Abstract
Effective handwashing with soap requires reliable access to water supplies. However, more than three billion persons do not have household-level access to piped water. This research addresses the challenge of improving hand hygiene within water-constrained environments. The antimicrobial efficacy of alcohol-based hand sanitizer, a waterless hand hygiene product, was evaluated and compared with handwashing with soap and water in field conditions in Dar es Salaam, Tanzania. Hand sanitizer use by mothers resulted in 0.66 and 0.64 log reductions per hand of Escherichia coli and fecal streptococci, respectively. In comparison, handwashing with soap resulted in 0.50 and 0.25 log reductions per hand of E. coli and fecal streptococci, respectively. Hand sanitizer was significantly better than handwashing with respect to reduction in levels of fecal streptococci (P = 0.01). The feasibility and health impacts of promoting hand sanitizer as an alternative hand hygiene option for water-constrained environments should be assessed.
Introduction
Diarrheal and respiratory diseases are leading causes of child mortality. Diarrhea claims the lives of 1.87 million children less than five years of age in the world per year, with more than two-thirds of these deaths occurring in Africa and Southeast Asia.1 In the countries with the highest mortality rates for children less than five years of age, approximately 40% of all child deaths are caused by diarrhea and the respiratory infection pneumonia.2 Diarrhea and respiratory diseases are caused by a variety of pathogens transmitted by the fecal-oral route, including Giardia lamblia, Cryptosporidium parvum, pathogenic Escherichia coli, Shigella, Salmonella spp., Vibrio cholerae, Streptococcus pneumonia, rotavirus, norovirus, and enteroviruses.
Contaminated hands play a key role in transferring fecal particles from one host to another.3 A person who practices inadequate hand hygiene after defecation can transfer pathogens to other persons through direct interpersonal contact, contact with inanimate objects and surfaces, and food preparation.4,5 In developing countries, where many households store the water they use for cooking and drinking in the home, dipping contaminated hands and cups into storage containers can also transfer pathogens to other family members.6 Hand-based transmission of pathogens is so ubiquitous that handwashing with soap has been argued to be the best intervention to prevent diarrhea,7 and the most cost-effective option for preventing the death of a child.8 Evidence from several meta-analyses suggest that handwashing education and promotion can reduce diarrhea incidence as much or more than improvements in water supply.9,10 A recent review of randomized controlled trials of handwashing interventions in developing countries found that handwashing can reduce diarrheal episodes by an average of 31%.11 Handwashing interventions have also been found to significantly reduce incidence of respiratory illness in community settings around the world by an average of 21%.12
However, the problem remains that most people do not wash their hands with soap at important times, such as after using the toilet, before preparing food, before eating, after cleaning up a child who has defecated, and before feeding a child. A review of handwashing behavior research from 11 countries found that only 17% of child caretakers wash their hands with soap after using the toilet.13 The quantity and proximity of water available to households have been demonstrated to correlate with frequency of handwashing.14–16 For example, households in east Africa that have individual piped water connections use more than twice the volume of water for personal hygiene compared with households that do not have piped supply.17 Globally, more than three billion persons do not have household-level access to piped water, which presents a formidable challenge to increasing rates of handwashing with soap and water.17
Identifying alternative hand hygiene methods for populations with limited water availability may be a critical step for reducing global child mortality. Alcohol-based hand sanitizers are waterless hand hygiene agents that have been widely accepted for use in hospitals and health care facilities in the United States and Europe, but have received little attention for their use in the developing world. Hand sanitizer formulations consist of ethanol, isopropanol, and/or n-propanol. Those sanitizers that contain 60–80% alcohol act as a skin disinfectant by denaturing proteins of pathogens.18 It is noted that hand sanitizer is not effective against bacterial spores or protozoan oocysts and has poor antimicrobial activity against certain nonenveloped viruses.19
The correct use of hand sanitizer does not require water, takes less time than handwashing, and does not require drying hands with potentially contaminated surfaces.20 A range of efficacy tests for hand sanitizer have been performed on hands artificially contaminated with bacteria and viruses. These studies have demonstrated hand sanitizer to be as or more efficacious than handwashing with plain (i.e., not antibacterial) soap and water.18,19,21,22 There is also evidence that hand sanitizer performs as well as handwashing with soap in field conditions among health care workers; however, the relative efficacy of hand sanitizer is not as well established among other populations.23,24 To the best of our knowledge there have been no published studies of the antimicrobial efficacy of hand sanitizer in a developing country, where conditions can be substantially different from those of health care settings in developed countries.
Only a handful of studies have used microbiologic methods to measure the efficacy of handwashing with soap and water in field conditions in a developing country.25–28 These and other previous studies have used the quantitative measurement of fecal indicator bacteria (FIB), such as fecal coliforms, fecal streptococci, and E. coli, on hands to evaluate hand hygiene.29–31 These organisms are not pathogens, but are used to indicate fecal contamination. Further work is needed to determine the relationship between the presence of these indicators on hands, hand hygiene behavior, and health. In addition, little is known regarding variation of FIB between the hands of the same person because of factors such as hand dominance or cultural hygiene practices such as anal cleansing. The present field study in Dar es Salaam, Tanzania addresses some of these knowledge gaps.
In this study, we investigate how the antimicrobial efficacy of hand sanitizer (ABHS) compares with handwashing with soap and water under field conditions in Dar es Salaam, Tanzania. We also explore the correlation between baseline levels of fecal indicator bacteria on hands with self-reported hand hygiene behavior and health status, assess the relationship between levels of bacteria on the right and left hand of the same person, and evaluate user perceptions of hand sanitizer as an alternative hand hygiene method.
Methods
Sampling frame.
Two types of study sites were identified in Dar es Salaam by local partner non-governmental organizations. The first study site was a set of six secondary schools within the Temeke Municipality of Dar es Salaam. Informed consent forms were distributed to parents before the study, and all participating students were required to present a signed parental consent form and to give oral assent before participating. A total of 53 students and 9 teachers were recruited for the study. The second study site was a local health clinic in the Ilala Municipality of Dar es Salaam. Women who were visiting the health clinic for their own or their child's health were approached by enumerators who explained the purpose of the study and asked for informed consent to participate. Only mothers with children less than 10 years of age and nurses at the health clinic were invited to participate. A total of 127 mothers and 10 nurses were recruited. The study was reviewed and approved by the Stanford Human Research Protection Program and the Tanzanian Commission for Science and Technology.
A total of 204 persons participated in the study, with 118 (58%) participating in a hand sanitizer efficacy test and 53 (26%) participating in a handwashing with soap efficacy test (Table 1). To characterize any difference in baseline levels of bacteria between the left and right hands of the same person, and to show what changes in FIB levels might be expected with the test method (caused by method precision and accuracy) when no hand hygiene product was used, a third group of 33 (16%) respondents was assigned to a control cohort. The handwashing with soap efficacy test was not performed at the school study site because of time constraints at that location. Therefore, comparisons of efficacy results between ABHS and handwashing are made from tests among adult mothers from the clinic site only.
Table 1.
Study sample by type of efficacy test*
| Group | Hand sanitizer | Handwashing | Control | Total |
|---|---|---|---|---|
| Students | 38 | 0 | 15 | 53 |
| Adults | 80 | 53 | 18 | 151 |
| Total | 118 (58) | 53 (26) | 33 (16) | 204 |
Values are no. (%). The control group did not use a hand hygiene method before hand sampling.
Data collection.
Data were collected during August 2008. A team of enumerators (university students and graduates from Dar es Salaam) completed an extensive training program during which they received instruction on conducting interviews with personal digital assistants (PDAs), obtaining microbial hand samples using sterile technique, as well as on the characteristics of hand sanitizer (ABHS) and its correct use. Questionnaires were developed and translated into local language to collect data on the demographic characteristics of respondents, current hand hygiene practices, water supply and sanitation services, and user perceptions of hand sanitizer. Incidence of gastrointestinal (GI) and acute respiratory illness (ARI) was also assessed for the past 48 hours before data collection. Gastrointestinal illness was classified as the respondent reporting one or more of the following symptoms: stomach pain, three or more bowel movements in 24 hours, watery or loose stools, blood in the stool, or vomiting. Acute respiratory illness was classified as the respondent reporting one or more of the following symptoms: coughing, congestion or runny nose, or difficulty breathing.
The translated survey was programmed into PDAs by using The Survey System software (Creative Research Systems, Peteluma, CA). Enumerators worked in pairs when collecting data, with 18 enumerators forming 9 teams. Survey answer files were downloaded onto a field laptop at the conclusion of each day of field work and reviewed each night for enumerator errors.
Hand rinse samples.
The ABHS and handwashing efficacy tests were conducted by obtaining hand rinse samples before and after correct use of the hand hygiene agent. Enumerators wore sterile gloves for the duration of the hand sampling activities. First, enumerators performed a visual inspection of the hands to document visible dirt on the palm, finger pads, or underneath the fingernails, as well as the length of the fingernails. After recording these visual observations, enumerators received a random instruction from the PDA to sample either the left or right hand at baseline.
Hand rinsing was performed by using a modified glove juice method.32,33 Each subject inserted his or her hand into a 69-oz Whirl-Pak bag (NASCO Corp., Fort Atkinson, WI) containing 350 mL of clean water. The subject was instructed to shake his or her hand vigorously in the water and to rub his or her thumb and fingers together for 15 seconds, after which an enumerator massaged the hand through the plastic for an additional 15 seconds. When the subject removed his or her hand, he or she was provided with a clean paper towel to dry the hand completely.
The subject was then asked to use hand sanitizer or water with soap to cleanse his or her hands according to the protocols described above. Immediately after the use of hand sanitizer or water and soap, the opposite hand, i.e., the one not selected at baseline, was then rinsed using the same method.34 The same hand was not sampled twice because the hand sampling method used could be considered similar to washing the hand with water and our objective was to determine the efficacy of hand sanitizer or hand washing with soap only. Although the hands of the same person may have had different levels of bacteria at baseline, randomizing the hand to be sampled first was carried out to minimize bias associated with comparisons across hands.
Hand hygiene protocols.
Each respondent in the hand sanitizer cohort was provided with an oral description of hand sanitizer and its use as an alternative hand hygiene method. The hand sanitizer product used in this study was Purell® instant hand sanitizer (GOJO Industries, Akron, OH), which contains 62% ethanol. Two mL of hand sanitizer product was placed on the palm of one of the respondent's hands, and the enumerator provided specific oral instructions on how to rub his or her hands together using a diagram of hand rubbing motions published by the World Health Organization.21 Each subject performed the same sequence of hand rubbing motions to standardize use across respondents and ensure that all hand surfaces were covered with the product. Enumerators also made certain that subjects allowed their hands to fully dry after application.
For the handwashing with soap trial, each enumerator team was equipped with an identical, portable handwashing station to standardize technique across respondents. Clean handwashing rinse water was stored in a covered 10-liter plastic bucket fitted with a spigot at the base. Field blanks of handwashing rinse water were collected to confirm absence of FIB contamination. A plastic measuring device was placed in a plastic open catch bucket to indicate when 500 mL of rinse water had been released from the bucket. After wetting the subject's hand with approximately 10 mL of rinse water, the enumerator placed approximately 1.4 g of liquid non-antimicrobial soap (Colgate-Palmolive, New York, NY) on one of the subject's hands.
As with the hand sanitizer application, enumerators provided oral instruction and used a sequence of diagrams published by the World Health Organization that showed correct handwashing procedure to coach subjects so that each would thoroughly cover his or her hands in soap and work it into a lather.21 After the subject finished the sequence of motions, an enumerator turned the bucket spigot handle to a marked position to regulate flow of the rinse water. The subject was asked to rub his or her hands together under the running water using the same motions used when applying the soap.
Enumerators used a stopwatch to record the number of seconds that elapsed during rinsing. When 500 mL of rinse water had been released, the enumerators closed the spigot, stopped the watch, and gave the subject clean paper towels to dry his or her hands. The mean rinse water flow rate documented by enumerators was 1.1 liters/minute (SD = 0.38 liters/minute, range = 0.56–2.31 liters/minute). We did not find any significant correlation between efficacy of handwashing with soap and variation in rinse water flow rate.
For those subjects assigned to the control group, their left and right hands were sampled separately to capture baseline levels of bacteria. Those respondents recruited to participate in the control test or the handwashing efficacy test were introduced and allowed to use hand sanitizer after these respective tests had been completed to obtain user perception data.
Microbiologic sample processing.
All hand rinse samples were kept on ice and processed within four hours of collection. Samples were processed in a field laboratory by membrane filtration to detect levels of the FIB, E. coli and fecal streptococci. The choice of fecal streptococci is supported by recommendations from previous reports to consider their use as an indicator of hand hygiene behavior.35,36 Each sample was passed through a 47-mm-diameter 0.45-μm cellulose filter (Millipore Inc., Billerica, MA) and then placed on growth media. When sample volumes less than 10 mL were filtered, approximately 10 mL of autoclaved water was added to the filtration funnel before filtering to facilitate uniform dispersion over the filter surface.
Samples were analyzed for concentrations of E. coli on MI media (BD Difco, Franklin Lakes, NJ) and incubated at 35 ± 0.5°C for 24 hours according to the United States Environmental Protection Agency method 1604 for enumerating E. coli in drinking water.37 Samples were also analyzed for concentrations of fecal streptococci by enumeration on mENT media (BD Difco) and incubated at 35 ± 0.5°C for 48 hours according to the American Public Health Association Standard Method 9230 C for enumeration of fecal streptococci in water.38
A 50-mL volume of water was filtered to detect E. coli for all samples, except for baseline samples obtained from teachers and students at the school study site, for which 10 mL was filtered because of the expectation for high baseline concentrations of E. coli. Based on results from data collection at the school site, the baseline hand rinse sample volume for the health clinic was adjusted to 50 mL. The lower detection limit was calculated by dividing 1 colony-forming unit (CFU)/plate by the volume filtered after a hand hygiene method had been performed and then multiplying by the sample volume (350 mL). The upper detection limit was calculated by dividing the maximum plate count of 500 CFU/plate by the volume filtered for baseline hand samples and then multiplying by the sample volume (350 mL). The detectable range of E. coli for mothers and nurses at the health clinic was from 7 CFU (0.85 log units) per hand to 3,500 CFU (3.54 log units) per hand, with the potential to observe a maximum reduction of 3,493 CFU per hand (2.70 log units). At the school study site, the maximum reduction was higher for students and teachers, 17,493 CFU per hand (3.40 log units per hand) because of the higher upper detection limit of 17,500 CFU per hand for baseline samples.
To minimize the probability of nondetectable results, different volumes were filtered for fecal streptococci assays. To enumerate fecal streptococci, a volume of 1 mL was processed for all samples obtained at baseline. A volume of 5 mL was processed for samples obtained after a hand hygiene method was carried out, except for samples obtained after the use of hand sanitizer from students and teachers at the school study site. For these samples, a volume of 10 mL was filtered because of the expectation that levels of fecal streptococci post hand sanitation would be lower. After analyzing results from the school study site, this volume was adjusted to 5 mL for data collection at the health clinic to minimize results above the detection limit. The lower and upper limits of detection were calculated using the same technique as for E. coli. The detectable range of fecal streptococci for mothers and nurses at the health clinic was from 70 CFU (1.85 log) per hand to 175,000 CFU (5.24 log) per hand, with the potential to observe a maximum reduction of 174,930 CFU per hand (3.40 log units) between hands sampled before and after hand hygiene. The maximum reduction that could have been observed for teachers and students was slightly higher, 174,965 CFU/hand (3.70 log units), because of the lower minimum detection limit of 35 CFU/hand for samples obtained after the use of ABHS.
Samples with results below the detection limit were assigned a value of 0.5 CFU/plate. A total of 2% of all fecal streptococci tests and 15% of all E. coli tests had a result below the detection limit. Samples that were too numerous to count were included in the analysis and assigned a value of 500 CFU/plate (the maximum plate count). A total of 12% of E. coli plates were above the maximum plate count and 19% of fecal streptococci plates were above the maximum plate count. These analysis methods for dealing with data below and above the detection limit are well documented in the literature.27,39–46 Tests were not performed to confirm E. coli or fecal streptococci. Thus, concentrations and reductions reported in this paper should be viewed as those of presumptive E. coli and fecal streptococci.
Statistical analyses.
All bacterial concentrations were normalized to CFU per hand and then log10 (hereafter referred to as log) transformed before analysis. The units we report are log CFU/hand. One-way analysis of variance, independent and paired sample t-tests, and the Pearson correlation coefficient were used to analyze data. All tests performed were two-tailed and probabilities of P < 0.05 were considered statistically significant. Statistical analysis was performed with SPSS Statistics software version 17.0 (SPSS Inc., Chicago, IL).
Results
Description of sample population.
The mean age of the high school students was 12 years (SD = 1.85 years, range = 9–15 years). The mean age of adults participating in the study was 27 years (SD = 6.9 years, range = 16–54 years) (Table 2).
Table 2.
Description of sample population*
| Demographic information | Students | Adults |
|---|---|---|
| Number | 53 | 151 |
| Mean age, years | 12 | 27 |
| Mean number of people in family | 6 | 5 |
| Female, % | 59 | 93 |
| Muslim, % | 62 | 72 |
| Christian, % | 38 | 28 |
| Have you felt sick with a stomach illness or respiratory infection in the past 48 hours? | ||
| Yes, reported GI symptoms, % | 15 | 13 |
| Yes, reported ARI symptoms, % | 23 | 28 |
GI = gastrointestinal illness; ARI = acute respiratory illness.
The percentage of female students enrolled (59%) was somewhat higher than the percentage of male students enrolled (41%). Most adults that participated in the study were female (93%), which was expected given our objective to enroll mothers with children, teachers, and nurses. Almost 75% of adults and 66.7% of students were Muslims, with the remainder Christians.
Symptoms of infectious illnesses commonly transmitted by the fecal-oral route were documented among student and adult participants. At time of data collection, the incidence of GI symptoms in the past 48 hours was 15% among students and 13% among adults. Symptoms indicating ARI were reported by 23% of students and 28% of adults.
Reported hand hygiene behavior.
Respondents were questioned regarding their handwashing habits (Table 3). However, it should be noted that self-reported data are often unreliable and may represent an overestimation of true handwashing behavior.47 Respondents were asked how many times they had washed their hands yesterday to get an estimate of a typical daily handwashing rate. Most students (68%) reported washing their hands 2–4 times per day, and 42% of adult respondents reported washing their hands ≥ 5 times per day. When asked about their most recent hand washing event, adults were more than twice as likely as students to report handwashing in the past hour, and no adults reported a time length greater than 12 hours.
Table 3.
Reported hand hygiene behavior
| Behavior | Students, % | Adults, % |
|---|---|---|
| How often do you use soap to wash your hands? | ||
| Always | 32 | 43 |
| Occasionally | 42 | 42 |
| Rarely or never | 21 | 15 |
| Do not wash hands | 3 | 0 |
| How many times did you wash your hands yesterday? | ||
| ≥ 5 times | 4 | 42 |
| 4 times | 14 | 12 |
| 3 times | 36 | 16 |
| Twice | 18 | 11 |
| Once | 12 | 2 |
| None | 2 | 1 |
| Do not know | 14 | 17 |
| How long ago was the last time you washed your hands? | ||
| < 1 hour | 17 | 37 |
| 1–4 hours before | 40 | 59 |
| 4–12 hours before | 23 | 2 |
| > 12 hours | 6 | 0 |
| Do not remember | 14 | 2 |
When subjects were asked how often they use soap when washing their hands, 43% of adults reported always using soap, 42% reported occasionally or sometimes using soap, and 15% reported rarely or never using soap. Fewer students (33%) than adults reported always using soap and approximately 25% reported rarely or never using soap. Those respondents who reported rarely or never using soap were asked if there was a particular reason they did not use soap. Reasons cited more than once included: soap is not available, not enough time, not a habit, soap is not necessary for cleaning hands, and don’t like soap/bad smell. A total of 10% of adults and 16% of students reported not always having enough water in their home to wash their hands, and 22% of adults reported soap for hand washing was not always available in their home. Only 23% of students reported always having access to soap for handwashing at school, and 54% reported that they do not always have enough water at school for handwashing.
Baseline levels of indicator bacteria on hands.
The mean log transformed concentrations (CFU) of E. coli and fecal streptococci per hand among all subjects were 2.49 (SD = 0.90, range = 0.54–4.21) and 4.23 (SD = 0.78, range = 2.24–5.24), respectively. Fecal streptococci levels were consistently higher than E. coli on the same hand at baseline by an average of 1.74 log CFU/hand (t = 28.2, degrees of freedom [df] = 197, P < 0.001, by paired sample t-test), although the two indicator concentrations were positively correlated (Pearson's r = 0.47, n = 198, P < 0.001).
There was a significant difference in baseline levels of E. coli (F = 10.20, df = 3, P < 0.001, by analysis of variance) and fecal streptococci (F = 8.56, df = 3, P < 0.001) between respondent types. Students were found to have 0.66 log CFU/hand less E. coli (df = 197, t = 4.85, P < 0.001) and 0.40 log CFU/hand less fecal streptococci (df = 197, t = 3.28, P = 0.001) than adults (Figure 1). We also found that female students had significantly lower levels of bacteria than male students at baseline (E. coli df = 50, t = 2.15, P = 0.036; fecal streptococci df = 50, t = 2.96, P = 0.005, by independent sample t-test). On average, females had 0.43 log CFU/hand less E. coli than males and 0.50 log CFU/hand less fecal streptococci.
Figure 1.
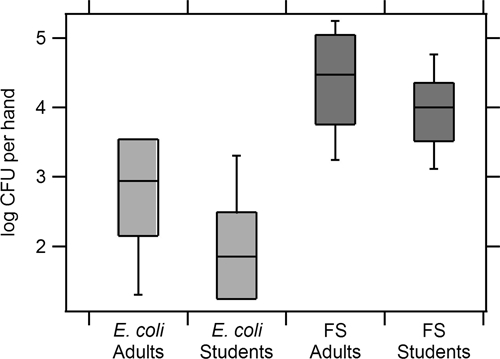
Box and whisker plots showing baseline levels of Escherichia coli and fecal streptococci (FS) per hand of respondents. The line within each box represents the median, the top and bottom of the box represent the 75th and 25th percentiles, and the top and bottom whisker extend to the 90th and 10th percentiles, respectively.
The dominant hands of respondents were found to have significantly higher levels of fecal streptococci than non-dominant hands at baseline by an average of 0.29 log CFU/hand (t = 2.64, df = 197, P = 0.009). Notably, the right hand was documented as the dominant hand for 98% of participants, and 100% of respondents reported using their left hand to clean themselves after using the toilet. There was no significant difference found in E. coli levels between the dominant and non-dominant hands. Among adults, those that reported always using soap to wash their hands had significantly lower levels of fecal streptococci and E. coli at baseline than those adults who reported occasionally or rarely using soap to wash their hands (E. coli t = −2.19, df = 145, P = 0.030; fecal streptococci t = −2.29, df = 145, P = 0.024, by t-test); mean differences were −0.32 log CFU E. coli and −0.30 log CFU fecal streptococci per hand.
Among all respondents, those reporting symptoms in the past 48 hours indicating a respiratory illness had significantly higher levels of fecal streptococci at baseline than respondents who did not report a respiratory illness (mean difference = 0.29 log CFU/hand, t = 2.39, df = 197, P = 0.018). Using bivariate analysis, we did not find any significant correlation between baseline hand contamination and diarrheal illness, religion, visible dirt on the hands (palms, finger pads or underneath the fingernails), length of fingernails, time since last handwashing, and reported daily rate of handwashing.
Bacteria variability between left and right hands in control group.
Among the 33 respondents participating in the control test (in which both hands were sampled separately at baseline and no hand hygiene method was tested), bacteria levels varied across hands of the same person, with mean absolute differences of 0.51 log CFU/hand E. coli and 0.66 log CFU/hand fecal streptococci found between hands. However, levels of E. coli and fecal streptococci on the left and right hands of the same person were found to be significantly correlated (E. coli Pearson's r = 0.63, n = 32, P < 0.001; fecal streptococci Pearson's r = 0.46, n = 32, P = 0.008), implying that if one of the subject's hands had high levels of bacteria, it was likely that his or her other hand also had high levels. Among the respondents participating in the control test, the trend described above for concentrations of fecal streptococci on right hands to be higher than on left hands was observed, but does not reach statistical significance (mean difference = 0.22 log CFU/hand, t = 1.56, P = 0.13, by paired t-test). These control test results are shown in Figure 2 as a basis for comparison with the hand hygiene efficacy tests.
Figure 2.
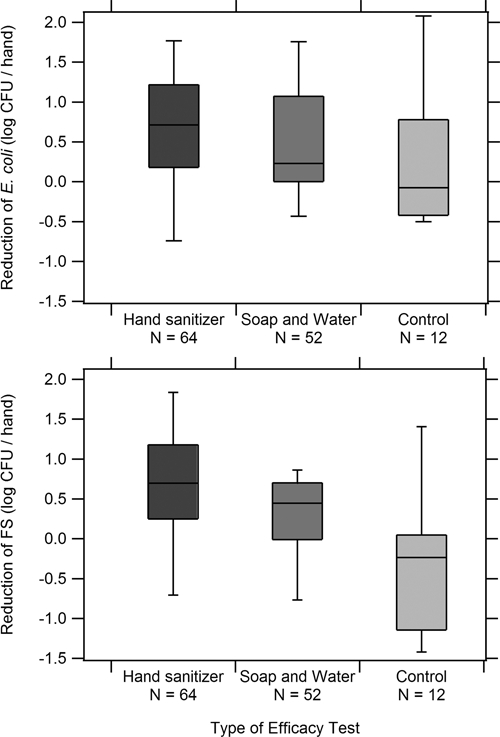
Box and whisker plots of reductions in concentrations (calculated as log colony-forming units [CFU]/hand before − log CFU/hand after) of Escherichia coli and fecal streptococci (FS) on hands after the use of hand sanitizer, after handwashing with soap and water, or after no hand hygiene method.
Efficacy of hand sanitizer in field conditions.
Among adults, paired t-tests showed significant differences in the levels of E. coli and fecal streptococci concentrations on the same person's hands before and after the use of hand sanitizer (Table 4). The mean log reductions of E. coli and fecal streptococci after use of hand sanitizer by adults were 0.66 log CFU/hand (SD = 0.89, t = 6.47, df = 75, P < 0.001) and 0.71 log CFU/hand (SD = 0.94, t = 6.57, df = 75, P < 0.001), respectively. Mean log reductions observed among students were lower than among adults. Levels of fecal streptococci were significantly reduced on students’ hands after use of hand sanitizer by a mean of 0.40 log CFU/hand (SD = 0.67, t = 3.68, df = 36, P = 0.003). Changes in levels of E. coli from student hand sanitizer use were found to be approaching statistical significance with a mean reduction of 0.25 log CFU/hand (SD = 0.84, t = 1.78, df = 36, P = 0.083).
Table 4.
Efficacy (SD) of hand sanitizer as measured by mean log reduction (log colony-forming units/hand) of Escherichia coli and fecal streptococci when used by adults and students under field conditions
| Organism | Students | Adults |
|---|---|---|
| E. coli | 0.25 (0.84) | 0.66 (0.89) |
| Fecal streptococci | 0.40 (0.67) | 0.71 (0.94) |
Efficacy of hand sanitizer compared with handwashing.
Efficacy tests of handwashing with soap and hand sanitizer were compared using data collected from the mothers as test subjects (Figure 2 and Table 5). Statistically significant reductions (mean of 0.66 log CFU/hand E. coli and 0.64 log CFU/hand fecal streptococci) were observed after use of hand sanitizer (E. coli t = 5.75, df = 63, P < 0.001; fecal streptococci t = 5.60, df = 62, P < 0.001). Mean reductions of 0.50 log CFU/hand for E. coli and 0.25 log CFU/hand for fecal streptococci were observed after handwashing with soap and water (E. coli t = 4.00, df = 51, P < 0.001; fecal streptococci t = 2.76, df = 51, P = 0.008).
Table 5.
Comparison of mean log reduction of Escherichia coli and fecal streptococci for hand sanitizer versus handwashing with soap among adult mothers*
| Organism | Hand sanitizer (n = 64) | Handwashing (n = 52) | Difference |
|---|---|---|---|
| E. coli | 0.66 (0.92), P < 0.001 | 0.50 (0.90), P < 0.001 | 0.16, P = 0.35 |
| Fecal streptococci | 0.64 (0.91), P < 0.001 | 0.25 (0.64), P = 0.008 | 0.39, P = 0.010 |
Differences were calculated by subtracting mean log reduction for handwashing from mean log reduction for hand sanitizer. Values in parentheses are standard deviations.
In a comparison of the efficacy of hand sanitizer versus handwashing with soap and water among adult mothers, hand sanitizer performed significantly better than handwashing with soap with respect to mean log reductions of fecal streptococci (mean difference = 0.39 log CFU/hand, t = 2.63, df = 114, P = 0.010, by independent samples t-test). Hand sanitizer also reduced levels of E. coli by an average of 0.16 log CFU/hand more than handwashing with soap, although this result was not statistically significant (P = 0.347). No statistically significant differences in baseline levels of E. coli and fecal streptococci were found between those mothers who participated in the hand sanitizer efficacy test and those who participated in the handwashing efficacy test.
We analyzed reductions in microbial contamination on test subjects whose hand rinse samples before and after treatment were within range of the lower and upper detection limits in the analysis. Even within this subset of data, hand sanitizer outperformed handwashing by providing an average 0.50 log CFU/hand greater reduction of E. coli (analysis includes 25 handwashing tests and 38 hand sanitizer tests; P = 0.016) and 0.29 log CFU/hand greater reduction of fecal streptococci (analysis includes 19 handwashing tests and 47 hand sanitizer tests; P = 0.103), although the latter did not achieve statistical significance.
User perceptions of hand sanitizer.
A total of 94% of all 204 respondents reported they would use hand sanitizer in their home, despite the fact that 97% had never seen a similar product before in Tanzania. In addition, 98% stated there was nothing about the hand sanitizer product that they did not like, and 96% reported that hand sanitizer had a pleasant smell. Respondents perceived hand sanitizer to have similar cleaning abilities to handwashing with soap. Of those respondents participating in the hand sanitizer efficacy test or the control test, 87% reported their hands were much cleaner after using hand sanitizer, and 81% of those respondents participating in the handwashing efficacy test reported that their hands were much cleaner after using soap and water.
Among the 53 respondents asked to compare directly the use of hand sanitizer with hand washing with soap and water, 85% reported that hand sanitizer was easier to use, 76% said hand sanitizer felt better on their hands, and 74% thought that hand sanitizer was more effective in cleaning their hands.
Discussion
In this study, hand sanitizer (ABHS) performed as well or better than handwashing with soap at reducing concentrations of E. coli and fecal streptococci on hands in field settings of a country where morbidity and mortality from diarrheal illness is high. Handwashing with soap appeared to be less efficacious for reducing levels of fecal streptococci on hands compared with its efficacy against E. coli. In contrast, hand sanitizer was observed to reduce levels of E. coli and levels of fecal streptococci comparably. Enterococcus is a Gram-positive bacterium, and E. coli is a Gram-negative bacterium. This finding suggests that further work should be done to assess whether handwashing has limited efficacy under field conditions against Gram-positive bacteria.
The mean log reductions of E. coli and fecal streptococci for adults using hand sanitizer were 0.66 log units (SD = 0.89) and 0.71 log units (SD = 0.97), respectively. It should be noted that because these changes were calculated by replacing plate counts below and above the detection limit with 0.5 and 500 CFU per plate, actual changes may be greater. However, these values are considerably lower than log reductions of 3.4–3.7 reported in the literature for alcohol-based hand sanitizer formulations consisting of 70% ethanol on hands artificially contaminated with E. coli and Staphylococcus aureus (a common hand hygiene agent test organism).22
The lower efficacy observed in this study may be caused by the real use conditions under which the efficacy tests were implemented. Higher efficacy has been documented in studies in which larger volumes of hand sanitizer were applied to the hands, sanitizer with higher concentrations of alcohol was used, application of sanitizer to the hands was carried out for a longer period of time, and reduction of artificial contamination (as opposed to existing hand microflora) was measured.48 It should also be noted that the log reductions observed in the present study may be greater than what is achieved under normal use conditions in Tanzania, during which mothers may not follow the World Health Organization recommended hand motions or have clean water from a spigot for rinsing.
Fecal streptococci were approximately two orders of magnitude higher than E. coli in hand rinse samples (4.2 log CFU/hand versus 2.5 log CFU/hand, respectively). The concentration of fecal streptococci is approximately 1.5 orders of magnitude lower than E. coli in human feces (5.2 versus 6.7 log CFU/gram, respectively).49 Assuming that the source of these fecal indicators on hands is feces, our results imply that fecal streptococci are more readily eluted from the hand by the modified glove juice method than E. coli. Another possibility is that fecal streptococci are more persistent on skin than E. coli after contamination, as reported by Pinfold.31 Alternatively, the higher ratio of fecal streptococci to E. coli in hand rinse samples relative to feces could suggest that sources other than feces are contributing the indicators on the hands. Possibilities include soil50 or the skin itself. Because streptococci are commensal cutaneous flora,51 some of these organisms may be fecal streptococci. More studies should be conducted to identify the source of fecal indicators on hands. In the present study, the source of the fecal indicator bacteria need not be known because we compared concentrations of the same organisms before and after a treatment.
The antimicrobial efficacy of hand sanitizer was not found to be correlated with levels of visible dirt observed on participants’ palms, finger pads, or underneath the fingernails. No evidence was observed of visible dirt having a significant shielding effect on hand sanitizer efficacy. This finding may have implications for the current recommendations by the World Health Organization or the Centers for Disease Control and Prevention to avoid using hand sanitizer when hands are visibly soiled.21,52 The efficacy of handwashing with soap was also not associated with visible dirt on the hands, an expected result given that soap cleans hands by dissolving fats, oils, and proteins and washing them away.18
We found that baseline levels of fecal streptococci on hands were positively associated with incidence of self-reported respiratory illness, i.e., persons who reported symptoms at the time of interview had relatively higher levels of hand contamination compared with persons who reported no symptoms of respiratory illness. We also observed that lower baseline levels of E. coli and fecal streptococci were associated with self-reported consistent soap use when handwashing. However, no significant correlation was found between baseline levels of FIB and reported time since last handwashing or daily rate of handwashing. Further research is needed to investigate the causal relationship between fecal streptococci concentrations on hands and health.
Despite a moderately high correlation between contamination levels of subjects’ right and left hands, considerable variation was found in bacteria levels between the hands. This finding has important implications for implementing hand rinse collection protocols in the field. In particular, our finding that the dominant hands of persons in Dar es Salaam may systematically have higher fecal streptococci levels than non-dominant hands indicates that researchers and practitioners should take steps to minimize the possibility of bias. This could be achieved by randomizing the hand to be tested (as was done in our study) or testing both hands, when assessing the efficacy of hand hygiene agents or evaluating baseline contamination levels.
Overall, study respondents displayed positive reactions to alcohol-based hand sanitizer as a hand hygiene technique. Additional research is needed to understand the potential challenges to the promotion of alcohol-based hand hygiene agents among particular groups. For example, some Muslim healthcare workers have expressed reluctance to the use of alcohol-based hand sanitizer as a hand hygiene agent.53 In addition, recent research suggests that even initially successful programs to promote handwashing with soap face challenges in continued compliance among households after 18 months.54 Similar study is needed to evaluate the relative sustainability of ABHS use, which to our knowledge, has not been assessed to date.
Although alcohol-based hand sanitizer is not currently produced at affordable rates in Tanzania or other developing countries, the formulation is not difficult to manufacture. The World Health Organization has developed guidelines for in-house manufacturing of hand sanitizer that could feasibly be implemented in Dar es Salaam.21 In settings that enable economies of scale (e.g., school classrooms), the cost of hand sanitizer has been estimated to be a few dollars per person per year for daily use in the United States.55 This cost is comparable to the per-person estimated annual cost of handwashing with soap in developing countries.55 Further research is necessary to evaluate the financial viability and potential health benefits of promoting hand sanitizer as an alternative hand hygiene option in developing countries, where quantities of water available for handwashing are often constrained.
Acknowledgments
We thank Mwajuma Mbaga, Fred George Njegeja, Helena Horak, Joshua Chynoweth, Kirsten Rogers, Annalise Blum, Rachelle Strickfaden, Jessie Liu, Sara Marks, Iain Clark, our Tanzanian laboratory assistants, the enumerator field team, and the study participants for their assistance with the study; The Health and Environmental Rescue Organization and the Tanzanian office of Population Services International for help in implementing the project; and three anonymous reviewers for their feedback in improving the manuscript.
Footnotes
Financial support: This study was supported by the American Public Health Association International Health Section Award Program (sponsored by the Colgate-Palmolive Company, 2008). GOJO Industries supplied the hand sanitizer product tested in this investigation.
Authors’ addresses: Amy J. Pickering, Alexandria B. Boehm, and Jennifer Davis, The Jerry Yang and Akiko Yamazaki Environment and Energy Building, Stanford University, Stanford, CA, E-mails: amyjanel@stanford.edu, aboehm@stanford.edu, and jennadavis@stanford.edu. Mathew Mwanjali, Population Services International, Dar es Salaam, Tanzania, E-mail: mmwanjali@psi.or.tz.
References
- 1.Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. 2008;86:710–717. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–2234. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 3.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 4.Curtis V, Biran A, Deverell K, Hughes C, Bellamy K, Drasar B. Hygiene in the home: relating bugs and behaviour. Soc Sci Med. 2003;57:657–672. doi: 10.1016/s0277-9536(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 5.Curtis V, Cairncross S, Yonli R. Review: domestic hygiene and diarrhoea- pinpointing the problem. Trop Med Int Health. 2000;5:22–32. doi: 10.1046/j.1365-3156.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- 6.Trevett AF, Carter RC, Tyrrel SF. Water quality deterioration: a study of household drinking water quality in rural Honduras. Int J Environ Health Res. 2004;14:273–283. doi: 10.1080/09603120410001725612. [DOI] [PubMed] [Google Scholar]
- 7.Curtis V. Talking dirty: how to save a million lives. Int J Environ Health Res. 2003;13((Suppl 1)):S73–S79. doi: 10.1080/0960312031000102822. [DOI] [PubMed] [Google Scholar]
- 8.Larsen Hygiene and health in developing countries: defining priorities through cost-benefit assessments. Int J Environ Health Res. 2003;13:37–46. doi: 10.1080/0960312031000122172. [DOI] [PubMed] [Google Scholar]
- 9.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 10.Esrey SA, Feachem RG, Hughes JM. Interventions for the control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bull World Health Organ. 1985;63:757–772. [PMC free article] [PubMed] [Google Scholar]
- 11.Ejemot R, Ehiri J, Meremikwu M, Critchley J. Hand washing for preventing diarrhoea. Cochrane Database Syst Rev. 2008;23:CD004265. doi: 10.1002/14651858.CD004265.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98:1372–1381. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis VA, Danquah LO, Aunger RV. Planned, motivated and habitual hygiene behaviour: an eleven country review. Health Educ Res. 2009;24:655–673. doi: 10.1093/her/cyp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis V, Kanki B, Mertens T, Traore E, Diallo I, Tall F, Cousens S. Potties, pits and pipes: Explaining hygiene behaviour in Burkina Faso. Soc Sci Med. 1995;41:383–393. doi: 10.1016/0277-9536(94)00341-p. [DOI] [PubMed] [Google Scholar]
- 15.Gilman RH, Marquis GS, Ventura G, Campos M, Spira W, Diaz F. Water cost and availability: key determinants of family hygiene in a Peruvian shantytown. Am J Public Health. 1993;83:1554–1558. doi: 10.2105/ajph.83.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oswald WE, Hunter GC, Lescano AG, Cabrera L, Leontsini E, Pan WK, Soldan VP, Gilman RH. Direct observation of hygiene in a Peruvian shantytown: not enough handwashing and too little water. Trop Med Int Health. 2008;13:1421. doi: 10.1111/j.1365-3156.2008.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumwine JK, Katui-Katua M, Munguti KK. Drawers of Water. II: 30 Years of Change in Domestic Use and Environmental Health in East Africa. London: International Institute for Environment and Development; 2002. [Google Scholar]
- 18.Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17:863–893. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1–45. [PubMed] [Google Scholar]
- 20.Pittet D. Improving adherence to hand hygiene practice: a multidisciplinary approach. Emerg Infect Dis. 2001;7:234–240. doi: 10.3201/eid0702.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO Guidelines on Hand Hygiene in Health Care (Advanced Draft) Geneva: World Health Organization, World Alliance for Patient Safety; 2006. [Google Scholar]
- 22.Bloomfield SF, Aiello AE, Cookson B, O’Boyle C, Larson EL.2007The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers Am J Infect Control 35S27– S64 [Google Scholar]
- 23.Traub-Dargatz JL, Weese JS, Rousseau JD, Dunowska M, Morley PS, Dargatz DA. Pilot study to evaluate 3 hygiene protocols on the reduction of bacterial load on the hands of veterinary staff performing routine equine physical examinations. Can Vet J. 2006;47:671–676. [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MA, Sheng H, Newman J, Hancock DD, Hovde CJ. Comparison of a waterless hand-hygiene preparation and soap-and-water hand washing to reduce coliforms on hands in animal exhibit settings. Epidemiol Infect. 2006;134:1024–1028. doi: 10.1017/S095026880600598X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoque BA. Handwashing practices and challenges in Bangladesh. Int J Environ Health Res. 2003;13((Suppl 1)):S81–S87. doi: 10.1080/0960312031000102831. [DOI] [PubMed] [Google Scholar]
- 26.Kaltenthaler E, Waterman R, Cross P. Faecal indicator bacteria on the hands and the effectiveness of hand-washing in Zimbabwe. Trop Med Int Health. 1991;94:358–363. [PubMed] [Google Scholar]
- 27.Sobel J, Mahon B, Mendoza CE, Passaro D, Cano F, Baier K, Racioppi F, Hutwagner L, Mintz E. Reduction of fecal contamination of street-vended beverages in Guatemala by a simple system for water purification and storage, handwashing, and beverage storage. Am J Trop Med Hyg. 1998;59:380–387. doi: 10.4269/ajtmh.1998.59.380. [DOI] [PubMed] [Google Scholar]
- 28.Luby SP, Agboatwalla M, Raza A, Sobel J, Mintz ED, Baier K, Hoekstra RM, Rahbar MH, Hassan R, Qureshi SM. Microbiologic effectiveness of hand washing with soap in an urban squatter settlement, Karachi, Pakistan. Epidemiol Infect. 2001;127:237–244. doi: 10.1017/s0950268801005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaltenthaler EC, Drasar BS. The study of hygiene behaviour in Botswana: a combination of qualitative and quantitative methods. Trop Med Int Health. 1996;1:690–698. doi: 10.1111/j.1365-3156.1996.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinfold JV, Horan NJ. Measuring the effect of a hygiene behaviour intervention by indicators of behaviour and diarrhoeal disease. Trans R Soc Trop Med Hyg. 1996;90:366–371. doi: 10.1016/s0035-9203(96)90507-6. [DOI] [PubMed] [Google Scholar]
- 31.Pinfold JV. Faecal contamination of water and fingertip-rinses as a method for evaluating the effect of low-cost water supply and sanitation activities on faeco-oral disease transmission. I. A case study in rural north-east Thailand. Epidemiol Infect. 1990;105:363–375. doi: 10.1017/s0950268800047956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larson EL, Gomez-Duarte C, Lee LV, Della-Latta P, Kain DJ, Keswick BH. Microbial flora of hands of homemakers. Am J Infect Control. 2003;31:72–79. doi: 10.1067/mic.2003.33. [DOI] [PubMed] [Google Scholar]
- 33.Larson EL, Strom MS, Evans CA. Analysis of three variables in sampling solutions used to assay bacteria of hands: type of solution, use of antiseptic neutralizers, and solution temperature. J Clin Microbiol. 1980;12:355–360. doi: 10.1128/jcm.12.3.355-360.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trick WE, Vernon MO, Hayes RA, Nathan C, Rice TW, Peterson BJ, Segreti J, Welbel SF, Solomon SL, Weinstein RA. Impact of ring wearing on hand contamination and comparison of hand hygiene agents in a hospital. Clin Infect Dis. 2003;36:1383–1390. doi: 10.1086/374852. [DOI] [PubMed] [Google Scholar]
- 35.Kaltenthaler EC, Pinfold JV. Microbiological methods for assessing handwashing practice in hygiene behaviour studies. J Trop Med Hyg. 1995;98:101–106. [PubMed] [Google Scholar]
- 36.Luby SP, Agboatwalla M, Billhimer W, Hoekstra RM. Field trial of a low cost method to evaluate hand cleanliness. Trop Med Int Health. 2007;12:765–771. doi: 10.1111/j.1365-3156.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 37.USEPA . Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium) Washington, DC: United States Environmental Protection Agency; 2002. [Google Scholar]
- 38.Clesceri LS, Greenberg AE, Eaton AD. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association; 1998. [Google Scholar]
- 39.Clasen TF, Brown J, Collin S, Suntura O, Cairncross S. Reducing diarrhea through the use of household-based ceramic water filters: a randomized, controlled trial in rural Bolivia. Am J Trop Med Hyg. 2004;70:651–657. [PubMed] [Google Scholar]
- 40.Levy K, Nelson KL, Hubbard A, Eisenberg JN. Following the water: a controlled study of drinking water storage in northern coastal Ecuador. Environ Health Perspect. 2008;116:1533–1540. doi: 10.1289/ehp.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horak HM, Chynoweth JS, Myers WP, Davis JA, Fendorf S, Boehm AB. Microbial and metal water quality in rain catchments compared with traditional drinking water sources in the East Sepik Province, Papua New Guinea. J Water Health. 2009 doi: 10.2166/wh.2009.233. (in press). doi:10.2166/wh.2009.233. [DOI] [PubMed] [Google Scholar]
- 42.Rainey RC, Harding AK. Drinking water quality and solar disinfection: effectiveness in peri-urban households in Nepal. J Water Health. 2005;3:239–248. doi: 10.2166/wh.2005.036. [DOI] [PubMed] [Google Scholar]
- 43.Clasen TF, Bastable A. Faecal contamination of drinking water during collection and household storage: the need to extend protection to the point of use. J Water Health. 2003;1:109–116. [PubMed] [Google Scholar]
- 44.Laborde DL, Weigle KA, Weber DJ, Kotch JB. Effect of fecal contamination on diarrheal illness rates in day-care centers. Am J Epidemiol. 1993;138:243–255. doi: 10.1093/oxfordjournals.aje.a116853. [DOI] [PubMed] [Google Scholar]
- 45.Kac G, Podglajen I, Gueneret M, Vaupré S, Bissery A, Meyer G. Microbiological evaluation of two hand hygiene procedures achieved by healthcare workers during routine patient care: a randomized study. J Hosp Infect. 2005;60:32–39. doi: 10.1016/j.jhin.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Hoque BA, Mahalanabis D, Alam MJ, Islam MS. Post-defecation handwashing in Bangladesh: practice and efficiency perspectives. Public Health. 1995;109:15–24. doi: 10.1016/s0033-3506(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 47.Manun’Ebo M, Cousens S, Haggerty P, Kalengaie M, Ashworth A, Kirkwood B. Measuring hygiene practices: a comparison of questionnaires with direct observations in rural Zaire. Trop Med Int Health. 1997;2:1015–1021. doi: 10.1046/j.1365-3156.1997.d01-180.x. [DOI] [PubMed] [Google Scholar]
- 48.Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Rutala WA. The effects of test variables on the efficacy of hand hygiene agents. Am J Infect Control. 2004;32:69–83. doi: 10.1016/j.ajic.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Todar K. The Bacterial Flora of Humans. Madison, WI: Department of Bacteriology, University of Wisconsin; 2002. [Google Scholar]
- 50.Hardina CM, Fujioka RS. Soil: the environmental source of Escherichia coli and enterococci in Hawaii's streams. Environ Toxicol Water Qual. 1991;6:185–195. [Google Scholar]
- 51.Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA. 2008;105:17994–17999. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention . Guidelines for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Atlanta: Centers for Disease Control and Prevention; 2002. [Google Scholar]
- 53.Ahmed QA, Memish ZA, Allegranzi B, Pittet D. Muslim health-care workers and alcohol-based handrubs. Lancet. 2006;367:1025–1027. doi: 10.1016/S0140-6736(06)68431-6. [DOI] [PubMed] [Google Scholar]
- 54.Luby SP, Agboatwalla M, Bowen A, Kenah E, Sharker Y, Hoekstra RM. Difficulties in maintaining improved handwashing behavior, Karachi, Pakistan. Am J Trop Med Hyg. 2009;81:140–145. [PubMed] [Google Scholar]
- 55.Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer use on elementary school absenteeism. Am J Infect Control. 2000;28:340–346. doi: 10.1067/mic.2000.107276. [DOI] [PubMed] [Google Scholar]


