Abstract
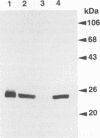
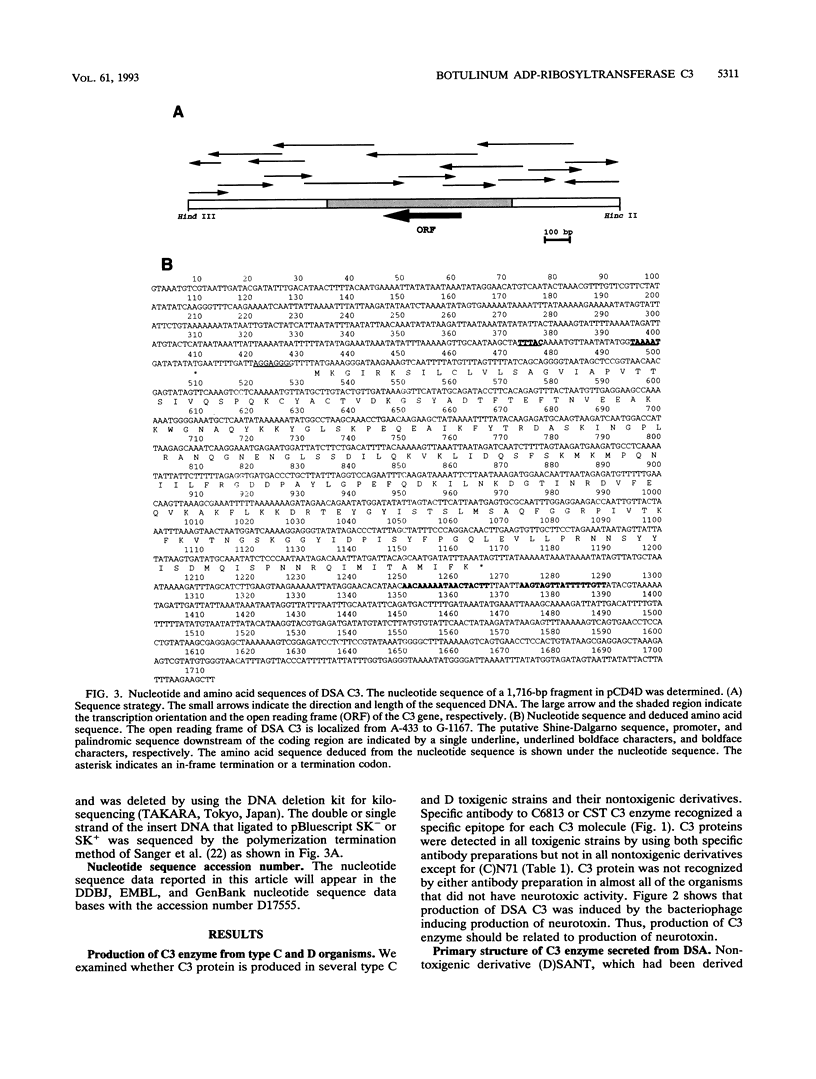
We examined production of ADP-ribosyltransferase C3 in 11 strains of Clostridium botulinum type C and D and their nontoxigenic derivatives. Antisera to C3 proteins of type C organisms divided C3 proteins roughly into at least two groups, bearing no relation to their bacterial types. The C3 gene of type D strain South African was isolated from a toxigenic phage library, and the complete sequence of the C3 gene was determined. The C3 protein of type D strain South African had 98% homology to the C3 protein of type C strain 003-9 and 66% homology to that of type D strain 1873. These results indicate that there are two types of C3 protein in type D organisms, as there are in type C organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Frevert J. ADP-ribosylation of a 21-24 kDa eukaryotic protein(s) by C3, a novel botulinum ADP-ribosyltransferase, is regulated by guanine nucleotide. Biochem J. 1987 Oct 15;247(2):363–368. doi: 10.1042/bj2470363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T., Kurazono H., Popoff M. R., Eklund M. W., Sakaguchi G., Kozaki S., Krieglstein K., Henschen A., Gill D. M., Niemann H. Nucleotide sequence of the gene encoding Clostridium botulinum neurotoxin type D. Nucleic Acids Res. 1990 Sep 25;18(18):5556–5556. doi: 10.1093/nar/18.18.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Habermann B., Just I., Aktories K., Vandekerckhove J. Purification of the 22 kDa protein substrate of botulinum ADP-ribosyltransferase C3 from porcine brain cytosol and its characterization as a GTP-binding protein highly homologous to the rho gene product. FEBS Lett. 1989 Jan 16;243(1):70–76. doi: 10.1016/0014-5793(89)81220-7. [DOI] [PubMed] [Google Scholar]
- Chardin P., Boquet P., Madaule P., Popoff M. R., Rubin E. J., Gill D. M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989 Apr;8(4):1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J., Weber R. F., Bokoch G. M., Evans T., Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989 Oct 5;264(28):16378–16382. [PubMed] [Google Scholar]
- Inoue S., Sugai M., Murooka Y., Paik S. Y., Hong Y. M., Ohgai H., Suginaka H. Molecular cloning and sequencing of the epidermal cell differentiation inhibitor gene from Staphylococcus aureus. Biochem Biophys Res Commun. 1991 Jan 31;174(2):459–464. doi: 10.1016/0006-291x(91)91438-i. [DOI] [PubMed] [Google Scholar]
- Just I., Mohr C., Schallehn G., Menard L., Didsbury J. R., Vandekerckhove J., van Damme J., Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992 May 25;267(15):10274–10280. [PubMed] [Google Scholar]
- Just I., Schallehn G., Aktories K. ADP-ribosylation of small GTP-binding proteins by Bacillus cereus. Biochem Biophys Res Commun. 1992 Mar 31;183(3):931–936. doi: 10.1016/s0006-291x(05)80279-7. [DOI] [PubMed] [Google Scholar]
- Kimura K., Fujii N., Tsuzuki K., Murakami T., Indoh T., Yokosawa N., Takeshi K., Syuto B., Oguma K. The complete nucleotide sequence of the gene coding for botulinum type C1 toxin in the C-ST phage genome. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1304–1311. doi: 10.1016/0006-291x(90)90828-b. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maehama T., Takahashi K., Ohoka Y., Ohtsuka T., Ui M., Katada T. Identification of a botulinum C3-like enzyme in bovine brain that catalyzes ADP-ribosylation of GTP-binding proteins. J Biol Chem. 1991 Jun 5;266(16):10062–10065. [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Kozaki S., Narumiya S. Clostridium botulinum C3 ADP-ribosyltransferase gene. Cloning, sequencing, and expression of a functional protein in Escherichia coli. J Biol Chem. 1991 Oct 15;266(29):19312–19319. [PubMed] [Google Scholar]
- Oguma K., Iida H., Shiozaki M., Inoue K. Antigenicity of converting phages obtained from Clostridium botulinum types C and D. Infect Immun. 1976 Mar;13(3):855–860. doi: 10.1128/iai.13.3.855-860.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Iida H., Shiozaki M. Phage conversion to hemagglutinin production in Clostridium botulinum types C and D. Infect Immun. 1976 Sep;14(3):597–602. doi: 10.1128/iai.14.3.597-602.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Yamaguchi T., Sudou K., Yokosawa N., Fujikawa Y. Biochemical classification of Clostridium botulinum type C and D strains and their nontoxigenic derivatives. Appl Environ Microbiol. 1986 Feb;51(2):256–260. doi: 10.1128/aem.51.2.256-260.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson H. F., Self A. J., Garrett M. D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990 Sep;111(3):1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M. R., Hauser D., Boquet P., Eklund M. W., Gill D. M. Characterization of the C3 gene of Clostridium botulinum types C and D and its expression in Escherichia coli. Infect Immun. 1991 Oct;59(10):3673–3679. doi: 10.1128/iai.59.10.3673-3679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M., Boquet P., Gill D. M., Eklund M. W. DNA sequence of exoenzyme C3, an ADP-ribosyltransferase encoded by Clostridium botulinum C and D phages. Nucleic Acids Res. 1990 Mar 11;18(5):1291–1291. doi: 10.1093/nar/18.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösener S., Chhatwal G. S., Aktories K. Botulinum ADP-ribosyltransferase C3 but not botulinum neurotoxins C1 and D ADP-ribosylates low molecular mass GTP-binding proteins. FEBS Lett. 1987 Nov 16;224(1):38–42. doi: 10.1016/0014-5793(87)80418-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981 Sep;33(3):155–188. [PubMed] [Google Scholar]
- Sugai M., Hashimoto K., Kikuchi A., Inoue S., Okumura H., Matsumoto K., Goto Y., Ohgai H., Moriishi K., Syuto B. Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J Biol Chem. 1992 Feb 5;267(4):2600–2604. [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki K., Kimura K., Fujii N., Yokosawa N., Indoh T., Murakami T., Oguma K. Cloning and complete nucleotide sequence of the gene for the main component of hemagglutinin produced by Clostridium botulinum type C. Infect Immun. 1990 Oct;58(10):3173–3177. doi: 10.1128/iai.58.10.3173-3177.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]