Abstract
The spread of Plasmodium falciparum drug resistance is outpacing new antimalarial development and compromising effective malaria treatment. Combination therapy is widely implemented to prolong the effectiveness of currently approved antimalarials. To maximize utility of available drugs, periodic monitoring of drug efficacy and gathering of accurate information regarding parasite-sensitivity changes are essential. We describe a high-throughput, non-radioactive, field-based assay to evaluate in vitro antimalarial drug sensitivity of P. falciparum isolates from 40 Senegalese patients. Compared with earlier years, we found a significant decrease in chloroquine in vitro and in genotypic resistances (> 50% and > 65%, respectively, in previous studies) with only 23% of isolates showing resistance. This is possibly caused by a withdrawal of chloroquine from Senegal in 2002. We also found a range of artemisinin responses. Prevalence of drug resistance is dynamic and varies by region. Therefore, the implementation of non-radioactive, robust, high-throughput antimalarial sensitivity assays is critical for defining region-specific prophylaxis and treatment guidelines.
Plasmodium falciparum, which causes the most virulent human malaria, is responsible for approximately one million deaths annually. Malaria related morbidity and mortality in sub-Saharan Africa presents a significant obstacle for economic development in this part of the world.1,2 Although a number of chemotherapeutic options are available for treating P. falciparum malaria, the rapid spread of drug resistance has marginalized the utility of many of these drugs. Chloroquine (CQ), quinine (QN), pyrimethamine, amodiaquine (AMQ), and artemisinin (ART) are among the most effective antimalarial agents. The latter two have been used in combination in many malaria endemic regions to thwart the emergence of drug resistance.3 In fact, the World Health Organization recommends the use of ART-based combination therapy (ACT) as a first-line regimen throughout much of Africa.4 However, monotherapy with ART and its derivatives has caused the emergence of parasites that show decreased sensitivity to these drugs, which is reflected in the higher IC50 values of some P. falciparum clinical isolates.5,6 Although clinical resistance to ART has not been adequately defined and reported, these decreased sensitivities may be the harbinger of clinical resistance. Periodic surveillance of drug efficacy through in vitro drug-sensitivity assays is essential for optimal selection of drug combinations, which will ensure successful administration of antimalarial treatment.
High-throughput methods that enable analyses of drug responsiveness of clinical isolates from different countries where malaria is endemic are essential to define antimalarial treatment regimens in those regions. For example, ACT is routinely and widely used for managing most malaria infections, sulfadoxine-pyrimethamine is still used for intermittent preventative treatment in pregnancy, and QN remains an important option for parenteral use in severe disease.4 In addition, a wide variation in drug sensitivity may be present even within the same country,7 and thus, drug-sensitivity testing would be needed for each geographically distinct region. Moreover, such high-throughput methods will allow screening of lead compounds that could potentially be developed into novel antimalarials.
Herein, we describe the field implementation of a non-radioactive, high-throughput assay to evaluate drug sensitivity on ex vivo isolates of P. falciparum collected from infected Senegalese patients. Previously, the only means of evaluating inhibition of parasite proliferation entailed microscopic examination of blood smears stained with modified Wright-Giemsa or use of the [3H]-hypoxanthine incorporation assay.8 The assays are either laborious or require the use of radioactive isotopes, whose safe disposal is difficult in a resource-limited setting. More recently, methods for quantifying parasite histidine-rich protein-2 (HRP-2) as well as non-radioactive fluorescence-based methods using SYBR green and 4–6-diamidino-2-phenylindole (DAPI) that measure parasite DNA have been developed.9–11 We have adapted the DAPI-based method to evaluate drug sensitivity of ex vivo P. falciparum parasite cultures, thus directly assessing parasite samples collected from patients under field laboratory conditions in Senegal.
We examined the drug-sensitivity profiles of P. falciparum parasites derived from Senegalese patients presenting during transmission season in the fall of 2007 to the Anti-Parasite Service in Thiès (SLAP), a city ~75 km southeast of Dakar, against a panel of antimalarial agents. In this region, malaria transmission is perennial and hypoendemic with an increase in cases during the end of the rainy season from September to December.12 Approximately one-third of all outpatient consultations and 20–30% of mortality in healthcare facilities in Senegal can be attributed to infection with P. falciparum. CQ was recommended as the first-line malaria therapy in Senegal until 2002. However, in 2003, the Senegalese Ministry of Health altered the national treatment policy to include ACT using artesunate, a more soluble derivative of ART, plus AMQ as the first-line antimalarial regimen.
Consenting outpatients with clinical signs suggestive of mild malaria and a positive Giemsa-stained thick film for P. falciparum were identified and enrolled from SLAP. Patients presenting with fever or those who had a history of fever and symptoms indicative of malaria were enrolled. The study protocol was approved by the Ethics Committee of the Ministry of Health in Senegal and the Human Subject Committee of the Harvard School of Public Health.
Before the start of the transmission season, microtiter plates were pre-loaded with antimalarial drug dilutions (serial dilutions of CQ [0.02–400 nM], AMQ [0.02–20 μM], ART [3.12–35 nM], and QN [234.37–75 μM] and stored at −20°C until needed. Blood was collected from patients and transported on ice to the central laboratory. Only samples with 0.3% parasitemia or greater were used for the study. Inasmuch as this may introduce some bias, we were unable to use our method to obtain standard threshold 50% inhibitory concentration (IC50) values for isolates with lower parasitemias. Packed erythrocytes were washed twice with RPMI (pH 7.4). The hematocrit of all samples was set to 2%, and samples with parasitemia greater than 1% were adjusted to 0.5–1.0% in complete medium using standard tissue-culture media.10 The cell suspension (180 μL per well) was dispensed into the thawed, pre-loaded, 96-well microtiter plates. The plates were incubated at 37°C in a gas environment of 5% CO2, 1% O2, and 94% N2 for 72 hours. Smears prepared from zero drug wells were stained with Giemsa to ensure parasite growth at 72 hours. Samples that did not show any parasite growth in culture were eliminated from the subsequent analyses. At the end of the assay, the plates were frozen at −20°C. The microtiter plates were then thawed at room temperature and centrifuged, and media were aspirated with care to avoid disturbing the red cell pellet. The fluorochrome mixture was prepared as previously described10 and dispensed in each well at a final dilution of 1:100,000 of DAPI (5 mg/mL stock; Molecular Probes, Inc., Eugene, OR). The microtiter plates were then incubated in the dark for 30 minutes and centrifuged at 4,000 rpm for 10 minutes. Excess fluorochrome mixture was aspirated, and 30 μL of 1× phosphate buffered saline (PBS) was dispensed into each well. The microtiter plates were read using a Fluoroskan plate reader (ThermoFisher Scientific, Milford, MA) with excitation and emission wavelengths of 355 nm and 460 nm, respectively. IC50 values were calculated by non-linear regression analysis (GraphPad Prism, La Jolla, CA). Each antimalarial compound was evaluated in duplicate.
Parasite resistance to CQ, QN, and AMQ was defined based on standard threshold IC50 values, above which parasites are classified as resistant (Table 1). The P. falciparum 3D7 laboratory strain was used to validate the DAPI assay under field laboratory conditions (Table 1). The IC50 values for 3D7 parallel those obtained in other studies.13
Table 1.
Median IC50 values of field isolates from Senegal for a panel of antimalarials
| CQ | AMQ | QN | ART | |
|---|---|---|---|---|
| IC50 of field isolates | 20.3 (23) | 21.0 (0) | 77.6 (7) | 4.6 |
| 3D7 | 11.1 | 37.9 | 23.0 | 0.3 |
| No. of isolates tested | 40 | 31 | 44 | 41 |
Resistance against CQ, AMQ, and QN are defined as IC50 values > 100 nM, > 60 nM, and > 800 nM, respectively.13 No clinical resistance threshold for ART has been established. Values in parenthesis indicate prevalence of resistance against each drug in the field isolates as a percentage.
Fifty-one patient isolates with 0.3% parasitemia or greater were plated, and of these, 44 samples showed adequate growth at 72 hours. Of these, 40 samples had adequate data points to generate a non-linear regression curve and calculation of IC50 for chloroquine. Fifty percent of these evaluable isolates were from female patients with an overall average age of 25 years and 2% parasitemia. Our analysis revealed that CQ resistance, defined as IC50 values > 100 nM, was prevalent at the rate of 23% (9/40; Figure 1 and Table 1). The P. falciparum CQ-resistance transporter (PfCRT) K76T polymorphism has been shown to be correlated with in vitro and in vivo CQ resistance and CQ treatment failure.14,15 We used standardized restriction fragment-length polymorphism to detect the PfCRT K76T polymorphism in the 40 isolates.16 All of the nine isolates that were resistant to CQ (IC50 > 100 nM) possessed the PfCRT K76T polymorphism, and all of the 31 in vitro sensitive isolates were wild-type, resulting in an overall 23% genotypic resistance to CQ.
Figure 1.
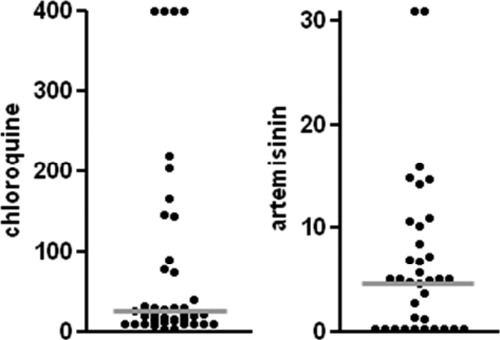
Dot plots of IC50 (nM) values for CQ and ART. Each dot represents one isolate. The gray lines represent the median IC50 value of the drug for the isolates tested (20.3 nM for CQ and 4.6 nM for ART).
Interestingly, the in vitro and genotypic resistance rate was significantly reduced compared with resistance rates reported previously in this region. The in vitro CQ resistance in Pikine, a suburb of Dakar, was 53% in 2001 (P = 0.0088) and 52% in 2002 (P = 0.0258).17,18 Furthermore, the PfCRT K76T polymorphism was 66% and 65%, respectively, in these previous studies. This reduction in two measures of CQ resistance rate parallels the withdrawal of CQ and introduction of other antimalarials in 2002 in Senegal. A similar reduction of CQ resistance after withdrawal of CQ had earlier been reported in Malawi.19 In that study, a rapid and progressive decrease in CQ resistance was observed after CQ withdrawal. In 1992, 85% of the isolates contained PfCRT K76T. In 1993, the prevalence was 50% and had reduced to 13% by 2000. This observation prompted a CQ in vivo efficacy study in Malawi in 2005 and was found to be 99% effective.20 Thus, CQ, a cheap and well-tolerated antimalarial, could potentially be reintroduced as part of combination therapy in regions where CQ-sensitive strains of P. falciparum have reemerged. Indeed, Senegal may find itself in a similar position so long as the country refrains from using CQ monotherapy for antimalarial therapy for the time being and undertakes constant surveillance of prevalence of drug resistance.
To protect the longevity and efficacy of ART and its derivatives, critical components of current antimalarial treatment options, it is essential for the partner drug in the combination therapy to be clinically effective. For example, in Cambodia, artesunate–mefloquine treatment failure was correlated with in vitro mefloquine resistance.6 In Senegal, artesunate–AMQ is a commonly used combination therapy.21 We, therefore, determined the drug responsiveness of our isolates to AMQ and found the IC50 values of all of them to be less than the resistance threshold of 60 nM.13
We identified a larger range of IC50 values among the isolates for ART with the highest value being > 30 nM (Figure 1). Our data are consistent with those of Jambou and others,5 which also found a large range of IC50 values (0.1–45 nM) for the ART-derivative artemether among clinical isolates in Senegal. No clinical resistance to date has been reported for the ART class of drugs in Africa. Thus, establishing baseline ART IC50 values is critical to be able to monitor any future decrease in ART sensitivity because of widespread use of ACT in this region, and our data could potentially serve as baseline values for future artemisinin-sensitivity analysis.
QN continues to have important clinical indications for parenteral treatment and severe disease. Our analysis revealed minimal resistance levels against QN. These levels have remained essentially unchanged (5% in Dakar in 2002).18 This absence of significant resistance may reflect the minimal selective pressure of QN (it is more commonly used than other drugs) and/or suggest that QN resistance does not arise as readily in the natural setting.
Drug-efficacy surveillance is critical for detecting the emergence of drug resistance and preventing its dissemination. Here, we describe an additional non-radioactive DAPI-based method to allow high-throughput screening for the prevalence of drug resistance in the field. We have used this method to determine the effect of withdrawal of CQ in 2002 on the prevalence of CQ resistance in Dakar, Senegal. Based on ex vivo culture of parasites from patient isolates, our data indicate that the prevalence of CQ resistance has decreased from 53% in 2002 to 23% currently in Dakar. Our data also confirm the sensitivity of P. falciparum in Dakar to AMQ, a commonly used partner drug in ACT in Senegal. However, we identified several isolates that exhibited elevated ART IC50 values. Periodic screening should enable us to keep track of the spread of these isolates. Finally, in vitro resistance does not necessarily correlate with clinical treatment failure, because antimalarial immunity (and perhaps other factors) impact in vivo parasite clearance.22 Nonetheless, in vitro drug-resistance patterns generally reflect parasite capacity to withstand chemotherapy in the field.23 Thus, extensive and periodic monitoring of drug efficacy and better control of unregulated drug usage is imperative.
Acknowledgments
We thank Sarah Volkman for helpful comments on the manuscript.
Footnotes
Authors’ addresses: Daouda Ndiaye, Allison Demas, Omar Ndir, and Souleymane Mboup, Department of Medicine and Pharmacy, Cheikh Anta Diop University, Dakar, Senegal. Vishal Patel and Jon Clardy, Harvard Medical School, Boston, MA. Michele LeRoux and Dyann F. Wirth, Harvard School of Public Health, Boston, MA. Viswanathan Lakshmanan and Johanna P. Daily, Albert Einstein College of Medicine, Bronx, NY.
References
- 1.Kondrachine AV, Trigg PI. Global overview of malaria. Indian J Med Res. 1997;106::39–52. [PubMed] [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–192. [PubMed] [Google Scholar]
- 4.World Health Organization . WHO guidelines for the treatment of malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 5.Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 6.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndiaye D, Daily JP, Sarr O, Ndir O, Gaye O, Mboup S, Wirth DF. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in Senegal. Trop Med Int Health. 2005;10:1176–1179. doi: 10.1111/j.1365-3156.2005.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baniecki ML, Wirth DF, Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother. 2007;51::716–723. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurth F, Pongratz P, Belard S, Mordmuller B, Kremsner PG, Ramharter M. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti-malarial drug susceptibility assays. Malar J. 2009;8:79. doi: 10.1186/1475-2875-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pages F, Texier G, Pradines B, Gadiaga L, Machault V, Jarjaval F, Penhoat K, Berger F, Trape JF, Rogier C, Sokhna C. Malaria transmission in Dakar: a two-year survey. Malar J. 2008;7:178. doi: 10.1186/1475-2875-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard D, Madji N, Manirakiza A, Djalle D, Koula MR, Talarmin A. Efficacy of chloroquine, amodiaquine, sulfadoxine-pyrimethamine, chloroquine-sulfadoxine-pyrimethamine combination, and amodiaquine-sulfadoxine-pyrimethamine combination in Central African children with noncomplicated malaria. Am J Trop Med Hyg. 2005;72:581–585. [PubMed] [Google Scholar]
- 14.Basco LK, Ringwald P. Analysis of the key pfcrt point mutation and in vitro and in vivo response to chloroquine in Yaounde, Cameroon. J Infect Dis. 2001;183:1828–1831. doi: 10.1086/320726. [DOI] [PubMed] [Google Scholar]
- 15.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SM, Ndir O, Dieng T, Mboup S, Wypij D, Maguire JH, Wirth DF. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am J Trop Med Hyg. 2002;66:474–480. doi: 10.4269/ajtmh.2002.66.474. [DOI] [PubMed] [Google Scholar]
- 17.Sarr O, Myrick A, Daily J, Diop BM, Dieng T, Ndir O, Sow PS, Mboup S, Wirth DF. In vivo and in vitro analysis of chloroquine resistance in Plasmodium falciparum isolates from Senegal. Parasitol Res. 2005;97:136–140. doi: 10.1007/s00436-005-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry M, Diallo I, Bordes J, Ka S, Pradines B, Diatta B, M’Baye PS, Sane M, Thiam M, Gueye PM, Wade B, Touze JE, Debonne JM, Rogier C, Fusai T. Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am J Trop Med Hyg. 2006;75:146–151. [PubMed] [Google Scholar]
- 19.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 20.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, Taylor TE, Plowe CV. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 21.Brasseur P, Agnamey P, Gaye O, Cisse M, Badiane M, Vaillant M, Taylor WR, Olliaro P. Dosing accuracy of artesunate and amodiaquine as treatment for falciparum malaria in Casamance, Senegal. Trop Med Int Health. 2009;14:79–87. doi: 10.1111/j.1365-3156.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 22.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 23.Ekland EH, Fidock DA. In vitro evaluations of antimalarial drugs and their relevance to clinical outcomes. Int J Parasitol. 2008;38:743–747. doi: 10.1016/j.ijpara.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


