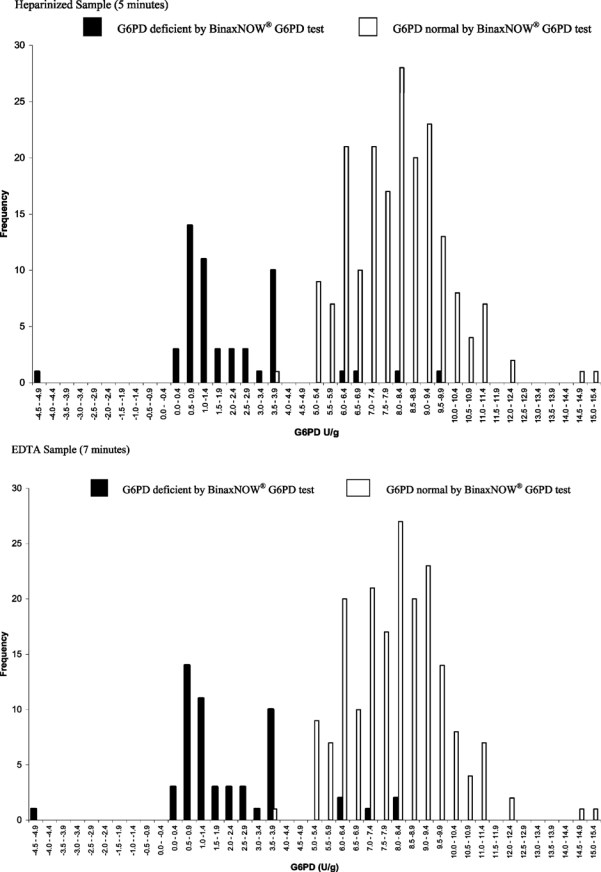
Distribution of glucose-6-phosphate dehydrogenase (G6PD) values determined by using the Trinity Biotech Quantitative G6PD Assay and the BinaxNOW® G6PD test result.
Sensitivity, specificity, and positive and negative predictive values.
The sensitivity of the BinaxNOW® G6PD test using heparinized whole blood was 0.98 and the specificity was 0.98. The positive predictive value for heparin samples was 0.72 and the negative predictive value was 1.00. The sensitivity of the test using EDTA-preserved whole blood was 0.98 and the specificity was 0.97. The positive predictive value for EDTA-preserved samples was 0.65 and the negative predictive value was 1.00 (Table 2). No significant differences in sensitivity or specificity were present when evaluated within the subgroups of race and sex.
Table 2.
Sensitivity and specificity of the BinaxNOW® G6PD test*
| Heparinized whole blood (5 minutes) | EDTA whole blood (7 minutes) | ||||
|---|---|---|---|---|---|
| BinaxNOW® | G6PD (Trinity Assay) | BinaxNOW® | G6PD (Trinity Assay) | ||
| ≤ 4 U/g hemoglobin | > 4.0 U/g hemoglobin | ≤ 4 U/g hemoglobin | > 4.0 U/g hemoglobin | ||
| Deficient | 49 | 4 | Deficient | 49 | 5 |
| Normal | 1 | 192 | Normal | 1 | 191 |
| Sensitivity = 0.98 | Sensitivity = 0.98 | ||||
| Specificity = 0.98 | Specificity = 0.97 | ||||
| PPV = 0.72 | NPV = 1.00 | PPV = 0.65 | NPV = 1.00 | ||
G6PD = glucose-6-phosphate dehydrogenase; PPV = positive predictive value; NPV = negative predictive value.
The BinaxNOW® G6PD test gave a false-normal result on the heparin- and EDTA-preserved samples from a six-year-old multiracial girl with a G6PD activity of 3.72 U/g of hemoglobin. There were four false-deficient results with heparin-preserved samples and five false-deficient results with EDTA-preserved samples. The G6PD activity of these samples ranged from 6.09 to 9.50 U/g of hemoglobin. The subjects that provided these samples were six females between the ages of 22 and 42. All discrepancies were among females, with normal hemoglobin values, from various race/ethnicity groups.
DISCUSSION
The BinaxNOW® G6PD test has a high sensitivity (0.98 with heparin-preseved samples and 0.98 with EDTA-preserved samples) and specificity (0.98 with heparin-preserved samples and 0.97 with EDTA-preserved samples) if the Trinity quantitative assessment of G6PD activity is used as a method for comparison, and distinguishes accurately between samples with G6PD activity less than 4.0 U/g of hemoglobin and those with greater enzyme activity. The World Health Organization (WHO) defines G6PD deficiency as less than 60% of normal activity.7 The cut-off value chosen in this study is 49% of the average activity in samples that had intermediate to normal results according to the Trinity assay. In contrast, the fluorescent spot method, the screening method recommended by the International Committee for Standardization in Hematology, identifies only those samples with 10–20% of normal G6PD activity classified by WHO as “severe enzyme deficiency”.6,8 The false-normal results in this study were for a sample with a G6PD level (3.72 U/g of hemoglobin) close to the threshold value. Using 4.0 U/g of hemoglobin as the cut-off value for G6PD deficiency, thereby including patients with moderate enzyme deficiency, classifies more patients as deficient than the fluorescent spot method, decreasing the risk of false-normal results.
Not surprisingly, most G6PD-deficient individuals were black, which corresponds to the known increased prevalence of G6PD deficiency in persons of African descent. Because of the risk of hemolytic anemia in persons with G6PD deficiency, the G6PD activity level of a person should be known before prescribing certain drugs, such as primaquine for prevention or treatment of malaria. Current G6PD deficiency screening methods are impractical for use in rural areas where malaria is endemic because of cost or need for specialized equipment and highly trained personnel. Because of these problems and the potential to cause hemolytic anemia in patients with G6PD deficiency, most malaria-endemic countries do not use primaquine in routine practice.9 However, primaquine is now being recommended for primary malaria prophylaxis in certain specific situations, and is the only drug available for preventing relapses of Plasmodium vivax and P. ovale malaria. The new recommendations call for primaquine as a first-line drug for malaria prophylaxis in areas where P. vivax malaria predominates.10 Finally, tafenoquine is an effective alternative to primaquine and is convenient because of its long half-life, but it is also contraindicated by G6PD deficiency.11 Easily available rapid screening for G6PD deficiency could make the use of this alternative possible in a wider variety of clinical settings.
The need for an inexpensive and easy to use screening test is of interest to the United States Army because troops are stationed in malaria-endemic areas around the world. With a prevalence of G6PD deficiency more than 12% among African American male soldiers, screening is warranted before use of primaquine.12,13 Carr and others described two recent cases of soldiers from Operation Iraqi Freedom in whom hemolytic anemia developed after primaquine therapy for malaria.14 Availability of a more convenient screening method that can be performed rapidly by personnel with minimal training would be desirable, especially in situations of rapid deployment.
Ability to test for G6PD deficiency is also critical to current efforts for eradication of malaria. Strategies that have resulted in success against P. falciparum malaria not may be equally applicable to P. vivax malaria, for which elimination of hypnozoites is required. A significant problem in controlling P. vivax malaria is that limitations imposed by primaquine, the only drug available for eradication of the liver phase of the parasite, prevent many areas from using the drug because of safety concerns.15 A rapid, inexpensive test for G6PD deficiency that can be used in the field, thus enabling more widespread use of primaquine, will be critical to eradication of P. vivax malaria. The product insert of this test reports low interobserver variability and high precision with a single operator.
One significant limitation of the BinaxNOW® G6PD test is the need to perform the test within a temperature range of 18°C to 25°C (64°F to 77°F). Although this temperature range is feasible in most U.S. laboratories, it may not be possible to use the device in settings without adequate temperature control or in locations where the ambient temperature is outside this range. This problem should be addressed in the development of future generations of the BinaxNOW® G6PD test, but we are not aware of the manufacturer's plans to do so. Another limitation of use in low income countries is cost. Although the manufacturer has not established the pricing of the test, it is expected to be less than $25 per test. Although this price may be acceptable in higher income countries, it will limit use of the device in lower income regions. Finally, the test has not been validated in samples of blood containing malaria parasites.
This study could not address whether the BinaxNOW® G6PD test performs well for the full range of genetic variants of G6PD deficiency. In our cohort, 86% of the G6PD-deficient subjects were African or African American, and there were no G6PD deficient Asians, although G6PD deficiency occurs at a rate of 5.2% in refugees from southeast Asia according to a survey performed by Schwartz et al.16 Genotyping was not performed in this study, and we are therefore unable to know the number of variants included in this cohort.
The BinaxNOW® G6PD rapid ECT is a sensitive screen for G6PD deficiency that requires minimal training and equipment and enables rapid diagnosis of G6PD deficiency. The simple procedure and minimal equipment requirements make this a useful test for situations in which prompt diagnosis of G6PD deficiency is imperative for safe treatment of malaria. It also presents advantages for testing travelers with imminent departures who may be candidates for primaquine prophylaxis against malaria.
Footnotes
Financial support: This study was supported by a research grant from Inverness Medical (Scarborough, ME).
Disclosure: Anne Jepson is employed by Inverness Medical, which manufactures the device studied. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Authors’ addresses: Kathleen E. Tinley, Creighton University School of Medicine, Omaha, NE. Anita M. Loughlin, Center for Pediatric and Vaccine Research, Boston Medical Center, Boston, MA. Anne Jepson, Inverness Medical, Scarborough, ME. Elizabeth D. Barnett, Maxwell Finland Laboratory for Infectious Diseases, Boston Medical Center, Boston, MA, E-mails: Elizabeth.barnett@bmc.org or ebarnett@bu.edu.
References
- 1.Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood. 2008;111:16–24. doi: 10.1182/blood-2007-04-077412. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 3.Beutler E, Yoshida A. Genetic variation of glucose-6-phosphate dehydrogenase: a catalog and future prospects. Medicine (Baltimore) 1988;67:311–334. doi: 10.1097/00005792-198809000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 5.Beutler E. A series of new screening procedures for pyruvate kinase deficiency, glucose-6-phosphate dehydrogenase deficiency, and glutathione reductase deficiency. Blood. 1966;28:553–562. [PubMed] [Google Scholar]
- 6.Beutler E, Blume KG, Kaplan C, Löhr GW, Ramot B, Valentine WN. International Committee for Standardization in Haematology: recommended screening test for glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Br J Haematol. 1979;43:469–477. doi: 10.1111/j.1365-2141.1979.tb03774.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazza J. Manual of Clinical Hematology. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 101–102. [Google Scholar]
- 8.Beutler E, Mitchell M. Special modifications of the fluorescent screening method for glucose-6-phosphate dehydrogenase deficiency. Blood. 1968;32:816–818. [PubMed] [Google Scholar]
- 9.Tantular IS, Iwai K, Khin L, Basuki S, Horie T, Htay HH, Matsuoka H, Marwoto H, Wongsrichanalai C, Dachlan YP, Kojima S, Ishii A, Kawamoto F. Field trials of a rapid test for G6PD deficiency in combination with a rapid diagnosis of malaria. Trop Med Int Health. 1999;4:245–250. doi: 10.1046/j.1365-3156.1999.00395.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . CDC Health Information for International Travel 2010. Atlanta: US Department of Health and Human Services, Public Health Service; 2009. pp. 128–159. [Google Scholar]
- 11.Shanks GD, Oloo AJ, Aleman GM, Ohrt C, Klotz FW, Braitman D, Horton J, Brueckner E. A new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malaria. Clin Infect Dis. 2001;33:1968–1974. doi: 10.1086/324081. [DOI] [PubMed] [Google Scholar]
- 12.Uddin DE, Dickson LG, Brodine CE. Glucose-6-phosphate dehydrogenase deficiency in military recruits. JAMA. 1974;227:1408–1409. [PubMed] [Google Scholar]
- 13.Chinevere TD, Murray CK, Grant E, Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006;171:905–907. doi: 10.7205/milmed.171.9.905. [DOI] [PubMed] [Google Scholar]
- 14.Carr ME, Jr, Fandre MN, Oduwa FO. Glucose-6-phosphate dehydrogenase deficiency in two returning Operation Iraqi Freedom soldiers who developed hemolytic anemia while receiving primaquine prophylaxis for malaria. Mil Med. 2005;170:273–276. doi: 10.7205/milmed.170.4.273. [DOI] [PubMed] [Google Scholar]
- 15.Mueller I, Galinski MR, Baird K, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz IK, Chin W, Newman J, Roberts JM. Glucose-6-phosphate dehydrogenase deficiency in Southeast Asian refugees entering the United States. Am J Trop Med Hyg. 1984;33:182–184. doi: 10.4269/ajtmh.1984.33.182. [DOI] [PubMed] [Google Scholar]


