Abstract
Alveolar echinococcosis cases diagnosed histopathologically in 2002, 2006, 2007, and 2009 in Ulaanbaatar, Mongolia were reconfirmed by evaluating the cytochrome c oxidase subunit I gene of mitochondrial DNA. The most recent three cases using paraffin-embedded and ethanol-fixed specimens revealed that one was of the “Asian” haplotype, whereas two others were of the “Inner Mongolian” type. All patients were born in the western provinces of Mongolia, they never resided outside of Mongolia, and they were given a preliminary diagnosis of malignant hepatic tumor or abscess. The most recent two cases were also confirmed serologically to be active alveolar echinococcosis.
Alveolar echinococcosis (AE), often misdiagnosed as hepatocellular carcinoma, is caused by the accidental ingestion of eggs of the fox tapeworm, Echinococcus multilocularis. AE is one of the most lethal parasitic zoonoses and is prevalent in most areas of the Northern Hemisphere. Endemic areas include parts of North America (Canada, Alaska, and some of the lower contiguous states of the United States),1 Asia (Russia and other states of the former Soviet Union,1,2 China,1,3–5 and Japan1,6), and the majority of Europe.1,7 One of the largest known foci of AE is in China.1,3–5 Although E. multilocularis has been reported from Russia1,2 and China,1,3–5 only two AE cases have previously been reported from Mongolia (the first case in 1982 and the second in 2002).8,9
Recently, we have confirmed three additional indigenous AE cases at the State Central Clinical Hospital (SCCH) in Ulaanbaatar (UB), Mongolia in 2006, 2007, and 2009 with ethical approvals in Mongolia. Here, we summarize the three most recent AE cases as well as the case from 2002. Cases were confirmed by histopathological observation (Figure 1), serology (Figure 2), and molecular analysis based on cytochrome c oxidase subunit I (cox1) of mitochondrial DNA (Figure 3).
Figure 1.
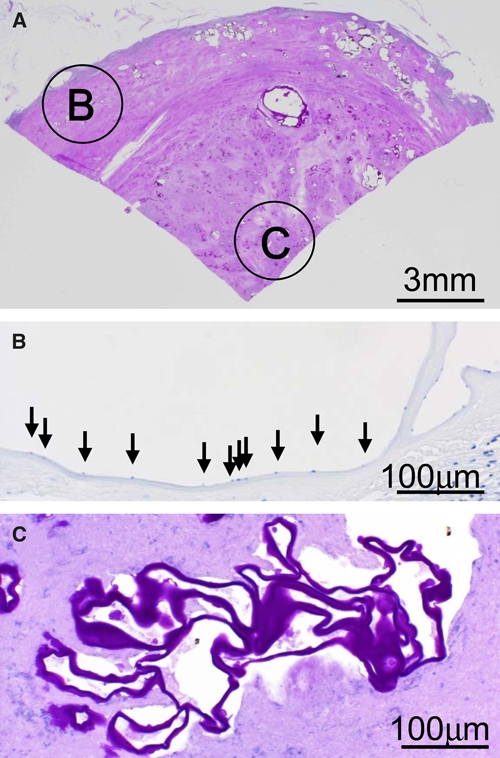
Histopathological pictures of AE case 4 showing (A) characteristic laminated layers of E. multilocularis (Periodic Acid Schiff [PAS] staining), (B) active outer lesion with germinal cells and germinal layer (Hematoxylin staining), and (C) inactive or necrotic inner lesion without germinal cell or germinal layer but PAS-positive laminated layers (PAS staining). This figure appears in color at www.ajtmh.org.
Figure 2.
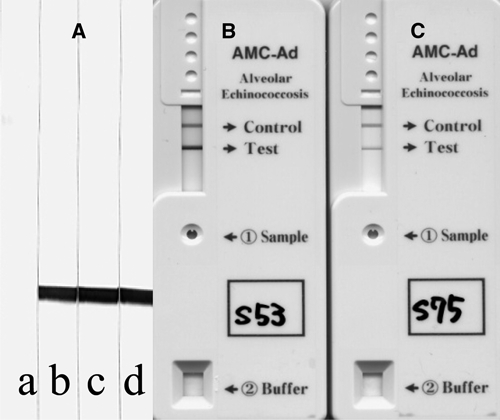
Immunoblot and immunochromatographic figures of the two AE cases in Mongolia using a recombinant Em18.11,12 (A) Immunoblot figures. Lane a, shows a pooled negative control serum in 1:50 dilution, lane b, shows confirmed AE serum in 1:1000 dilution, and lanes c and d show sera from cases 4 and 5, respectively, in 1:20 dilution. (B and C) Immunochromatographic figures of cases 4 and 5, respectively.10
Figure 3.
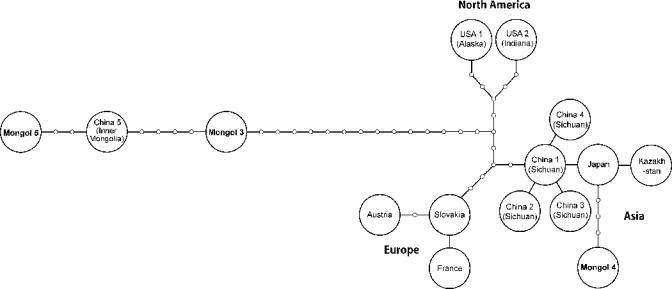
Haplotype network constructed with the program TCS 1.2118 based on 1543 base pairs of cox1 sequences.17 Each node indicates a mutational change, and the geographic names inside circles represent the origins of haplotypes. GenBank accession numbers of each sequence used in the present study are as follows: Austria (AB461412), France (AB461413), Slovakia (AB461414), Kazakhstan (AB461415), Japan (AB461416), China 1 (AB461417), China 2 (AB477010), China 3 (AB477011), China 4 (AB477012), USA 1 (AB461418), USA 2 (AB461419), China 5 (AB461420), Mongol 2 (AB510022), Mongol 3 (AB510023), Mongol 4 (AB510024), and Mongol 5 (AB510025).
Case 2, a 28-year-old policeman born in Orkhon-Uul province, was admitted to the SCCH in 2002. Pre-surgical diagnosis was a suspected chronic liver abscess. During surgery, the patient died of heart failure. Post-mortem diagnosis was hepatic AE with a 13.8 × 7.7-cm lesion on the right lobe of the liver.
Case 3, a 25-year-old disabled man born in Uvs province but living in UB since 1995, was admitted to the SCCH in 2006. He had a 15 × 9.5-cm lesion on the right lobe of the liver with invasion to the diaphragm. The lesion was not resectable, and he died 5 days post-operatively from liver failure. Post-mortem diagnosis was hepatic AE.
Case 4, a 22-year-old female student born in Khovd province, was admitted to the SCCH in May 2007. She had a 7 × 7.5-cm lesion on the left lobe of the liver and a 4 × 4.5-cm lesion on the upper lobe of the left lung. The hepatic lesion was resected but was deemed too large for radical resection, and therefore, the pulmonary cyst was left without surgical treatment. Histopathology revealed hepatic AE (Figure 1).
Case 5, a 20-year-old unemployed female born in Bayan-Ulgii province, was admitted to the SCCH in January 2009 with hepatic and pulmonary lesions. She had a 6.3 × 6.2-cm lesion on the right lobe of the liver. Histopathology revealed hepatic AE. Among the five confirmed AE cases in Mongolia, three patients (cases 1–3) died before, during, or within 1 week of surgery because of the advanced stage of disease.8
A serum sample from case 4 was obtained in September 2008, 1 year after surgery, without any clinical background information. Serology (Figure 2) was carried out by immunoblot (IB; Figure 2A) and a commercially available rapid immunochromatography (ICT) kit10 (Figures 2B–C) using a recombinant Em18 (RecEm18).11,12 Case 4 showed very strong antibody responses to RecEm18, the highly specific diagnostic antigen for detection of active AE by both IB and ICT.10–15 This finding indicates that this woman still had active lesions, because serology becomes negative within 1 year of successful radical resection.12,13 A pre-surgical serum sample from case 5 also showed very strong antibody responses to RecEm18 in IB and ICT, suggesting that AE could have been easily confirmed if serology was introduced for diagnosis before surgery. Antibody responses in case 4 seemed to be much stronger than that in case 5 when we tested these sera by the rapid ICT kit (Figure 2B–C), because the strength of the band is a quantitative result (Sako Y and others, unpublished data). All known Mongolian AE cases,8,9,16 including the three most recent cases, were diagnosed as malignant hepatic tumors or abscesses before surgery, but they were confirmed as AE after histopathological examination.
A formalin-fixed specimen was evaluated for the case diagnosed in 2002 (case 2), paraffin-embedded specimens were evaluated for the cases diagnosed in 2006 and 2007 (cases 3 and 4), and an ethanol-fixed specimen was evaluated for the case diagnosed in 2009 (case 5). No specimen was available from the 1982 case (case 1) because of the sociopolitical crisis that occurred around 1990.
A DNA tissue kit (Qiagen, Hilden, Germany) was used for extracting DNA from each specimen. For paraffin-embedded specimens, histological sections were processed using xylene and ethanol for paraffin removal and were then rehydrated before DNA extraction. Because of the degradation of DNA, it is difficult to obtain long-fragment DNA from formalin-fixed tissues. Thus, for cases 2–4, we performed polymerase chain reaction (PCR) using primer pairs, which can amplify small (100–200 bp) fragments of cox1. For case 5, complete cox1 was amplified. Some of the primers were already reported elsewhere,17 and others were newly designed for the present study (Table 1).
Table 1.
Primers used for the amplification of cox1 gene fragments of E. multilocularis
| Primers | Sequence (5¢ to 3¢) | Positions | Reference |
|---|---|---|---|
| Em cox1 F1 | gactttctctttggttggtgtaag | tRNA-ser | 17 |
| Em cox1 F11 | tgtgattaggtagttgggtattga | 11–34 | PS |
| Em cox1 F100 | ttaagttttagtttgttgattcgtg | 100–124 | PS |
| Em cox1 F9 | tgactaatcatggtataataatgatc | 182–207 | PS |
| Em cox1 F400 | tcttcttcatatttttctaggagtag | 400–425 | PS |
| Em cox1 F500 | gtactttgtatagtgtttttatgact | 500–525 | PS |
| Em cox1 F4 | gttttggctgctgctattactatgc | 601–625 | PS |
| Em cox1 F2 | gtttatgttttgattctgcctggatt | 727–752 | PS |
| Em cox1 F5 | tgggttggatgtgaagacggcggt | 885–908 | PS |
| Em cox1 F1000 | aagagtgatcctattttgtggtgggt | 1000–1025 | PS |
| Em cox1 F1100 | ttttacacgatacttgatttgtggtg | 1100–1125 | PS |
| Em cox1 F6 | attactggtttgaggttgaataagt | 1201–1225 | PS |
| Em cox1 F10 | cctatgcattattttggtttatgtg | 1282–1306 | PS |
| Em cox1 F7 | tcttttatatctgcgtttagtgggtg | 1375–1400 | PS |
| Em cox1 R1 | aacctaaacaaccaacttcacag | rrnL | 17 |
| Em cox1 R11 | gtaattatgtatttgctgttggttag | 1583–1608 | PS |
| Em cox1 R10 | tagtggattatttaatgagtcctgta | 1475–1500 | PS |
| Em cox1 R1400 | cttttatatctgcgtttagtgggtg | 1376–1400 | PS |
| Em cox1 R9 | tatgtgggttgcctcgtcgtgtgtg | 1301–1325 | PS |
| Em cox1 R1200 | ttatgtttatttgatggtgaccgttg | 1175–1200 | PS |
| Em cox1 R4 | cgatacttgatttgtggtggctcat | 1107–1131 | PS |
| Em cox1 R8 | atgttgcttaattctagtgtaaata | 976–1000 | PS |
| Em cox1 R7 | gtttgggttttatggtttgttgttt | 801–825 | PS |
| Em cox1 R2 | ttcagcatatgttttggttttttgg | 692–716 | PS |
| Em cox1 R600 | tattttattgttagtgacgttgcct | 576–600 | PS |
| Em cox1 R400 | tggttggactttttatcctccattgt | 375–400 | PS |
| Em cox1 R12 | ggtggtttgtctgatttgaatttgcc | 268–293 | PS |
| Em cox1 R200 | tttttggtgactaatcatggtataat | 175–200 | PS |
PS = present study; tRNA-ser = tRNA gene for serine; rrnL = large subunit ribosomal RNA.
All PCRs were performed in 20-μL volumes containing 0.5 units of Ex Taq Hot Start Version (TaKaRa, Ohtsu, Japan), 0.2 mM of dNTP, 1 × Ex Taq Buffer with a final MgCl2 concentration of 2.0 mM, 15 pmol of each primer, and 1.0 μL of genomic DNA. PCR amplification consisted of initial denaturation at 95°C for 2 minutes, 35 cycles of 95°C for 15 seconds, 53°C for 15 seconds, and 72°C for 20 seconds, and a terminal extension at 72°C for 1 minute. PCR products were directly sequenced, and the obtained sequences were concatenated. Partial cox1 sequences (1,543 bp) from cases 3 and 4 and a complete cox1 sequence (1,608 bp) from case 5 were obtained. To estimate the genealogical relationship among the haplotypes in the world, the statistical parsimony network of cox1 haplotypes was constructed by TCS 1.21.18 Mitochondrial DNA studies have already revealed that isolates of E. multilocularis occur in the Northern Hemisphere, and they are divided into four clades: European, Asian, North American, and Inner Mongolian.17 Case 4 was of the “Asian” type, whereas cases 3 and 5 were of the “Inner Mongolian” type (Figure 3). Case 2 was also confirmed as AE based on cox1 sequence, but it was not included in the haplotype network analysis because of the short sequence (659 bp).
All AE cases confirmed from the western parts of Mongolia, which are located between Russia and China where highly endemic AE foci were previously described by WHO,1 had no history of going abroad and were concluded to be indigenous AE cases. This leads to the hypothesis that there may be one large focus of AE in the mountainous region that includes parts of Mongolia, China, and Russia.
The coexistence of the Asian and North American haplotypes was reported from the St. Lawrence Island in the Bering Sea, and a evolutionary scenario in which distinct parasite populations derived from glacial refugia have been maintained by indigenous host mammals was discussed.17 The present results indicate that human AE cases in Mongolia show additional and different coexistence of the Asian and Inner Mongolian haplotypes. Therefore, further studies on the genetic diversities of the parasite through identification of the natural intermediate and definitive host animals in Mongolia are necessary and essential to discuss the evolution of this parasite.1,17 Surveillance of E. multilocularis in humans and animals, such as rodents (intermediate hosts), red foxes (Vulpes vulpes), corsac foxes (Vulpes corsac),19 wolves, and dogs (definitive hosts), is currently under discussion between the National Center for Communicable Diseases in Mongolia and Asahikawa Medical College in Japan.
Acknowledgment
We thank Dr. Christine Budke for editing this manuscript.
Footnotes
Financial support: This study was supported in part by a Grant-in-Aid for international research projects on cestode zoonoses in Asia (17256002, 21256003) and by the Asia-Africa Science Platform Fund (2006-2011) from the Japan Society for the Promotion of Science (to A.I.). N.M. received a World Health Organization/Special Programme for Research and Training in Tropical Diseases (WHO/TDR) Grant (200075162). Nucleotide sequence data reported in this paper are available in the DNA Data Bank of Japan/GenBank/European Molecular Biology Laboratory (DDBJ/GenBank/EMBL) databases under accession numbers AB510022 (Mongol 2), AB510023 (Mongol 3), AB510024 (Mongol 4), and AB510025 (Mongol 5).
Authors’ addresses: Akira Ito, Tetsuya Yanagida, Yasuhito Sako, Minoru Nakao, and Yuji Ishikawa, Department of Parasitology, Asahikawa Medical College, Asahikawa, 078-8510, Japan, E-mails: akiraito@asahikawa-med.ac.jp, yanagida@asahikawa-med.ac.jp, yasusako@asahikawa-med.ac.jp, nakao@asahikawa-med.ac.jp, and iyuji@mvc.biglobe.ne.jp. Gurbadam Agvaandaram, Narankhajid Myadagsuren, and Temuulen Dorjsuren, Department of Medical Biology and Histology, School of Biomedicine, Health Sciences University of Mongolia, Ulaanbaatar 210648, Mongolia, E-mails: agurbadam@yahoo.com, narankhajid@yahoo.com, and dtemuulen@yahoo.com. Oyun-Erdene Bat-Ochir and Batsaikhan Chuluunbaatar, Center of Pathology, Ministry of Health, Ulaanbaatar 210648, Mongolia, E-mails: oerdene@yahoo.com and info@pathologycenter.emoh.mn. Nyamkhuu Gonchigsenghe, Department of Surgery, State Central Clinical Hospital, Ulaanbaatar 210648, Mongolia, E-mail: davka64@yahoo.com. Kazuhiro Nakaya, Animal Laboratory for Medical Research, Asahikawa Medical College, Asahikawa, 078-8510, Japan, E-mail: nky48@asahikawa-med.ac.jp. Abmed Davaajav and Nyamkhuu Dulmaa, National Center for Communicable Diseases, Ministry of Health, Ulaanbaatar 210648, Mongolia, E-mails: abmed99@yahoo.com and dnyamkhuu@nccd.gov.mn.
References
- 1.Eckert J, Schantz PM, Gasser RB, Torgerson PR, Bessonov AS, Movsessian SO, Thakur A, Grimm F, Nikogossian MA. In: WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS, editors. Paris: World Organization for Animal Health; 2001. pp. 100–142. (Geographic distribution and prevalence). [Google Scholar]
- 2.Bessonov AS. Echinococcus multilocularis infection in Russia and neighboring countries. Helminthologia. 1998;35:73–78. [Google Scholar]
- 3.Craig PS, Deshan L, MacPherson CN, Dazhong S, Reynolds D, Barnish G, Gottstein B, Zhorong W. A large focus of alveolar echinococcosis in central China. Lancet. 1992;340:826–831. doi: 10.1016/0140-6736(92)92693-a. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Urbani C, Qiu JM, Vuitton DA, Qiu DC, Heath DD, Craig PS, Zheng F, Schantz PM. Control of echinococcosis and cysticercosis: a public health challenge to international cooperation in China. Acta Trop. 2003;86:3–17. doi: 10.1016/s0001-706x(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 5.Craig PS. The Echinococcosis Working Group in China, 2006. Epidemiology of human alveolar echinococcosis in China. Parasitol Int. 55:S221–S225. doi: 10.1016/j.parint.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Ito A, Romig T, Takahashi K. Perspective on control options for Echinococcus multilocularis with particular reference to Japan. Parasitology. 2003;127:S159–S172. [PubMed] [Google Scholar]
- 7.Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55:S187–S191. doi: 10.1016/j.parint.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Galtsog L, Enkhtsetseg O, Gurbadam A, Narankhajid M, Temuulen D. First case of Alveococcus multilocularis was diagnosed in Mongolia. Mongolian Med Sci J. 2002;122:27–30. [Google Scholar]
- 9.Ebright JR, Altantsetseg T, Oyungerel R. Emerging infectious diseases in Mongolia. Emerg Infect Dis. 2003;9:1509–1515. doi: 10.3201/eid0912.020520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sako Y, Fukuda K, Kobayashi Y, Ito A. Development of an immunochromatographic test to detect antibodies against recombinant Em18 for diagnosis of alveolar echinococcosis. J Clin Microbiol. 2009;47:252–254. doi: 10.1128/JCM.01476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sako Y, Nakao M, Nakaya K, Yamasaki H, Gottstein B, Lightowlers MW, Schantz PM, Ito A. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J Clin Microbiol. 2002;40:2760–2765. doi: 10.1128/JCM.40.8.2760-2765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A, Nakao M, Sako Y. Echinococcosis: serological detection of patients and molecular identification of parasites. Future Microbiol. 2007;2:439–449. doi: 10.2217/17460913.2.4.439. [DOI] [PubMed] [Google Scholar]
- 13.Tappe D, Frosch M, Sako Y, Itoh S, Grüner B, Reuter S, Nakao M, Ito A, Kern P. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am J Trop Med Hyg. 2009;80:792–797. [PubMed] [Google Scholar]
- 14.Fujimoto N, Ito A, Ishikawa Y, Inoue M, Suzuki Y, Ohhira M, Ohtake T, Kohgo Y. Usefulness of recombinant Em18-ELISA to evaluate efficacy of treatment in patients with alveolar echinococcosis. J Gastroenterol. 2005;40:426–431. doi: 10.1007/s00535-004-1559-7. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa Y, Sako Y, Itoh S, Ohtake T, Kohgo Y, Matsuno T, Ohsaki Y, Miyokawa N, Nakao M, Nakaya K, Ito A. Serological monitoring of progression of alveolar echinococcosis with multi-organ involvement using recombinant Em18. J Clin Microbiol. 2009;47:3191–3196. doi: 10.1128/JCM.01111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davaatseren N, Otogondalai A, Nyamkhuu G, Susher AH. Management of echinococcosis in Mongolia. J Ark Med Soc. 1995;92:122–125. [PubMed] [Google Scholar]
- 17.Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y, Ito A. Geographic patterns of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int. 2009;58:384–389. doi: 10.1016/j.parint.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 19.Tang CT, Wang YH, Peng WF, Tang L, Chen D. Alveolar Echinococcus species from Vulpes corsac in Hulunbeier, Inner Mongolia, China, and differential development of the metacestodes in experimental rodents. J Parasitol. 2006;92:719–724. doi: 10.1645/GE-3526.1. [DOI] [PubMed] [Google Scholar]


