Abstract
Infants are a vulnerable and unique population at risk for dengue in endemic areas. This report describes the incidence and presenting clinical features of infant dengue virus (DENV) infections from a prospective community-based study performed between January 2007 and May 2009 in the Philippines. DENV3 was the predominant infecting serotype over a wide spectrum of disease severity, ranging from inapparent infection to dengue hemorrhagic fever (DHF). In 2007, the incidence of inapparent DENV infections during infancy was 103 per 1,000 persons person-years and 6-fold higher than symptomatic dengue. The age-specific incidence of infant DHF was 0.5 per 1,000 persons over the age of 3–8 months, and it disappeared by age 9 months. A febrile seizure, macular rash, petechiae, and lower platelet count were presenting clinical features associated with DENV infection among infants with acute undifferentiated febrile illnesses. Community-based studies can help to delineate the incidence rates, disease spectrum, and clinical features of DENV infections during infancy.
Introduction
Dengue is caused by infection with any one of the four dengue virus (DENV) serotypes (DENV1-4), and it is the most prevalent arboviral disease worldwide.1,2 DENV infections cause illness in tens of millions each year throughout the tropics and subtropics, severe morbidity in approximately 2 million persons/year, and approximately 20,000 deaths/year.3 The clinical manifestations of a DENV infection can range from an inapparent or mild febrile illness to the more symptomatic dengue fever (DF) to the potentially life-threatening illness, dengue hemorrhagic fever (DHF). Classic DF is characterized by fever, headache, myalgias/arthralgias, and asthenia as prominent symptoms. DF may be associated with severe thrombocytopenia and clinically significant bleeding, but it is rarely life-threatening.4,5 DHF is an entity characterized by a transient and rapid increase in vascular permeability with hemoconcentration, thrombocytopenia, and in the most severe cases, hypovolemic shock and coagulopathy.1,6
Primary infection with a DENV serotype generates long-term protective immunity against the homologous serotype. Primary DENV infections infrequently produce life-threatening disease in children and adults. After a short period of cross-protection, individuals who have recovered from a primary DENV infection are then fully susceptible to infection and disease by heterologous serotypes (secondary infection).7,8 The relative risk of developing severe disease and DHF is enhanced by sequential, heterologous DENV infections.9–11 As such, dengue disease characteristics and severity have been extensively studied in children and adults with secondary infections. Primary DENV infections in infants may have unique clinical characteristics4,12 and more readily lead to DHF and life-threatening illness than in older children and adults.13,14 We are conducting a prospective clinical study in the Philippines of DENV infections during infancy. This ongoing, community-based study provides a unique and broad perspective on infant dengue. Here, we present the initial report of incidence rates, disease-severity characteristics, and presenting features of infant DENV infections captured in our prospective study.
Materials and Methods
Ethics statement.
The study protocol was approved by the institutional review boards of the Research Institute for Tropical Medicine, Philippines, and the University of Massachusetts Medical School. Mothers and their healthy infants were recruited and enrolled after providing written informed consent. The clinical study is registered at www.clinicaltrials.gov (Identifier NCT00377754).
Study design.
The prospective clinical study is being conducted in San Pablo, Laguna, the Philippines—a semi-urban community south of metro Manila. Study enrollment began in October 2006, and surveillance for acute febrile illnesses began in January 2007. The infants with acute febrile illnesses in this report were identified in surveillance between January 2007 and May 2009. Healthy infants were enrolled in the study between the ages of 6 and 18 weeks old. Clinical and epidemiological data and a blood sample were collected at the enrollment study visit. Clinical and epidemiological information and a second blood sample were next collected from infants at their second study visit between the ages of 4 and 7 months. A third study visit and blood sample collection were arranged for a subset of 250 infants in December 2007. These 250 infants were randomly selected from infants ≤ 16 months old in December 2007 with two prior study visits before the onset of the rainy season and without any reported febrile illnesses between January 2007 and December 2007. We conducted surveillance year-round for hospitalized acute febrile illnesses in study infants across the seven hospitals serving San Pablo. During the rainy seasons (June–November), mothers were encouraged to bring their infants to the San Pablo City Health Office for evaluation of outpatient febrile illnesses. Acute illness and convalescent-phase (day 14) blood samples were obtained from study infants with febrile illnesses that did not have an obvious source at time of presentation (e.g., lobar pneumonia, bacterial meningitis, or pyelonephritis). Routine clinical information was abstracted daily during any hospitalization and at the acute and convalescent time points for all febrile study infants. Additional details of the study protocol and information on some of the infants in this report have been previously published.15
Identification and characterization of DENV infections.
A DENV infection was identified in febrile infants by serotype-specific reverse transcriptase-polymerase chain reaction (RT-PCR) in acute-phase sera16 and DENV IgM/IgG enzyme-linked immunosorbent assay (ELISA)17 in paired acute and convalescent phase sera. Primary or secondary DENV infections were identified by previously established serologic criteria for the paired IgM/IgG ELISA results.17 The infecting DENV serotype was identified by RT-PCR for all except one of the symptomatic infants. A sample for RT-PCR was not collected for this infant, but a monotypic rise in anti-DENV3 neutralizing antibody titers was seen in the paired acute and convalescent phase sera. Serial blood samples (study visits 1, 2, and 3) from a subset of 250 infants without reported febrile illnesses in 2007 were screened for evidence of clinically inapparent DENV infection using a hemagglutination-inhibition (HAI) assay to DENV1-4 and Japanese encephalitis virus (JEV).18 Infants with a ≥ 4-fold rise in DENV HAI titers between two time points were then tested by plaque-reduction neutralizing-antibody assay to DENV1-4 and JEV.10 A primary DENV infection was identified by a > 4-fold rise in DENV 50% plaque-reduction neutralization titers (PRNT50) between two time points with a monotypic pattern.10 The DENV serotype with the highest PRNT50 in a monotypic pattern was assumed to be the serotype that produced the clinically inapparent infection.
Laboratory and radiographic investigations for hospitalized infants with DENV infections were directed by the treating physicians. There were at least three serial determinations of hematocrit and platelets performed for all the hospitalized infants that covered the period of defervescence. Hemoconcentration was measured by comparing the maximum recorded hematocrit (around the time of defervescence and platelet nadir) with the minimum recorded hematocrit at either the beginning or end of hospitalization. None of the infants received red blood cell or whole blood transfusions. Hospitalized infants with DENV infection were classified as having DHF only when review of their clinical course and all clinical data strictly met the World Health Organization (WHO) classification criteria.19 Hospitalized infants with DENV infection that did not strictly meet the above criteria were classified as having DF. All the non-hospitalized infants with DENV infections had mild self-resolving febrile illnesses less than 1 week in duration.
Statistical analysis.
SPSS (version 17.0) software was used for the statistical analyses. Parametric tests (student's t test and ANOVA) were used for comparisons and correlations among normally distributed variables. Wilcoxon ranked-sum and Kruskal–Wallis tests were used for comparisons of variables that were not normally distributed. Pearson χ2 test was used for comparisons of categorical variables among disease-severity groups. A logistic regression model was used to determine the multivariate odds ratios for categorical and ordinal variables. Mean, median, and proportional values are presented with their respective 95% confidence intervals (CIs) in brackets. P values < 0.05 were considered significant; P values ≥ 0.05 and < 0.10 were considered a non-significant trend.
Results
Incidence of infant DENV infections during 2007 in San Pablo, Philippines.
Infants (4,441) and their mothers participated in the prospective study of DENV infections between January 2007 and January 2008. We captured 353 infants with acute undifferentiated febrile illnesses during surveillance in this time period. Forty (11%) of these acute febrile illnesses were caused by DENV infections. We also screened a subset of 250 study infants without reported febrile illnesses between January 2007 and December 2007 and identified 20 (8%) with inapparent DENV infections. The incidence rate of inapparent infant DENV infections in our study during 2007 was 103/1,000 person-years (64–155; mean [95% CI]) and 6-fold higher than the incidence rate of symptomatic infant DENV infections (16/1,000 person-years; 11–22; mean [95% CI]). The incidence rates of hospitalized and outpatient symptomatic infant DENV infections were equivalent in 2007 (approximately 8/1,000 person-years). The age-specific incidence of symptomatic DENV infections without DHF varied between 0.7/1,000 (0–1.6) and 2.8/1,000 (0–6.7) in ages of 2–15 months. The age-specific incidence of infant DHF was 0.5/1,000 (0.2–0.8) in ages of 3–8 months. There were no DHF cases seen in infants ≥ 9 months old (Figure 1A).
Figure 1.
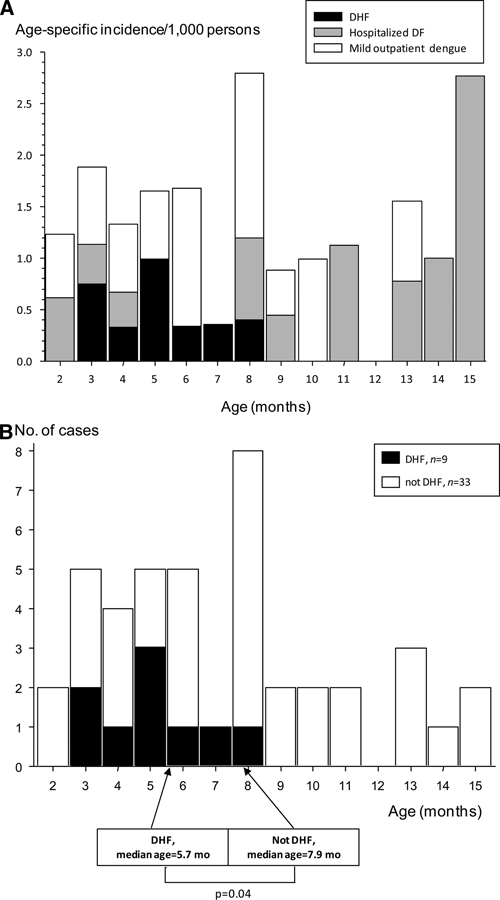
(A) Age-specific incidence of symptomatic DENV infections in infants. Black filled bars, hospitalized DHF; gray filled bars, hospitalized DF; open bars, mild outpatient dengue illness. (B) Age distribution of infants with symptomatic DENV infections. Black filled bars, number of hospitalized infants with DHF; open bars, number of symptomatic infants without DHF (hospitalized and non-hospitalized).
Clinical characteristics and disease severity of infant DENV infections.
From January 2007 to May 2009, we have identified 42 infants with symptomatic DENV infections and 20 infants with inapparent DENV infections in our prospective study. DENV3 was the predominant serotype among the symptomatic (37/42) and inapparent (15/20) infant dengue cases. All the inapparent and all the symptomatic cases except one were primary DENV infections (Table 1). The severity of symptomatic DENV infections during infancy ranged from mild outpatient febrile illnesses (N = 19) to hospitalized DF (N = 14) to hospitalized unambiguous DHF (N = 9, all primary infections). Five of the infants with unambiguous DHF had dengue shock syndrome (DSS), including one infant who died. The remaining four infants were classified as DHF grade I with hemoconcentration ranging from 27% to 44% (one DHF Grade I infant also had a large pleural effusion on a chest radiograph). Hypotension or hemoconcentration occurred around the time of defervescence in eight of nine DHF infants. One DHF infant had prolonged fevers, and another had a biphasic febrile illness. None of the DENV-infected infants had clinically significant bleeding. One hospitalized infant had mild hematemesis, and another had mild epistaxis. Only 1 of 42 symptomatic DENV-infected infants developed a classic dengue convalescent rash. The most common initial clinical diagnosis given to infants with DENV infections was a respiratory illness (upper respiratory infection, bronchitis, or pneumonia; Table 1). The modal age for infants with symptomatic dengue was 8 months. The infants with unambiguous DHF were younger than the symptomatic infants without DHF (median ages DHF versus not DHF were 175 versus 241 days old, respectively; P = 0.04; Figure 1B). The same trend was seen when only primary DENV infections were included (median ages primary DHF versus not DHF were 175 versus 240 days old, respectively; P = 0.05).
Table 1.
Characteristics of infants with DENV infections (January 2007 to May 2009)
| Study subject characteristics | Symptomatic DENV infection* (N = 42) | Inapparent DENV infection (N = 20) | |||
|---|---|---|---|---|---|
| Hospitalized (N = 23) | Outpatient (N = 19) | ||||
| DHFb (N = 9) | DFb (N = 14) | ||||
| DENV serotypes | DENV3: N = 9 | DENV3: N = 14 | DENV1: N = 1 | DENV2: N = 5 | |
| DENV2: N = 4 | DENV3: N = 15 | ||||
| DENV3: N = 14 | |||||
| 1° or 2° DENV infection | 1°: N = 9 | 1°: N = 13 | 1°: N = 19 | 1°: N = 20 | |
| 2°: N = 1 | |||||
| Gender ratio (male/female) | 6/3 | 8/6 | 11/8 | 10/10 | P = 0.9 |
| Initial clinical diagnoses | Respiratory illness: N = 9 | Respiratory illness: N = 13 | |||
| Dengue: N = 8 | Fever, unknown etiology: N = 2 | ||||
| Febrile seizure: N = 3 | Dengue: N = 1 | ||||
| Viral infection: N = 2 | Viral exanthem: N = 1 | ||||
| Acute gastroenteritis: N = 1 | Herpangina: N = 1 | ||||
| Impetigo: N = 1 | |||||
Infants with symptomatic and inapparent DENV infections were identified as described in Materials and Methods.
Initial signs and symptoms in febrile DENV-infected infants.
We compared the presenting signs and symptoms in the 42 symptomatic DENV-infected infants with 423 non-dengue acute undifferentiated febrile illnesses captured over the same time period (Table 2). The duration of fever before presentation was similar between the two groups (2–3 days). The most common presenting signs and symptoms in infants with dengue and non-dengue acute febrile illnesses were respiratory (cough and/or nasal congestion and dyspnea), circulatory (cold hands and feet), and gastrointestinal (vomiting/spitting up and refusal to feed). The frequency of these presenting symptoms was not significantly different between infants with dengue or non-dengue acute febrile illnesses. A recent febrile seizure, drowsiness/lethargy, petechiae, and a macular rash on presentation were significantly and independently associated with a DENV infection (Table 2). There was a non-significant trend toward fewer days of fever and lower mean temperature on presentation in the infants with mild outpatient dengue compared with those with hospitalized DF or DHF (Table 2). Cough and/or nasal congestion were reported more frequently in the infants with mild outpatient dengue (90%) than in those with hospitalized dengue (hospitalized DF = 43%, hospitalized DHF = 33%; P = 0.003). No other presenting signs or symptoms were significantly different among the symptomatic dengue-disease severity groups.
Table 2.
Signs and symptoms at illness presentation
| Sign/symptom at illness presentation | Non-dengue acute febrile illnesses (N = 423) | Symptomatic dengue (N = 42) | Univariate P values* | Multivariate odds ratio(95% CI) for symptomatic dengue | Hospitalized DHF (N = 9) | Hospitalized DF (N = 14) | Mild outpatient dengue illness (N = 19) | Univariate P values* |
|---|---|---|---|---|---|---|---|---|
| Age (months) median (95% CI) | 7.9 (7.4, 8.4) | 7.2 (5.8, 8.3) | – | |||||
| Gender (male/female) | 242/181 | 25/17 | – | |||||
| Days of fever before presentation median (95% CI) | 2.0 (2.0, 2.0) | 3.0 (2.0, 3.0) | – | 3.0 (1.0, 5.0) | 3.5 (2.0, 5.0) | 2.0 (1.0, 3.0) | P = 0.05 | |
| Temperature (°C) mean ± SD | 38.7 ± 0.6 | 38.8 ± 0.6 | – | 39.1 ± 0.4 | 39.0 ± 0.8 | 38.6 ± 0.6 | P = 0.07 | |
| Cough and/or nasal congestion | 70% | 64% | – | 33% | 43% | 90% | P = 0.003 | |
| Cold hands and feet | 51% | 57% | – | 56% | 43% | 63% | – | |
| Dyspnea | 33% | 26% | – | 33% | 14% | 32% | – | |
| Vomiting/spitting up | 31% | 43% | – | 22% | 50% | 47% | – | |
| Refusal to feed | 27% | 36% | – | 22% | 43% | 37% | – | |
| Macular rash | 19% | 48% | P < 0.001 | 4.2 (2.1, 8.7) | 67% | 50% | 37% | – |
| Drowsiness/lethargy | 14% | 31% | P = 0.006 | 2.6 (1.2, 5.7) | 33% | 29% | 37% | – |
| Flushing | 9% | 21% | P = 0.01 | 2.0 (0.8, 5.0) | 11% | 43% | 11% | P = 0.06 |
| Febrile seizure | 5% | 21% | P = 0.001 | 8.1 (3.2, 20.9) | 22% | 29% | 16% | – |
| Petechiae | 3% | 12% | P = 0.01 | 4.8 (1.3, 17.1) | 11% | 21% | 5% | – |
| Agitation | 3% | 7% | – | 22% | 7% | 0% | – |
P values > 0.10 are not shown (–).
Laboratory and serological data in febrile DENV-infected infants.
Complete blood counts were obtained at the time of presentation in 31 primary DENV-infected febrile infants and 43 infants with non-dengue acute undifferentiated febrile illnesses. All of the non-dengue infants in this subset were hospitalized. Mean platelet and leukocyte counts were lower at the time of presentation in the febrile infants with dengue compared with those without dengue. There was also a non-significant trend toward a higher percentage of mononuclear leukocytes (lymphocytes or monocytes) in the DENV-infected infants. In a logistic regression model, the odds of a febrile infant having a DENV infection increased 2-fold for every 100,000/mm3 decrease in the platelet count on illness presentation (Table 3).
Table 3.
Laboratory findings at time of illness presentation
| Laboratory finding at illness presentation* | Non-dengue acute febrile illnesses (N = 43) | Symptomatic dengue (N = 31) | Univariate P values | Multivariate odds ratio (95% CI) for symptomatic dengue | Hospitalized DHF (N = 9) | Hospitalized DF (N = 13) | Mild outpatient dengue illness (N = 9) | Univariate P values |
|---|---|---|---|---|---|---|---|---|
| Hematocrit (%) | 33.6 (32.5, 34.6) | 34.5 (33.2, 35.8) | P = 0.3 | 36.3 (32.9, 39.8) | 33.6 (31.5, 35.6) | 33.9 (32.4, 35.4) | P = 0.2 | |
| Platelet count (×103/mm3) | 283 (246, 319) | 178 (141, 215) | P < 0.001 | 2.2 (1.2, 4.1 for every 100 × 103/mm3 decrease) | 123 (74, 173) | 202 (125, 278) | 201 (153, 250) | P = 0.11 |
| Leukocyte count (×103/mm3) | 11.7 (9.5, 13.9) | 7.7 (6.7, 8.8) | P = 0.004 | 1.3 (0.7, 2.7 for every 5 × 103/mm3 decrease) | 6.2 (4.9, 7.5) | 8.3 (6.1, 10.4) | 8.6 (6.6, 10.6) | P = 0.16 |
| Lymphocyte or monocyte percentage (%) | 49.0 (43.5, 54.5) | 58.4 (46.3, 70.5) | P = 0.09 | 63.3 (45.8, 80.7) | 54.8 (35.9, 73.8) | ND† | P = 0.4 |
Values are presented as mean (95% CI).
ND = not done.
We detected a positive DENV IgM in acute- or convalescent-phase sera in 34 of 41 (83%) of the infants with primary DENV infections. A positive DENV IgM was most likely to be found in infant sera collected ≥ 5 days and ≤ 30 days from the reported onset of illness (Figure 2). In two infants, DENV IgM levels were below the positive threshold in sera collected 3 and 13 days after reported illness onset. In one infant, serum samples were collected 1, 11, 17, and 30 days after dengue illness onset. The DENV IgM became positive 11 days after illness onset, but it dropped below the positive cutoff level 6 days later (17 days after illness onset). These cases illustrate the potentially narrow window of IgM seropositivity in infant primary DENV infections.
Figure 2.
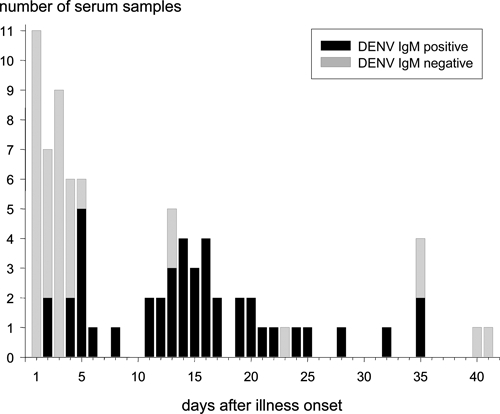
DENV IgM ELISA results from acute illness and convalescent phase sera for infants with symptomatic primary DENV infections (N = 80 serum samples). The number of samples with positive or negative DENV IgM levels is shown versus the number of days after illness onset when the samples were collected. Black filled bars, sera with DENV IgM ELISA values above the positive threshold value (DENV IgM positive); gray filled bars, sera with DENV IgM ELISA values below the positive threshold value (DENV IgM negative).
Discussion
We have examined the incidence rates and clinical characteristics of DENV infections in infants participating in a prospective community-wide study in San Pablo, Laguna, the Philippines. Previous studies have described clinical and laboratory features of dengue in infants, but they have been based on cohorts from referral pediatric hospitals.4,12,20–22 Our study has captured a wide spectrum of clinical disease severity among infants with DENV infections ranging from inapparent infections and mild outpatient febrile illnesses to hospitalized DF and unambiguous hospitalized DHF/DSS. DENV3 was the predominant infecting serotype in the symptomatic and inapparent cases in this report. All the infant dengue cases except one were primary DENV infections. Most of the infant DENV infections in this report occurred in 2007 when there was a high level of dengue activity throughout the Philippines.
Between January 2007 and January 2008, we found that the overall incidence of DENV infections in infants 2–15 months old was nearly 12% (119/1,000 person-years; 75–177). Asymptomatic or very minimally symptomatic DENV infections accounted for the majority of this high incidence rate (87%; 74–93%), which has been previously observed in older children with primary or secondary DENV infections.9,23 The incidence rates of hospitalized and non-hospitalized symptomatic dengue in infants were equivalent (8/1,000 person-years). The overall infant dengue hospitalization rate in our study during 2007 was similar to what was first reported from Bangkok in 1964 (5/1,000 person-years)14 but lower than a 2001 dengue outbreak in French Polynesia (15/1,000 person-years).24 The peak number of symptomatic primary dengue cases in our study was seen around 8 months of age. All the infants with unambiguous DHF had primary DENV infections, were clustered between 3 and 8 months old, and had a slightly lower median age than symptomatic infants without DHF. These observations are generally consistent with what has been previously described for infant dengue and DHF.13,22,24 In our study, there were no significant differences in the age-specific incidence of DHF over the age range 3–8 months. However, we cannot identify potentially significant differences in age-specific DHF incidence rates for 2007 ≤ 2.1/1,000. In two previous studies, the age-specific incidence rates of infant DHF were reported to peak around 7–9 months of age.14,24 In these population-based studies, monthly age-specific incidence rates were estimated using clinical dengue diagnoses divided by one-twelfth of available census data for infants < 1 year of age.14 The latter assumption ensures that the age distribution curves for the number of cases, and incidence rates are identical in these studies. In our prospective study, age-specific incidence rates were more accurately determined using laboratory-confirmed dengue cases divided by the actual number of study infants in a monthly age group during the surveillance period. We speculate that the true age-specific incidence rates of DHF over the first 6 months of life (i.e., number of DHF cases per unit of person-time at risk) are underestimated in this prospective study and the previous population-based studies. The numbers of infants susceptible to DENV infection (i.e., those at risk) are more likely to be overestimated in younger infants (e.g., 2–4 months old) than older infants (e.g., 7–10 months old), because younger infants are more likely to have protective levels of maternally derived anti-DENV antibodies.15 Continued prospective studies of infant dengue along with reliable correlates of immune protection will be needed to better assess the true age-specific incidence rates of DHF during early infancy.
The presenting signs and symptoms of DENV-infected febrile infants were generally similar to other infants with non-dengue acute undifferentiated febrile illnesses. The majority of febrile infants in both groups presented with some respiratory tract symptoms, particularly those with mild outpatient illnesses. The prominence of respiratory tract symptoms in our community-based study differs from other studies of hospitalized infant dengue in referral settings,4,12,21 but it is similar to a community-based study of dengue in older children.23 The results are a reminder that clinicians caring for infants in dengue-endemic regions should maintain a high index of suspicion for dengue even when the initial clinical impression may point to a respiratory illness. There were selected clinical features on presentation that distinguished dengue from the non-dengue acute febrile illnesses. Nearly one-half of all the DENV-infected infants presented with a macular rash, and the odds of a febrile infant having a DENV infection were increased 4-fold in the presence of a macular rash. A macular or maculopapular rash early in illness has been a consistent and common finding in studies of DENV-infected infants, and it is more prevalent than in older children or adults with dengue.4,12 On recovery, we observed the classic convalescent rash of dengue in only 1 of 41 infants with a primary infection (2%), although it was reported in 24% of infants with dengue admitted to the Children's Hospital in Bangkok.12 Infantile febrile seizures were the presenting clinical feature most strongly associated with a DENV infection in our study. The association of febrile seizures with DENV infection and the trend of increasing mean temperatures with increasing dengue disease severity may suggest that infant systemic inflammatory responses are more severe in DENV infections compared with non-dengue undifferentiated febrile illnesses. The presence of petechiae and a lower platelet and white blood cell count early in illness were also associated with dengue among the febrile infants in our study. These findings have been fairly common across multiple studies that have attempted to identify initial distinguishing clinical and laboratory features of dengue in children and adults.25
In our community-based study, 45% of the infants hospitalized for a primary DENV infection met criteria for unambiguous DHF, and none had clinically significant bleeding. We recognize that some hospitalized infants classified as DF in our study may have met the WHO criteria for DHF Grade I/II with more intensive investigations. Most reports of infant dengue from referral pediatric hospitals have classified 95–100% of hospitalized infants as having DHF.6,12,21 Our study has painted a more complete picture of an “iceberg” distribution for the manifestations of primary DENV infections during infancy. The vast majority of primary DENV infections during infancy were inapparent (i.e., asymptomatic or very mildly symptomatic). Among the 10–25% of infants with symptomatic dengue after primary DENV infection, approximately one-half were mild outpatient febrile illnesses and one-half were hospitalized in the community setting. Among these hospitalized infants, approximately one-half had obvious DHF or DSS. DENV3 was the pre-dominant infecting serotype in this report. The current data provide a foundation for comparisons of the disease-severity spectrum in primary DENV3 infections during infancy, childhood, and adulthood. Future data from the ongoing clinical study should also help to determine if the dengue-disease spectrum will be similar for primary infections caused by other serotypes during infancy.
DENV IgM ELISA assays are a common laboratory diagnostic test used by clinicians during acute febrile illnesses in infants. IgM does not cross the placenta, and it is a reliable marker of the infant's primary immune response. We found that DENV IgM levels were likely to remain below a well-established positive threshold within the first 5 days of illness in primary DENV-infected infants. Positive DENV IgM values were generally seen between 5 and 30 days after illness onset. However, several cases captured in this study highlighted that the window of IgM seropositivity in an infant with a primary DENV infection may be quite brief and easily missed with limited testing. The direct detection of virus (RT-PCR) or viral antigen (NS1) in infant sera22 is likely to become increasingly available for clinicians in dengue-endemic countries, and it may prove to be especially useful within the first 5 days of illness. Prospective studies of infant dengue provide the opportunity to better understand DENV transmission in households and communities and better delineate the clinical and laboratory features of dengue over a broad spectrum of disease severity in this vulnerable population.
Acknowledgments
The authors thank Linda Dexter-Fraser and Ronald Banez for protocol supervision and study coordination, Butsaya Thaisomboonsuk for supervising the neutralization and HAI assays, Analisa Bautista for supervising the dengue ELISA assays, Edelwisa Segubre-Mercado for supervising the dengue RT-PCR assays, and Veronica Tallo for supervising data entry and management.
Footnotes
Financial support: The study was supported by National Institutes of Health Grant U01 AI065654. The contents of this publication are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health or the U.S. Department of Defense.
Authors’ addresses: Rosario Z. Capeding, Research Institute for Tropical Medicine, Departments of Microbiology and Medicine, FCC, Alabang, Muntinlupa City, Metro Manila, Philippines, E-mail: lerosecap@yahoo.com.ph. Job D. Brion and Mercydina M. Caponpon, San Pablo City Health Office, Mabini Extension, San Pablo City, Laguna, Philippines, E-mails: jdbrion@yahoo.com and drdinamendoza@yahoo.com. Robert V. Gibbons, Richard G. Jarman, and In-Kyu Yoon, Armed Forces Research Institute of Medical Sciences, Department of Virology, Bangkok, Thailand, E-mails: robert.gibbons@afrims.org, richard.jarman@afrims.org, and InKyu.Yoon@afrims.org. Daniel H. Libraty, Center for Infectious Disease and Vaccine Research, University of Massachusetts Medical School, Worcester, MA, E-mail: daniel.libraty@umassmed.edu.
References
- 1.Nimmannitya S. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno G, editors. New York: CAB International; 1997. pp. 133–146. (Dengue hemorrhagic fever: diagnosis and management). [Google Scholar]
- 2.Pinheiro FP, Corber SJ. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997;50:161–169. [PubMed] [Google Scholar]
- 3.International Vaccine Institute Global burden of dengue. 2009. http://www.pdvi.org/about_dengue/GBD.asp Available at. Accessed July 16, 2009.
- 4.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 5.Tsai CJ, Kuo CH, Chen PC, Changcheng CS. Upper gastrointestinal bleeding in dengue fever. Am J Gastroenterol. 1991;86:33–35. [PubMed] [Google Scholar]
- 6.Wills BA, Oragui EE, Dung NM, Loan HT, Chau NV, Farrar JJ, Levin M. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis. 2004;190:810–818. doi: 10.1086/422754. [DOI] [PubMed] [Google Scholar]
- 7.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 8.Sabin A. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 9.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 10.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 11.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–184. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 12.Kalayanarooj S, Nimmannitya S. Clinical presentations of dengue hemorrhagic fever in infants compared to children. J Med Assoc Thai. 2003;86((Suppl 3)):S673–S680. [PubMed] [Google Scholar]
- 13.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg. 1969;18:997–1021. doi: 10.4269/ajtmh.1969.18.997. [DOI] [PubMed] [Google Scholar]
- 15.Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, Capeding RZ. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klungthong C, Gibbons RV, Thaisomboonsuk B, Nisalak A, Kalayanarooj S, Thirawuth V, Nutkumhang N, Mammen MP, Jr, Jarman RG. Dengue virus detection using whole blood for reverse transcriptase PCR and virus isolation. J Clin Microbiol. 2007;45:2480–2485. doi: 10.1128/JCM.00305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 18.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 19.Anonymous . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 1997. [Google Scholar]
- 20.Kabilan L, Balasubramanian S, Keshava SM, Thenmozhi V, Sekar G, Tewari SC, Arunachalam N, Rajendran R, Satyanarayana K. Dengue disease spectrum among infants in the 2001 dengue epidemic in Chennai, Tamil Nadu, India. J Clin Microbiol. 2003;41:3919–3921. doi: 10.1128/JCM.41.8.3919-3921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 22.Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–424. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 24.Hubert B, Halstead SB. Dengue 1 virus and dengue hemorrhagic fever, French Polynesia, 2001. Emerg Infect Dis. 2009;15:1265–1270. doi: 10.3201/eid1508.081500. [DOI] [PubMed] [Google Scholar]
- 25.Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328–1340. doi: 10.1111/j.1365-3156.2008.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


