Abstract
Monkeypox virus (MPXV), a member of the family Poxviridae and genus Orthopoxvirus, causes a smallpox-like disease in humans. A previously described pan-Orthopoxvirus assay, based on a broad-range polymerase chain reaction (PCR) coupled with electrospray ionization mass spectrometry (PCR/ESI-MS), was evaluated for its ability to detect MPXV from spiked human and aerosol-infected cynomolgous macaque (Macaca fascicularis) samples. Detection of MPXV DNA from macaque tissue, blood, and spiked human blood by the PCR/ESI-MS pan-Orthopoxvirus assay was comparable, albeit at slightly higher levels, to the current gold standard method of real-time PCR with the pan-Orthopoxvirus assay and had a limit of detection of 200 plaque-forming units. Furthermore, the platform was able to distinguish MPXV and vaccinia viruses that were spiked into macaque blood samples at various concentrations. This platform provides a new tool for the diagnosis and monitoring of orthopoxviral loads during vaccine or antiviral studies, but also could provide rapid identification during natural outbreaks or bioterrorism attacks.
INTRODUCTION
Orthopoxviruses are classified within the family Poxviridae and the subfamily Chordopoxvirinae, which contains the genus Orthopoxvirus. All members in the family Poxviridae have lipid envelopes and double-stranded DNA genomes. The most recognized member of this genus is variola virus (the causative agent of smallpox), which has notoriously been associated with large-scale human morbidity and mortality.1 Since the eradication of smallpox nearly three decades ago, most orthopoxviruses circulating today are zoonotic.2 Specifically, monkeypox virus (MPXV) is a zoonotic virus and has been reported to produce disease in humans after direct contact with infected blood, saliva, and raw meat.2–5 This virus was first isolated in 1958 from pock lesions that developed on laboratory monkeys, which were being quarantined before experimentation.6 Currently, MPXV is most prevalent in western and central Africa, with the more virulent MPXV strains found in the Congo River basin and the less virulent MPXV strains found in western Africa.2,7–10 In 2003, there was a MPXV outbreak in the United States in persons who came in contact with infected prairie dogs, which were housed with rodents imported from Africa.7 There were no deaths, but this was the first outbreak reported for MPXV infection in humans outside Africa.7,8,11
Understandably, the development of safer orthopoxvirus vaccines and antiviral drug therapies has been severely inhibited by the elimination of smallpox in the wild and the fact that working with variola virus is limited to two World Health Organization laboratories: the Centers for Disease Control and Prevention in the United States and the State Research Center of Virology and Biotechnology VECTOR in Koltsovo, Russia. Therefore, MPXV is often used as a surrogate for variola virus during vaccine and antiviral drug studies when non-human primates and other animal species, such as rodents, are used as the animal model because variola virus cannot infect them. Therefore, MPXV has been widely used as a challenge agent in nonhuman primate animal models during the development of new orthopox vaccines, such as the highly attenuated modified vaccinia virus (VACV) Ankara vaccine, and antiviral drugs, such as ST-246.12–14 Another viral therapeutic that is currently in clinical trials is CMX001 (HDP-CDV). CMX001 (HDP-CDV) is an ether-lipid analog of cidofovir (CDV) with equivalent efficacy to CDV and less side effects, such as nephrotoxicity.15
The threat of zoonotic orthopoxviruses being exploited as a bioweapon is a serious public health concern. Furthermore, the elimination of smallpox vaccination programs for the general public has established new generations of susceptible hosts, increasing the potential pathogenicity of zoonotic orthopoxviruses.
An integrated broad-range polymerase chain reaction coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) system (Ibis T5000; Ibis Biosciences, Carlsbad, CA) has been designed to rapidly detect and accurately identify emerging pathogens and biothreat agents without prior knowledge of the nucleic acid sequence of the pathogen.16–18 Previously, the PCR/ESI-MS technology has been applied for detecting influenza virus, alphaviruses, coronaviruses, and bacterial species such as Haemophilus influenzae, Neisseria meningitides, and Streptococcus pyogenes.17,19–21
Recently, a PCR/ESI-MS pan-Orthopoxvirus assay was developed to target all members of the genus Orthopoxvirus by using the T5000 platform.22 Some of the first genus-specific assays required conventional PCR followed by restriction endonuclease digestion and subsequent gel electrophoresis.23,24 More recently, quantitative real-time PCR assays have been described to determine viral loads in clinical samples, but lack broad-range orthopoxvirus detection capabilities in a single assay.7,25 For these assays, differentiation of the various Orthopoxvirus species requires the use of different TaqMan probes in separate reactions or melt-curve analysis of hybridization probes. The recently developed PCR/ESI-MS pan-Orthopoxvirus assay was successful in identifying all known orthopoxviruses tested, but its ability to detect viruses from human clinical samples or experimentally infected animals was not studied. In this study, the performance of the pan-Orthopoxvirus assay was evaluated to validate its ability to identify MPXV viral loads from human and nonhuman primate clinical samples.
MATERIALS AND METHODS
Viral isolates and DNA extraction.
The MPXV (strain Zaire 79) used for the aerosol infections of nonhuman primates was acquired from Biodefense and Emerging Infections Research Resources Repository/The American Type Culture Collection (Manassas, VA). Characterized MPXV DNA samples collected from virus cultures and macaque blood and tissues were extracted using the QIAamp DNA mini assay manual protocol (Qiagen, Valencia, CA). Also, DNA was isolated from whole blood and selected tissues by using the BioRobot M48 (Qiagen) in accordance with the manufacturer's instructions.
MPXV-spiked human samples.
Two hundred fifty microliters of MPXV, ranging in concentrations from 20 to 2 × 107 plaque-forming units (PFU), were spiked into 750 μL of culture medium, human sera, blood, and urine samples that were obtained from Bioreclamation, Inc. (Liverpool, NY). Samples were divided in half and extracted by using two methods: the QIAamp DNA mini assay and the BioRobot M48 (Qiagen). To determine the limit of detection (LOD), MPXV was serially diluted 10-fold and then spiked into the matrix for each sample type and tested in triplicate on the PCR/ESI-MS system using the pan-Orthopoxvirus assay.
Internal positive DNA control.
To determine the efficiency of the PCR, each reaction contained a synthetic internal positive DNA control previously described.22 This control was present at a predetermined concentration (100 copies/PCR) and acted as a calibrant to determine the efficiency of the PCR and provide quantitative information.22
Broad-range orthopoxvirus PCR.
A Janus robot (PerkinElmer, Waltham, MA) was used to set up each PCR. All PCRs were performed in volumes of 50 μL using 96-well microtiter plates and a Mastercycler® thermocycler (Eppendorf, Hamburg, Germany). The PCR buffer consisted of 2.5 units of FastStart Taq (Roche, Indianapolis, IN), 1× buffer II, 2.0 mM MgCl2, 0.4 M betaine, 800 µM dNTP mixture, and 250 nM propyne containing PCR primers.22 The following PCR conditions were used to amplify sequences: 95°C for 10 minutes, followed by 8 cycles at 95°C for 30 seconds, 48°C for 30 seconds, and 72°C for 30 seconds, followed by 37 cycles at 95°C for 15 seconds, 56°C for 20 seconds, and 72°C for 20 seconds.
MPXV-infected cynomolgus macaques.
Cynomolgus macaques (Macaca fascicularis) were exposed to 4 × 105 PFU/animal of aerosolized MPXV under an approved U.S. Army Medical Research Institute of Infectious Diseases animal use protocol. Starting with day zero, samples were collected every other day after challenge.22
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations related to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
MPXV- and VACV-spiked macaque blood.
Two orthopoxviruses (MPXV-Zaire 79 strain and VACV-WR strain) were cultured in Vero cells (African green monkey kidney cells) and their titers were determined by plaque assay using the same cell lines. MPXV and VACV were diluted in separate tubes so each virus had a final concentration of 1 × 108 PFU/mL. Approximately 100 µL of diluted MPXV and VACV were spiked into 1 mL of uninfected macaque blood to give a final concentration of 1 × 107 PFU/mL for each virus in sample one. In samples two and three, VACV and MPXV were spiked in various volumes so VACV (approximately 10 µL) had a final concentration of 1 × 106 PFU/mL and MPXV (approximately 100 µL) had a final concentration of 1 × 107 PFU/mL in sample two. Macaque blood sample three contained 1 × 106 PFU/mL of MPXV and 1 × 107 PFU/mL of VACV. DNA was extracted as described above using the QIAamp DNA mini assay manual protocol (Qiagen) and evaluated using the pan-Orthopoxvirus PCR/ESI-MS assay.
Mass spectrometry and signal processing.
After PCR, approximately 30 μL of each PCR was bound to a weak anion exchange matrix where a series of wash steps removed salts and excess reaction reagents. After clean up, the purified PCR products were eluted from the stationary phase using a volatile buffer. A Daltonics microToF (Bruker, Billerica, MA) mass spectrometer was used for analyzing purified DNA.16 Products from each reaction well were individually sprayed into the mass spectrometer by using an autosampler (LEAP Technologies, Carrboro, NC). Internal mass standards and plasmid calibrants were used to reach a mass accuracy of approximately 5–10 ppm and provided accurate measurements with high-resolution mass spectra for each sample by previously described protocols.16 Proprietary signal-processing software was used to deconvolute raw data from mass per charge (m/z). This molecular mass was then assigned to the empirical molecular mass and correlating base composition of the amplicon, which was matched with those in the database of the system. Using a number of statistical considerations and multi-primer results, we identified the organisms.26 For every PCR well, the signal amplitude of the calibrant and the sample were compared and interpreted to give quantitative results.16
Real-time PCR.
Real-time PCR was carried out with the Light Cycler (Roche) using a pan-Orthopoxvirus hemagglutinin (HA) assay as previously described.25 Briefly, the oligonucleotide primers and minor groove binder protein–containing TaqMan probe were selected from conserved regions of the HA gene.7 Cloned target DNA (HA gene) was prepared as a 10-fold serial dilution from 5 × 106 to five gene copies in 5 µL.
All reactions were run in duplicate. The LightCycler analysis software version 4.0 was used to generate linear regression curves and accompanying attributes (slope, intercept, error, and r value) for each assay and these curves were used as standard curve for measuring the sample in each run.
Statistical analyses.
To determine if there were any statistically significant differences between the manual and automated methods for any specimen types in Tables 1 and 2, we preformed a standard t-test. To determine if there were any statistically significant differences between real-time PCR and PCR/ESI-MS for any of the specimen types in Table 3, we preformed an analysis of variance overtime between the two methods; pairwise comparisons were performed at each time point. A t-test was performed to determine if there were any statistically significant differences between the data for the various tissues types between the two detection methods (Figure 1). For all statistical analyses, a P < 0.05 was considered statistically significant.
Table 1.
Polymerase chain reaction coupled with electrospray ionization mass spectrometry quantitation of human clinical specimens spiked with 2 × 106 plaque-forming units of monkeypoxvirus and comparison between manual and automated DNA extraction methods*
| Specimen | QIAamp (manual) | BioRobot (automated) |
|---|---|---|
| Media | 8.4 (0.3) | 8.1 (0.1) |
| Serum | 8.3 (0.1) | 8.0 (0.1) |
| Urine | 8.3 (0.1) | 7.7 (0.2) |
| Whole blood | 8.7 (0.3) | 8.6 (0.2) |
Values are mean log10 genome equivalents per milliliter (standard error).
Table 2.
Limit of detection of polymerase chain reaction coupled with electrospray ionization mass spectrometry for media and human sera clinical samples spiked with different concentrations of monkeypox virus and extracted using manual and robotic methods*
| PFU/mL | QIAamp (manual) | BioRobot (automated) | ||
|---|---|---|---|---|
| Media | Serum | Media | Serum | |
| 20 | 0 | 0 | 0 | 0 |
| 200 | 4.3 (0.3) | 4.8 (0.1) | 4.2 (0.1) | 4.0 (0.0) |
| 2,000 | 5.5 (0.1) | 5.6 (0.1) | 5.3 (0.2) | 5.0 (0.2) |
| 20,000 | 6.5 (0.2) | 6.6 (0.1) | 6.3 (0.3) | 6.1 (0.0) |
Values are mean log10 genome equivalents per milliliter (standard error). PFU = plaque-forming units.
Table 3.
Monkeypox virus DNA extracted from blood samples from experimentally infected macaques quantitated on two pan-Orthopoxvirus diagnostic platforms*
| Blood samples | Real-time PCR | PCR/ESI-MS |
|---|---|---|
| Macaque 1, day 4 | 4.1 | 4.6 |
| Macaque 1, day 6 | 5.9 | 8.0 |
| Macaque 1, day 9† | 6.1 | 8.0 |
| Macaque 2, day 4 | 5.0 | 4.5 |
| Macaque 2, day 6 | 5.2 | 5.2 |
| Macaque 2, day 8 | 6.0 | 8.2 |
| Macaque 3, day 4 | 3.4 | 4.2 |
| Macaque 3, day 6 | 4.5 | 4.9 |
| Macaque 3, day 8 | 5.1 | 5.3 |
Values are mean log10 genome equivalents per milliliter. PCR/ESI-MS = polymerase chain reaction coupled with electrospray ionization mass spectrometry.
This macaque was moribund and was killed on day 9.
Figure 1.
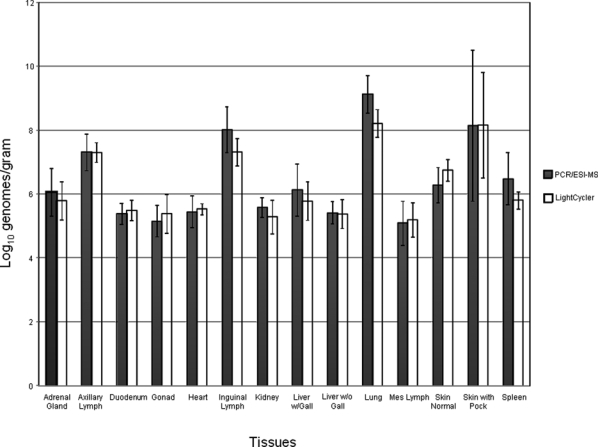
Comparison of real-time polymerase chain reaction (PCR) and PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) pan-Orthopoxvirus assays for detecting monkeypox virus (MPXV) in tissues from aerosolized MPXV-infected macaques (n = 2). Standard error bars are shown for each set of data.
RESULTS
Quantification of MPXV from spiked human clinical samples.
Table 1 depicts the DNA concentration (log10 genomes/mL) for the human clinical samples spiked with 2 × 106 PFU of MPXV and tested using the pan-Orthopoxvirus PCR/ESI-MS assay. Statistically, no differences were observed between the manual and automated methods for any of the specimen types tested.
Determination of the LOD of MPXV in spiked human clinical samples.
The DNA concentrations (log10 genomes/mL) for the human sera and medium samples spiked with MPXV ranging from 20 to 2 × 104 PFU are shown in Table 2. The DNA for each sample was either extracted using the QIAamp DNA mini assay or the BioRobot M48, serially diluted, and tested using PCR/ESI-MS. The LOD for all the samples tested was 200 PFU because all samples tested at 20 PFU were negative, based on the calculated DNA concentrations using Ibis software. Statistically, a standard t-test showed that there were only significant differences between the manual and automated methods for the serum specimen spiked at 200 and 20,000 PFU/mL. There were no statistically significant differences observed for the other samples.
Real-time PCR versus PCR/ESI-MS quantitative results for blood samples collected from MPXV-infected macaques.
The results for the pan-Orthopoxvirus real-time PCR and PCR/ESI-MS for the blood samples collected from the three macaques infected with MPXV are shown in Table 3. Blood samples of from macaque 1 obtained at day 9 post-inoculation had the highest amount of MPXV DNA detected from all three macaques by real-time PCR and PCR/ESI-MS detection systems. To determine if there were statistically significant differences among the data, an analysis of variance was performed with day as the repeated measure. No significant difference in log10-transformed genomes/mL between methods types (P = 0.124) and between day and method type (P = 0.657) were observed (Table 3).
Real-time PCR versus PCR/ESI-MS quantitative results for MPXV-infected macaque tissues.
The results for the extracted MPXV DNA collected from the two experimentally infected macaques from a variety of tissues and tested using real-time PCR and PCR/ESI-MS are shown in Figure 1. Statistically, for both animals, the PCR/ESI-MS quantitative results were comparable to those of the real-time PCR assay. Also, there were no tissue inhibitory effects observed for the real-time PCR or the PCR/ESI-MS assay from cellular macaque DNA, and the highest concentrations of MPXV DNA was detected from the lung tissue, as would be expected from an aerosol infection.
Quantification of MPXV and VACV from spiked macaque blood.
The concentrations of the two viral DNAs detected from the spiked macaque blood samples are shown in Table 4. MPXV was detected at a slightly higher concentration in sample one when equal concentrations of MPXV and VACV were spiked, but both viruses had clear identification signals in terms of spectra data (Figure 2). In sample two, MPXV was spiked at the highest concentration and was detected at 3.6 log10 genome equivalents/mL compared with VACV, which was detected at 3.0 log10 genome equivalents/mL. In sample 3, VACV was spiked at a higher concentration and was detected at 3.8 log10 genome equivalent/mL; MPXV was detected at 3.3 log10 genome equivalent/mL.
Table 4.
MPXV and VACV DNAs extracted from spiked macaque blood containing the same concentration (sample 1) or different concentrations of each virus (sample 2 and 3) and quantitated on the pan-Orthopoxvirus platform*
| Macaque blood sample | 1 × 106 PFU/mL | PCR/ESI-MS | 1 × 107 PFU/mL | PCR/ESI-MS |
|---|---|---|---|---|
| 1 | NA | NA | MPXV, VACV | 3.5, 3.4 |
| 2 | VACV | 3.0 | MPXV | 3.6 |
| 3 | MPXV | 3.3 | VACV | 3.8 |
Values are mean log10 genome equivalents per milliliter. MPXV = monkeypox virus; VACV = vaccinia virus; PFU = plaque-forming units; PCR/ESI-MS = polymerase chain reaction coupled with electrospray ionization mass spectrometry; NA = not available.
Figure 2.
Mass spectra data from the pan-Orthopoxvirus polymerase chain reaction coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) assay representing a single-strand amplicon of the DNA polymerase gene for both monkeypox virus and vaccinia virus from a mixed blood sample. This figure appears in color at www.ajtmh.org.
DISCUSSION
MPXV poses a significant threat as a zoonotic pathogen and potential bioterrorism agent. Currently, real-time PCR is the most widely accepted diagnostic system for detecting orthopoxviruses. A real-time PCR assay has been previously described to detect MPXV by DNA polymerase (E9L-NVAR) and extracellular enveloped protein (B6R) genes as primer targets.11 The E9L-NVAR assay detected only Eurasian orthopoxviruses and not variola, and the B6R assay detected only MPXV isolates.11 Also, two other real-time PCR assays have also been described for detecting different species of orthopoxviruses.7,25 One of the orthopoxvirus assays consists of primers that target the HA gene and the other assay uses primers specific for the vaccinia virus F3L and NR3 genes.7,25 Consequently, only the previously described pan-Orthopoxvirus real-time PCR assay, which are specific for the HA gene, can detect all orthopoxviral species in a single assay by melt curve analysis.25 However, because the HA assay cannot identify the exact species of orthopoxvirus being amplified, all amplicons for the currently described Orthopoxvirus real-time PCR assays need to be sequenced to determine their exact identity.7,25
In contrast, the pan-Orthopoxvirus PCR/ESI-MS assay can identify all members of the Orthopoxvirus genus in a single assay and can quantitatively identify MPXV DNA in clinical specimens without the need for sequencing. Spiked human samples containing 2 × 106 PFU of MPXV had comparable MPXV DNA viral loads detected between the automated and manual DNA extraction methods (Table 1). Specifically, when comparing the viral loads detected in each of the four biological backgrounds, all samples had comparable viral loads detected, except for the urine sample extracted by the automated method. This particular sample did not have a viral load detected at > 8.0 log10 genome equivalents/mL; this anomaly cannot be currently explained. For the LOD, the manual and automated methods were again comparable and the LOD was determined to be 200 PFU in medium and human sera spiked with MPXV (Table 2). Viral load detection directly corresponded in a logarithmic fashion to the amount of MPXV used to originally spike the sample. Additionally, MPXV viral loads could be detected from blood and tissue specimens that were collected from experimentally infected cynomolgus macaques and were comparable to data using a pan-Orthopoxvirus real-time assay (Table 3 and Figure 1). For all samples, we observed no inhibition with the pan-Orthopoxvirus PCR/ESI-MS platform and there was no inhibitory amplification from residual cellular DNA or tissue inhibition. The pan-Orthopoxvirus PCR/ESI-MS had slightly higher MPXV viral loads detected for most tissue samples compared with the pan-Orthopoxvirus real-time PCR assay (Table 3). Specifically, the MPXV DNA extracted from the lung tissues had the highest concentrations of MPXV detected compared with the rest of the tissue extracted DNA and this corresponded to the route of infection, which was by the aerosol route.
Finally, the pan-Orthopoxvirus PCR/ESI-MS platform was successful in discriminating MPXV and VACV viral DNAs that were spiked together in macaque blood. The three samples tested had similar (sample 1) and various concentrations (samples 2 and 3) of the two viruses spiked into them (Table 4). MPXV was detected at a slightly higher concentration in terms of log10 genome equivalents per milliliter, when equal concentrations of both viruses were spiked into the same sample, but VACV could still be clearly identified (Figure 2). Therefore, the pan-Orthopoxvirus PCR/ESI-MS assay has the capability to identify VACV and MPXV from mixed blood samples, which are present at various concentrations (Table 4).
Overall, this study strongly supports the application of the Ibis-T5000 Biosensor pan-Orthopoxvirus assay for the quantitative detection of orthopoxviruses from experimentally infected macaques, spiked human and macaque clinical samples, and potentially naturally infected human specimens. Furthermore, the PCR/ESI-MS platform has the capability to detect multiple orthopoxviruses from the same sample, thus being able to differentiate vaccine strains (i.e., VACV) from challenge virus (i.e., MPXV), which might be used concurrently during a trial testing for vaccine efficacy.
Acknowledgments
We thank Diana Fisher for technical assistance with the statistical analysis.
Footnotes
Financial support: This study was supported by the Defense Advanced Research Projects Agency, the Department of Homeland Security (contract no. W81XWH-05-C-0116), the U.S. Defense Threat Reduction Agency (U.S. Army Medical Research Institute of Infectious Diseases Research Plan #114538), and the Office of Biodefense Research Affairs National Institute of Allergy and Infectious Diseases (interagency agreement A120-B11). This research was performed while Rebecca J. Grant held a National Research Council Research Associateship Award at the U.S. Army Medical Research Institute of Infectious Diseases,
Disclaimer: Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
Disclosure: Some of the authors are employed by Abbott Molecular/Ibis Biosciences. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Authors’ addresses: Rebecca J. Grant, Carson D. Baldwin, Aysegul Nalca, and Chris A. Whitehouse, U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, E-mail: rebecca.j.grant@amedd.army.mil. Scott Zoll, Lawrence B. Blyn, Mark W. Eshoo, Heather Matthews, and Rangarajan Sampath, Ibis Biosciences, Carlsbad, CA.
References
- 1.Eyler JM. Smallpox in history: the birth, death, and impact of a dread disease. J Lab Clin Med. 2003;142:216–220. doi: 10.1016/S0022-2143(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 2.Jezek Z, Fenner F. Human Monkeypox. Basel: Karger; 1988. [Google Scholar]
- 3.Jezek Z, Grab B, Paluku KM, Szczeniowski MV. Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop Geogr Med. 1988;40:73–83. [PubMed] [Google Scholar]
- 4.Ladnyi ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 5.Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnus P, von Anderson EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acat Path Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 7.Kulesh DA, Loveless BM, Norwood D, Garrison J, Whitehouse CA, Hartmann C, Mucker E, Miller D, Wasieloski LP, Jr, Huggins J, Huhn G, Miser LL, Imig C, Martinez M, Larsen T, Rossi CA, Ludwig GV. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 9.Magnus PV, Anderson EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acat Path Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 10.Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, Schriewer J, Buck C, Wang C, Lefkowitz EJ, Esposito JJ, Harms T, Damon IK, Roper RL, Upton C, Buller RM. Virulence differences between monkeypox virus isolates from west Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sbrana E, Jordan R, Hruby DE, Mateo RI, Xiao SY, Siirin M, Newman PC, AP DAR, Tesh RB. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am J Trop Med Hyg. 2007;76:768–773. [PubMed] [Google Scholar]
- 13.Stittelaar KJ, Neyts J, Naesens L, van Amerongen G, van Lavieren RF, Holy A, De Clercq E, Niesters HG, Fries E, Maas C, Mulder PG, van der Zeijst BA, Osterhaus AD. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 14.Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, Niesters HG, van Doornum G, van der Zeijst BA, Mateo L, Chaplin PJ, Osterhaus AD. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker S, Touchette E, Oberle C, Almond M, Robertson A, Trost LC, Lampert B, Painter G, Buller RM. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77:39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, Drader JJ, Hannis JC, Sannes-Lowery KA, Cummins LL, Libby B, Walcott DJ, Schink A, Massire C, Ranken R, White N, Samant V, McNeil JA, Knize D, Robbins D, Rudnick K, Desai A, Moradi E, Ecker DJ. TIGER: the universal biosensor. Int J Mass Spectrom. 2005;242:23–41. [Google Scholar]
- 17.Ecker DJ, Sampath R, Blyn LB, Eshoo MW, Ivy C, Ecker JA, Libby B, Samant V, Sannes-Lowery KA, Melton RE, Russell K, Freed N, Barrozo C, Wu J, Rudnick K, Desai A, Moradi E, Knize DJ, Robbins DW, Hannis JC, Harrell PM, Massire C, Hall TA, Jiang Y, Ranken R, Drader JJ, White N, McNeil JA, Crooke ST, Hofstadler SA. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc Natl Acad Sci U S A. 2005;102:8012–8017. doi: 10.1073/pnas.0409920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ecker DJ, Drader J, Gutierrez J, Gutierrez A, Hannis J, Schink A, Sampath R, Ecker AJ, Blyn LB, Eshoo MW, Hall TA, Tobarmosquera M, Jiang Y, Sannes-Lowery K, Cummins L, Libby B, Walcott DJ, Massire C, Ranken R, Manalili SM, Ivy C, Melton R, Levene H, Harpin V, Li F, White N, Pear M, Samant V, Knize DJ, Robbins DW, Rudnick K, Hajjar F, Hofstadler SA. The Ibis T5000 universal biosensor: an automated platform for pathogen identification and strain typing. JALA. 2006;11:341–351. [Google Scholar]
- 19.Sampath R, Russell KL, Massire C, Eshoo MW, Harpin V, Blyn LB, Melton R, Ivy C, Pennella T, Li F, Levene H, Hall TA, Libby B, Fan N, Walcott DJ, Ranken R, Pear M, Schink A, Gutierrez J, Drader J, Moore D, Metzgar D, Addington L, Rothman R, Gaydos CA, Yang S, St George K, Fuschino ME, Dean AB, Stallknecht DE, Goekjian G, Yingst S, Monteville M, Saad MD, Whitehouse CA, Baldwin C, Rudnick KH, Hofstadler SA, Lemon SM, Ecker DJ. Global surveillance of emerging influenza virus genotypes by mass spectrometry. PLoS One. 2007;2:e489. doi: 10.1371/journal.pone.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshoo MW, Whitehouse CA, Zoll ST, Massire C, Pennella TT, Blyn LB, Sampath R, Hall TA, Ecker JA, Desai A, Wasieloski LP, Li F, Turell MJ, Schink A, Rudnick K, Otero G, Weaver SC, Ludwig GV, Hofstadler SA, Ecker DJ. Direct broad-range detection of alphaviruses in mosquito extracts. Virology. 2007;368:286–295. doi: 10.1016/j.virol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Sampath R, Hofstadler SA, Blyn LB, Eshoo MW, Hall TA, Massire C, Levene HM, Hannis JC, Harrell PM, Neuman B, Buchmeier MJ, Jiang Y, Ranken R, Drader JJ, Samant V, Griffey RH, McNeil JA, Crooke ST, Ecker DJ. Rapid identification of emerging pathogens: coronavirus. Emerg Infect Dis. 2005;11:373–379. doi: 10.3201/eid1103.040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshoo MW, Whitehouse CA, Nalca A, Zoll S, Ecker JA, Hall TA, Pennella TT, Duncan DD, Desai A, Moradi EK, R, Rudnick K, Libby B, Ranken R, Sampath R, Hofstadler SA, Ecker DJ, Blyn LB. Rapid and high-throughput pan-Orthopoxvirus detection and identification by broad-range PCR and mass spectrometry. PloS One. 2009;4:e6342. doi: 10.1371/journal.pone.0006342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer H, Pfeffer M, Rziha HJ. Sequence alterations within and downstream of the A-type inclusion protein genes allow differentiation of Orthopoxvirus species by polymerase chain reaction. J Gen Virol. 1994;75:1975–1981. doi: 10.1099/0022-1317-75-8-1975. [DOI] [PubMed] [Google Scholar]
- 24.Ropp SL, Jin Q, Knight JC, Massung RF, Esposito JJ. PCR strategy for identification and differentiation of small pox and other orthopoxviruses. J Clin Microbiol. 1995;33:2069–2076. doi: 10.1128/jcm.33.8.2069-2076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulesh DA, Baker RO, Loveless BM, Norwood D, Zwiers SH, Mucker E, Hartmann C, Herrera R, Miller D, Christensen D, Wasieloski LP, Jr, Huggins J, Jahrling PB. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J Clin Microbiol. 2004;42:601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muddiman DC, Anderson GA, Hofstadler SA, Smith RD. Length and base composition of PCR-amplified nucleic acids using mass measurements from electrospray ionization mass spectrometry. Anal Chem. 1997;69:1543–1549. doi: 10.1021/ac961134r. [DOI] [PubMed] [Google Scholar]


