Abstract
West Nile virus (WNV) perpetuates in an enzootic transmission cycle involving Culex mosquitoes and virus-competent avian hosts. In the northeastern United States, the enzootic vectors, Cx. pipiens and Cx. restuans, feed preferentially on American robins (Turdus migratorius), suggesting a key role for this bird species in the WNV transmission cycle. We examined the role of American robin communal roosts as virus amplification foci in greater New Haven, Connecticut. Robin communal roosts were located by radio tracking. After mid-August, when most robins were using the roosts, Cx. pipiens and Cx. restuans fed often on robins and were significantly more infected with WNV at communal roosts than at non-roosting sites. We also identified 6.4% human-derived blood meals in Aedes vexans in communal roosts. Our results indicate that communal roosts act as late-season amplification foci facilitating transmission to humans because of high infection rates, high abundance, and feeding patterns of enzootic and bridge vectors.
Introduction
Human risk of infection with a vector-borne zoonotic pathogen is associated with the intensity of enzootic transmission and the ability of the vector population to serve as a transmission bridge from infected reservoir hosts to humans. The frequency of vector–host contacts is a key determinant of pathogen transmission dynamics and can be highly variable in space and time. Heterogeneity in contact rates arises from vector preferential feeding on specific hosts1–3 and from spatial clustering of vectors and hosts at specific landscape features.4–8 Host aggregations may further increase biting rates if they result in a stronger attractive signal to vectors, increasing local vector density in relation to hosts.9 Virus amplification is then expected to be most intense if preferred, pathogen-competent hosts congregate in habitats suitable for vector breeding and host seeking.4
Since the introduction of West Nile virus (WNV; family Flaviviridae, genus Flavivirus) into North America in 1999, the number of human cases attributed to the virus has exceeded 29,000, including 1,154 fatalities in the United States.10 Culex pipiens serve as the primary enzootic vector11,12 and passerine birds serve as amplification hosts13 in the northeastern United States. Although WNV transmission was initially associated with large-scale crow mortality in North America, corvid-derived blood meals have been rarely identified in Cx. pipiens.14,15 Recent studies in the northeastern United States and elsewhere have found that a single host species, American robin (Turdus migratorius), comprises 5–71% of all Cx. pipiens blood meals, and the majority of studies report > 40%.14–21 Some of these studies describe a seasonal shift in Cx. pipiens feeding from robins to other bird species15,21 or humans17 during the transmission season. In a study in Maryland,17 this shift was presumed to be a consequence of robin migration out of urban areas.
Less frequent detection of robin-derived blood meals in late summer and fall may alternatively result from the aggregation of robins in nocturnal communal roosts. In the northeastern United States, robins, European starlings (Sturnus vulgaris), and common grackles (Quiscalus quiscula) form highly clustered communal roosts in mid-summer that continue through mid-fall.22,23 Aggregation in these communal roosts results in highly patchy availability of robins as hosts to crepuscular-feeding vectors. Unless collections of blood-fed mosquitoes specifically target these roosts, the probability of finding robin-derived blood meals is expected to be low. It has been suggested that starling communal roosts act as amplification foci for eastern equine encephalitis virus24,25 and that mosquitoes sampled from the vicinity of massive avian communal roosts of robins, starlings, and blackbirds were more frequently infected by an avian virus (Highlands J virus) than those sampled in swamps away from communal roosts (N. Komar, unpublished data). Communal roosting of crows26–29 and sparrows30 has been proposed as a factor influencing spatial and temporal patterns of WNV transmission, although systematic assessments have rarely been carried out.
The objective of this study was to evaluate the contribution of American robin roosting behavior on amplification and intra-urban spatial heterogeneity of WNV transmission in the northeastern United States. Spatial and temporal variations in infection prevalence and host feeding behavior of the primary mosquito vectors were investigated. Finally, the potential for bridging transmission to humans in areas around communal roosts was assessed based on the composition, WNV infection, and feeding patterns of the vector community.
Methods
Study sites.
The study was conducted between May 12th and October 19th of 2008 in 10 sites in greater New Haven, Connecticut. Three of the sites were located in suburban areas included in the Connecticut Agricultural Experiment Station (CAES) WNV surveillance network, and the other seven were identified as communal roosting sites (Figure 1). No avian communal roosts where observed at CAES sites, so we designated them as “non-roosting” sites for comparative purposes. Additional data were collected in 2009 to monitor the full timeline of bird-roosting behavior. A site was defined as a 500-m radius area around a pair of CO2-baited Center for Disease Control (CDC) light and gravid mosquito traps. At communal roosting sites, mosquito surveillance was started on or after August 10th when roosts were discovered, so comparisons between mosquito abundance and infection at communal roosting and non-roosting sites were restricted to after this date.
Figure 1.
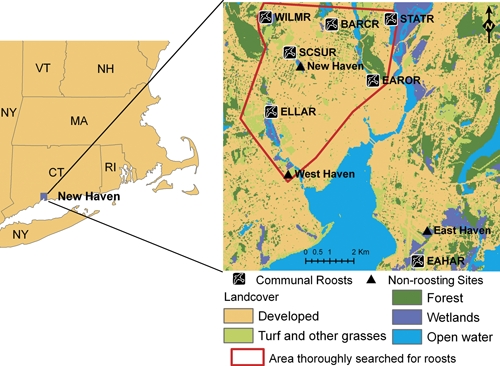
Location of communal roosts and non-roosting study sites in greater New Haven, Connecticut. This figure appears in color at www.ajtmh.org.
Breeding and roosting behavior of American robins.
Beginning May 12th, 2008, we mapped American robin territories and located nests at the three non-roosting sites (Figure 1). We mapped territories according to methods described elsewhere.31,32 To locate the nests, we walked the site watching for females returning to the nest and adults carrying food. Nests were monitored one day per week until the end of the nesting season (late July), and we recorded the presence of eggs, nestlings, or adults and the age of nestlings. Nests were considered active from discovery to the day of fledging or the midpoint between the last recorded active day and the first inactive day. From this data, the daily proportion of active nests was calculated.
To estimate bird density, 25 point counts were conducted during the breeding season at the three non-roosting sites at randomly distributed positions located more than 200 m apart. Counts were carried out between 5:30 and 10:00 am and were not performed when winds exceeded 16 km/hour or precipitation was more than a light drizzle. Each position was visited one time between June 11th and June 26th. During each visit, we recorded all birds seen or heard within a 50-m radius of a survey position during a 10-minute period.31 Point counts were only performed during the breeding season when males vocally defend territories and thus, were easier to detect. Birds that flew over the point circle or entered the circle mid-count were not included in the calculation of species densities. The density of each bird species at a site was estimated as the number of birds observed divided by the sum of the areas of all the point-count circles. No corrections for bias associated with point counts were made, because the available methods are not generally applicable to extensive, multiple-species surveys.33
We began searching for American robin communal roosts the week of July 14th. To locate communal roosts, we observed robins starting 1.5 hour before sunset from locations where they were feeding. When at least 20 birds headed in a certain direction, the compass direction of departure was recorded, and observers moved to another location in that direction and resumed watching for robins. Observers continued to follow the robins until their communal roosts were located (N. Komar, personal communication).
To aid in roost finding and monitor roost constancy, we placed 20 radio transmitters (model A2440, Advanced Telemetry Systems Inc., Isanti, MN) on 30 robins. These transmitters were designed for songbirds and weigh 2 g; this is < 5% of the bird's body weight, which is in accordance with the Ornithological Council guidelines.34 Robins were captured with mist nets after the breeding season. Transmitters were either glued to the feathers of the lower back of the bird35,36 or attached by a leg harness.37,38 An R410 scanning receiver and three-element folding yagi antenna (Advanced Telemetry Systems Inc., Isanti, MN) were used to locate robins fitted with radio transmitters. Appropriate state, federal, and Yale Institutional Animal Care and Use Committee (YACUC) permits were obtained before placing radio transmitters on American robins. Each night from August 5th to October 7th, we visited all communal roosts to locate radio-tagged birds. We estimated roost constancy as the proportion of times a radio-tagged bird returned to the same roost on successive nights (N. Komar, personal communication).
Additional information on usage of communal roosts from formation until peak was obtained in 2009 when systematic counts were conducted in June, July, and August. During a count, three to seven trained counters took positions around the perimeter of the roost 2 hours before sunset. Each counter focused attention on a section of the roost perimeter and recorded the number of birds that entered through that section each minute until all robins, starlings, and grackles had arrived. The number of counters needed for a count was roughly proportional to the size of the roost perimeter. The areas that were used for communal roosting in 2008 continued to be used in 2009, and we expected that the general timeline of roost usage was similar among years.
Avian exposure to WNV.
We collected blood samples from robin nestlings 5–11 days old and from fledgling and adult birds after the breeding season. Blood-sample collection was limited to nestlings in accessible nests, public parks, or residential yards. Fledglings and adults were trapped at both the roosting and non-roosting sites using mist nets. Blood samples were collected using a heparinized capillary tube after the pricking of the brachial vein. The quantity of blood drawn was limited to < 1% of the bird's weight.34 Exposure to WNV was assessed by serology and virus isolation. Blood samples were centrifuged to separate the serum fraction and tested for viral infection in Vero cell culture. Briefly, 10–20 μL of sera were absorbed onto confluent Vero cells growing in 25-cm2 flasks, and cell cultures were screened daily for cytopathic effect (CPE) for 7 days. The limit of virus detection in Vero cell culture is one plaque forming unit (PFU; P Armstrong, unpublished observations), and therefore, based on the volumes tested, we anticipated WNV detection when viremias exceeded 50–100 PFU/mL. Prior exposure to WNV was assessed by serological testing using the plaque-reduction neutralization test (PRNT). Bird sera were heat-inactivated at 56°C for 20 minutes, diluted 1:10 in phosphate-buffered saline, and then mixed with an equal volume of WNV (100 PFU) for a final serum dilution of 1:20. Virus–antibody mixtures were incubated at 37°C for 1 hour and then, were inoculated onto Vero cells growing in 12-well plates. Cell cultures were incubated at 37°C for 1 hour with periodic rocking to promote uniform absorption of virus and then, were overlaid with minimal essential medium, 5% fetal bovine serum, antibiotics, and 1% methyl-cellulose. Plates were returned to the incubator, and after 4 days, cell monolayers were fixed in 7.4% formaldehyde and stained with 1% crystal violet. Plaques were counted, and serum samples that neutralized > 80% of the virus inoculum were considered sero-reactive.
Mosquito abundance, WNV-infection prevalence, and feeding behavior.
To monitor mosquito-community composition and infection rates with WNV, CO2-baited CDC light and gravid traps containing a lactalbumin and hay infusion (one of each per site) were set at the three non-roosting sites from June 3 to October 9, 2008 (Figure 1). Trapping frequency was variable but occurred at least one time every 10 days throughout the entire season. Additional pairs of traps were placed at each communal roost site next to the roosting areas. These traps were set weekly as communal roosts were identified on or after August 10th until October 19th.
Mosquitoes were identified to species based on morphology39 and pooled in groups of up to 50 by trapping location, date of collection, trap type, and species. For virus isolation, mosquitoes were homogenized and inoculated onto confluent Vero cell cultures. Cells were maintained for 7 days and monitored daily for CPE. Infected cell cultures were harvested and identified by real-time reverse transcriptase polymerase chain reaction (RT-PCR) using primer sets targeting the envelope and 3¢ non-translated regions of the WNV genome.40
To examine feeding behavior, resting mosquitoes were collected at the three non-roosting and seven communal roosting sites between May 27th and October 9th using battery-powered modified CDC backpack aspirators (model 1412, John W. Hock Company, Gainesville, FL). For blood-meal analysis, blood-fed mosquitoes were dissected, and DNA was individually isolated from the abdomens using DNA-zol BD (Molecular Research Center, Cincinnati, OH) following the manufacturer's instructions with modifications. PCR was used to screen extracted DNA for vertebrate mitochondrial cytochrome b gene sequences using mammalian and avian-specific primers as described elsewhere.15 PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and sequenced at the W.M. Keck Foundation Biotechnology Resource Laboratory (Yale University, New Haven, CT), a core DNA sequencing facility. Sequences were identified and assigned to specific host species by comparison with available vertebrate sequences in GenBank.
Statistical analyses.
The transmission season was categorized into “early season” (before August 10th when robins were actively breeding) and “late season” (after August 10th, when the vast majority of robins were using the communal roosts).
A negative-binomial regression model was used to evaluate differences in late-season vector community composition between communal roosting and non-roosting sites. Mosquito-abundance estimates were derived from light-trap collections.41
The Pooled Infection Rate add-in developed for Excel42 was used to calculate early and late season infection rates (IR) for communal roosting sites and non-roosting sites. This software uses a bias-corrected maximum likelihood estimation (MLE) to compute the infection rate (per 1,000 mosquitoes tested) and confidence intervals of mosquito pools of different sizes. MLE IR data were derived from combined light- and gravid-trap collections.
The proportion of blood meals derived from each host species early and late in the transmission season was calculated for Cx. pipiens and Cx. restuans. Feeding index as a measure of mosquito-host feeding preference21 was calculated for the early transmission season as the fraction of total blood meals collected before August 10th from a specific host species divided by the quotient of its density and the total avian density estimated in the June point counts.21 For the early season data, a chi-square goodness of fit test was used to assess whether or not Cx. pipiens fed on a particular host species more than expected based on relative abundance of the host.
Results
Breeding and roosting behavior of American robins.
We mapped 107 robin territories and located 99 nests. Consistent with reports that robins generally produce two broods,43 two peaks of nesting activity were observed (Figure 2); however, the first peak in late May or early June was less defined.
Figure 2.
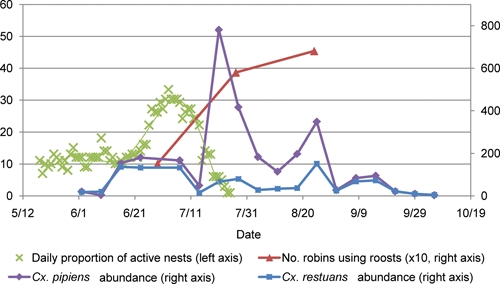
Seasonal patterns of Cx. pipiens and Cx. restuans abundance and American robin nesting behavior at sites not used for roosting in 2008 and seasonal patterns of American robin use of communal roosts in 2009 are shown. This figure appears in color at www.ajtmh.org.
Six robin communal roosts in greater New Haven were identified during the summer of 2008, five by observation and one by following a bird equipped with a radio transmitter. A seventh roost Southern CT State Univ. roost (SCSUR) was short lived, because no birds were using it by August 15th. The density of communal robin roosts in the study area that was thoroughly searched (31 km2; Figure 1) was one per 5.16 km2. Five of the major communal roosts were situated in wetlands dominated by Phragmites australis; the sixth communal roost Barraclough Ave. roost (BARCR) was located in a residential area.
In 2009, robins were first observed going to communal roosts in early June. The number of robins using the roosts was low in late June (approximately 1,500 birds at all roost combined), and it increased to approximately 6,000 birds by late July (Figure 2). In late August, some roosts continued to increase in size (BARCR and State St. roost [STATR]), whereas the other four reached a plateau or slightly decreased in size. Although we did not conduct counts in late September, the roosts were visited to determine if they were still active. All roosts were still active, but the number or robins at BARCR and East Haven roost (EAHAR) seemed smaller than in the previous counts. Reduced activity in September was also observed in 2008. From September 25th to October 17th 2008, the number of birds with transmitters returning to communal roosts decreased from nine to three, indicating the onset of migration. Roost constancy was estimated at 0.87 based on 240 observations of two successive nights derived from 26 radio-tagged birds (14 hatch year and 12 after hatch year).
Avian WNV-virus exposure.
Blood samples were collected from 35 robin nestlings from 16 nests representing 13.1% of territories from May 27 to July 26, 2008, as well as from 45 fledglings and 42 adults. Virus was not isolated from any samples; however, three fledglings (6.6%) and thirteen adults (30.9%) that were using communal roosts had neutralizing antibodies against WNV by PRNT.
Early season mosquito infection and feeding patterns at non-roosting sites.
Early season MLE-infection rates with WNV at the non-roosting sites for Cx. pipiens were 3.55 for New Haven, 3.44 for West Haven, and 0.0 for East Haven. For Cx. restuans, MLE-infection rate was 6.71 for East Haven, 3.05 for New Haven, and 0.0 for West Haven. Combined Cx. pipiens and Cx. restuans MLE-infection rate was 3.22 (CI = 1.52–6.09) for the three sites; eight mosquito pools out of 2,583 individuals (109 pools) tested positive.
Blood-meal sources were successfully identified from 50 Cx. pipiens and 45 Cx. restuans (Table 1). All blood meals identified from Cx. pipiens and all but one identified from Cx. restuans were avian-derived. Robin-derived blood meals were identified in 82.1% of Cx. pipiens and 57.7% of all Cx. restuans. We detected no more than six blood meals from any other single bird species (mourning dove; Zenaida macroura). The feeding index for robins at the three sites averaged 11.95 (±3.92) for Cx. pipiens and 8.87 (±0.29) for Cx. restuans. Both mosquito species fed on robins significantly more than would be expected based on their relative abundance in the host community at each of the three non-roosting sites (chi-square goodness-of-fit test; P < 0.005).
Table 1.
Blood meal source for Cx. pipiens and Cx. restuans at three non-roosting sites in greater New Haven, CT during the breeding period (before August 10th, 2008)
| Species | Cx. pipiens | Cx. restuans | ||||
|---|---|---|---|---|---|---|
| East | New | West | East | New | West | |
| American robin (Turdus migratorius) | 2 | 27 | 13 | 4 | 16 | 6 |
| Baltimore oriole (Icterus galbula) | 1 | |||||
| Barn swallow (Hirundo rustica) | 1 | |||||
| Black-crowned night heron (Nycticorax nycticorax) | 1 | |||||
| Cedar waxwing (Bombycilla cedrorum) | 1 | |||||
| European starling (Sturnus vulgaris) | 1 | 3 | 1 | |||
| Green heron (Butorides virescens) | 1 | |||||
| Grey catbird (Dumetella carolinensis) | 1 | |||||
| House sparrow (Passer domesticus) | 2 | |||||
| House wren (Troglodytes aedon) | 1 | |||||
| Mourning dove (Zenaida macroura) | 1 | 3 | 2 | |||
| Rose-breasted grosbeak (Pheucticus ludovicianus) | 1 | |||||
| Rock dove (Columba livia) | 1 | |||||
| Red-winged blackbird (Agelaius phoeniceus) | 1 | 1 | ||||
| Swamp sparrow (Melospiza georgiana) | 1 | |||||
| Northern flicker (Colaptes auratus) | 1 | |||||
| Mammal | 1 | |||||
Late season mosquito infection, feeding patterns, and vector abundance at communal roosting versus non-roosting sites.
Combined Cx. pipiens and Cx. restuans infection rate was significantly higher at the communal roosting sites (MLE IR = 26.97; CI = 15.59–44.46) than at non-roosting sites (MLE IR = 8.93; CI = 4.77–15.47; Figure 3). A total of 228 mosquito pools and 2,059 individuals were tested; 15 pools of 721 individuals (110 pools) tested positive at communal roosting sites, and 11 pools of 1,338 individuals (118 pools) tested positive at non-roosting sites. The last infected mosquito pool was collected on October 13th for roosting sites (Cx. restuans at East Rock roost [EAROR]) and on September 30th for non-roosting sites (Cx. pipiens in New Haven).
Figure 3.
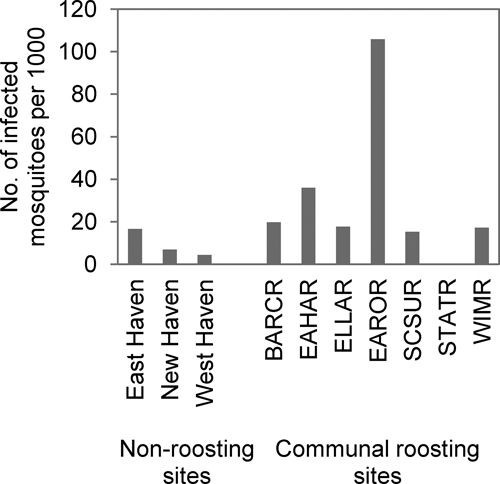
Maximum likelihood estimates of Cx. pipiens and Cx. restuans WNV infection rates at American robin and mixed-species communal roosts and non-roosting sites during the post-breeding period (after August 10th, 2008).
The proportion of robin-derived blood meals from Cx. pipiens and Cx. restuans was six of nine (66.6%) at communal roosting sites and two of eight (25%) at non-roosting sites. We detected robin-derived blood meals in mosquitoes collected at the roosts from late August through October (Table 2). By analysis of 115 engorged Aedes vexans collected in the immediate vicinity of three of the roosts (STATR, Ella T. Grasso Blvd. roost [ELLAR], and Wilmont Rd. roost [WILMR]), we identified avian- (5.2%) and mammalian-derived (94.8%) blood meals, 6.4% of which were from human hosts.
Table 2.
Dates of Cx. pipiens and Cx. restuans blood meals obtained at communal roosting and non-roosting sites during the post-breeding period (after August 10th, 2008)
| Collection | Date | Mosquito species | Blood meal source |
|---|---|---|---|
| Non-roosting | 13-Aug-08 | Cx. restuans | American robin |
| Non-roosting | 14-Aug-08 | Cx. pipiens | Northern cardinal (Cardinalis cardinalis) |
| Non-roosting | 14-Aug-08 | Cx. pipiens | American robin |
| Non-roosting | 14-Aug-08 | Cx. pipiens | House wren |
| Non-roosting | 14-Aug-08 | Cx. pipiens | Mourning dove |
| Non-roosting | 14-Aug-08 | Cx. pipiens | Northern cardinal |
| Non-roosting | 14-Aug-08 | Cx. pipiens | Rose-breasted grosbeak |
| Non-roosting | 30-Sep-08 | Cx. restuans | Wood duck (Aix sponsa) |
| Communal roost | 28-Aug-08 | Cx. pipiens | American robin |
| Communal roost | 28-Aug-08 | Cx. pipiens | American robin |
| Communal roost | 28-Aug-08 | Cx. restuans | American robin |
| Communal roost | 08-Sep-08 | Cx. restuans | American robin |
| Communal roost | 09-Sep-08 | Cx. restuans | American robin |
| Communal roost | 10-Sep-08 | Cx. restuans | Wood thrush (Hylocichla mustelina) |
| Communal roost | 10-Sep-08 | Cx. restuans | House wren |
| Communal roost | 03-Oct-08 | Cx. restuans | American robin |
| Communal roost | 03-Oct-08 | Cx. restuans | European starling |
Ae. vexans was the most abundant species in both roosting and non-roosting sites and Cx. pipiens and Cx. restuans were more abundant at non-roosting than at roosting sites (negative binomial regression; likelihood ratio χ2 test; P < 0.0001; Table 3; Figure 4). Cx. pipiens abundance peaked in late July with a secondary peak in late August when most birds were using the communal roosts; Cx. restuans did not show any obvious peaks in abundance (Figure 2).
Table 3.
Negative binomial regression model of mosquito abundance in light traps in relation to vector species and site type
| Coefficient | Standard error | z | P > |z| | 95% CI | |
|---|---|---|---|---|---|
| Site type | 0.05 | 0.31 | 0.14 | 0.886 | −0.57 0.66 |
| Cx. pipiens | −2.77 | 0.31 | −8.80 | 0.000 | −3.39 −2.15 |
| Cx. restuans | −3.97 | 0.32 | −12.36 | 0.000 | −4.60 −3.34 |
| Cx. salinarius | −1.73 | 0.31 | −5.55 | 0.000 | −2.35 −1.12 |
| Site type × Cx. pipiens | 1.30 | 0.46 | 2.82 | 0.005 | 0.40 2.21 |
| Site type × Cx. restuans | 1.23 | 0.47 | 2.60 | 0.009 | 0.30 2.16 |
| Site type × Cx. salinarius | 0.46 | 0.46 | 1.01 | 0.310 | −0.43 1.36 |
Ae. vexans was used as a reference group for comparison with the other vector species. 0, communal roosting site; 1, non-roosting site.
Figure 4.
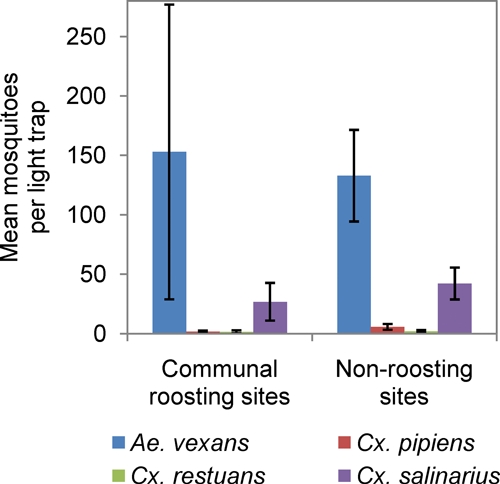
Mean number of putative WNV vectors collected from light traps at communal roosts and non-roosting sites during the postbreeding period (after August 10th, 2008). This figure appears in color at www.ajtmh.org.
Discussion
Our results indicate that robin communal roosts in greater New Haven play an important role in WNV amplification with increased potential for bridging transmission to humans. Mosquito-infection rates were significantly higher at communal roosting sites than at non-roosting sites late in the transmission season. Although we cannot discount the possibility that early transmission occurred at locations where communal roosts eventually formed, our collective evidence suggests that it is the use of these sites as roosts by robins that drives late-season amplification. Robin-roosting behavior results in large aggregations of virus-competent hosts where they are preferentially fed on by the main enzootic vectors, Cx. pipiens and Cx. restuans. Recruitment of viremic robins and immunologically naïve robin fledglings to these roosts would further intensify virus amplification later in the season. Identification of human-derived blood meals from Ae. vexans, an abundant and competent vector species at roosting sites, provides additional support for transmission to human hosts in the immediate vicinity.
Vector preferential feeding has been proposed as a mechanism driving heterogeneity in WNV transmission.21,30,44 Consistent with previous studies,15,17,21 we found evidence for Cx. pipiens and Cx. restuans preferential feeding on robins. However, the proportion of robin-derived blood meals did not decline later in the transmission season as previously reported in other studies where Cx. pipiens exhibited a shift to other avian species15,21 or humans.17 We note that these studies did not specifically target communal roosts for mosquito sampling. Continuous feeding on robins throughout the transmission season was reported in Tennessee and was attributed to robins being year-round residents.18 Our study shows that in urban locations in the northeastern United States, robins reside throughout the transmission season and cluster in communal roosts at night. This results in a patchy distribution of robins, which impacts their availability to host-seeking mosquitoes later in the transmission season. These communal roosts were likely missed by other studies conducting diurnal bird surveys.
The roosting and nesting behavior of various avian species has been proposed to drive spatial patterns of WNV transmission in California. In Los Angeles, risk of human infection was associated with the distribution of dead American crow (Corvus brachyrhynchos) clusters around communal crow roosts. In contrast, in nearby Kern County, human cases were more evenly distributed in areas where the dominant corvid species, western scrub-jay (Aphelocoma californica), shows a more uniform distribution pattern.26 Similarly, in the northeastern United States, robin communal-roosting behavior may also explain intra-urban spatial patterns of human risk of infection. It has been generally assumed that territorial birds such as American robins roost at their breeding territories.27 Although this is true for nesting females during the breeding season, robins increasingly roost communally as the season progresses, first the males and then the juveniles as they fledge; finally, the females join the communal roosts.43
In this study, the prevalence of WNV infection in mosquitoes collected at the roosting sites was variable but generally higher than at non-roosting sites. This finding would seem to be caused by the preferential interaction between robins and vector mosquitoes. Variability may arise from differences in communal roost size and host-species composition in mixed-species roosts. Frequent feeding on other virus-competent roosting birds such as grackles13 would result in WNV amplification, whereas feeding on low reservoir-competent starlings13 would likely result in decreased transmission or dilution (Ostfeld and Keesing45) of the virus. However, in our analysis, we did not identify frequent vector feeding on these hosts. A number of other factors may also impact the intensity of enzootic transmission, including roost constancy, herd immunity in avian hosts, and spatial and temporal abundance of competent mosquito vectors.
The potential of communal roosts to serve as sources of enhanced WNV infection to humans depends on the abundance, vectorial capacity, and feeding patterns of resident mosquito populations. In our study we did not identify any human-derived blood meals in Cx. pipiens, and based on previous blood-meal analyses in Connecticut, human feeding seems to be quite rare.15 However, in Chicago, Illinois, a number of human-derived blood meals were identified in Cx. pipiens, leading authors to incriminate this species as a bridge vector to humans; this could be caused by genetic differences among regional populations.46 Although we cannot discount the role of Cx. pipiens in transmission to humans, our study provides some additional support for the role of Ae. vexans, a moderately competent vector11 from which WNV has been repeatedly isolated in this region.12 We identified 7 of 109 (6.4%) human-derived blood meals in addition to limited avian feeding as a necessary prerequisite for transmission.
This study identifies the importance of American robins in WNV amplification in the northeastern United States during late-season transmission, which is when most human cases typically occur. Our study also identifies areas of intense arboviral amplification within urban localities with potential to serve as sources of infection to humans. The role of communal roosts in other geographical regions will depend on the roosting behavior of key reservoir hosts. Understanding the role of solitary versus communal roosting behavior in various WNV transmission scenarios could provide additional insights into the mechanisms driving the spatiotemporal patterns of WNV transmission.
Acknowledgments
We are grateful to CAES technical staff (John Sheppard, Michael Thomas, Shannon Finan, and the mosquito/arbovirus support group), Durland Fish for logistical support, Paul Cislo for statistical support, and Elyse LeeVan, Anna Milkowski, Lindsay Rollend, Leah and Ariel Simons, Gabrielle Gareau, Colter Fulman, Sarah Guagliardo, and Emily Fung for collecting mosquitoes, locating roosts, and assisting with mist netting and placement of transmitters on robins.
Footnotes
Financial support: Funding for this research was provided in part by Laboratory Capacity for Infectious Diseases Cooperative Agreement Number U50/CCU6806-01-1 from the Centers for Disease Control and Prevention, United States Department of Agriculture (USDA) Specific Cooperative Agreement Number 58-6615-1-218, USDA-administered Hatch funds CONH00768 to the CAES, USDA-Agricultural Research Service Cooperative Agreement 58-0790-5-068, the G. Harold and Leila Y. Mathers Charitable Foundation, and the Yale Institute for Biospheric Studies Faculty Support Endowment Fund.
Authors’ addresses: Maria A. Diuk-Wasser, Jennifer E. Simpson, and Corrine M. Folsom-O’Keefe, Yale School of Public Health, New Haven, CT, E-mails: maria.diuk@yale.edu, jennifer.simpson@yale.edu, and corrine.folsom@yale.edu. Goudarz Molaei, Philip M. Armstrong, and Theodore G. Andreadis, The Connecticut Agricultural Experiment Station, New Haven, CT, E-mails: Goudarz.Molaei@ct.gov, Philip.Armstrong@ct.gov, and Theodore.Andreadis@ct.gov.
References
- 1.Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press; 1991. [Google Scholar]
- 3.Grenfell BT, Dobson AP. Ecology of Infectious Diseases in Natural Populations. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 4.Shaman J. Amplification due to spatial clustering in an individual-based model of mosquito-avian arbovirus transmission. Trans R Soc Trop Med Hyg. 2007;101:469–483. doi: 10.1016/j.trstmh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Lothrop HD, Reisen WK. Landscape affects the host-seeking patterns of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J Med Entomol. 2001;38:325–332. doi: 10.1603/0022-2585-38.2.325. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen CF, Armijos MV, Wheeler S, Carpenter TE, Boyce WM, Kelley K, Brown D, Scott TW, Reisen WK. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in northern California. Am J Trop Med Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Reisen WK, Lundstrom JO, Scott TW, Eldridge BF, Chiles RE, Cusack R, Martinez VM, Lothrop HD, Gutierrez B, Wright SE, Boyce K, Hill BR. Patterns of avian seroprevalence to western equine encephalomyelitis and Saint Louis encephalitis viruses in California, USA. J Med Entomol. 2000;37:507–527. doi: 10.1603/0022-2585-37.4.507. [DOI] [PubMed] [Google Scholar]
- 8.Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Smith DL, Dushoff J, McKenzie FE. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2004;2:1957–1964. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Statistics, Surveillance and Control. US Department of Health and Human Services. 2009. (West Nile Virus).http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm Available at. Accessed November 9, 2009.
- 11.Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- 12.Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- 13.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molaei G, Andreadis TA, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patrican LA, Hackett LE, Briggs JE, McGowan JW, Unnasch TR, Lee JH. Host-feeding patterns of Culex mosquitoes in relation to trap habitat. Emerg Infect Dis. 2007;13:1921–1923. doi: 10.3201/eid1312.070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- 22.Caccamise DF, Fischl J. Patterns of association of secondary species in roosts of European Starlings and Common Grackles. Wilson Bull. 1985;97:173–182. [Google Scholar]
- 23.Morrison DW, Caccamise DF. Ephemeral roosts and stable patches—a radiotelemetry study of communally roosting starlings. The Auk. 1985;102:793–804. [Google Scholar]
- 24.Komar N, Dohm DJ, Turell MJ, Spielman A. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris) Am J Trop Med Hyg. 1999;60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- 25.Komar N, Spielman A. Emergence of Eastern Encephalitis in Massachusetts. Ann NY Acad Sci. 1994;740:157–168. doi: 10.1111/j.1749-6632.1994.tb19866.x. [DOI] [PubMed] [Google Scholar]
- 26.Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, Fang Y, Shafii M, Kahl N, Ashtari S, Kramer V, Glaser C, Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Ward MP, Raim A, Yaremych-Hamer S, Lampman R, Novak RJ. Does the roosting behavior of birds affect transmission dynamics of West Nile virus? Am J Trop Med Hyg. 2006;75:350–355. [PubMed] [Google Scholar]
- 28.Yaremych SA, Novak RJ, Raim AJ, Mankin PC, Warner RE. Home range and habitat use by American Crows in relation to transmission of West Nile Virus. Wilson Bull. 2004;116:232–239. [Google Scholar]
- 29.Wheeler SS, Barker CM, Fang Y, Armijos MV, Carroll BD, Husted S, Johnson WO, Reisen WK. Differential impact of West Nile virus on California birds. The Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- 31.Bibby CJ, Burgess ND, Hill DA, Mustoe SH. Bird Census Techniques. 2nd ed. New York: Academic Press; 2000. [Google Scholar]
- 32.Gale GA, Hanners LA, Patton SR. Reproductive success of worm-eating Warblers in a forested landscape. Conservation Biology. 1997;11:246–250. [Google Scholar]
- 33.Johnson DH. In defense of indices: the case of bird surveys. J Wildl Manage. 2008;72:857–868. [Google Scholar]
- 34.Gaunt AS, Oring LW. The Ornithological Council. 1999. (Guidelines to the Use of Wild Birds in Research).http://www.nmnh.si.edu/BIRDNET/GuideToUse/ Available at. Accessed November 9, 2009.
- 35.Johnson GD, Pebworth JL, Krueger HO. Retention of transmitters attached to passerines using a glue-on technique. Journal of Field Ornithology. 1991;62:486–491. [Google Scholar]
- 36.Perry MC, Haas GH, Carpenter JW. Radio transmitters for mourning doves: a comparison of attachment techniques. J Wildl Manage. 1981;45:524–527. [Google Scholar]
- 37.Rappole JH, Tipton AR. New harness design for attachment of radio transmitters to small passerines. Journal of Field Ornithology. 1991;62:335–337. [Google Scholar]
- 38.Mennill D. How to Radiotrack Chickadees or Other Small Birds. Windsor; Canada: 2001. http://web2.uwindsor.ca/courses/biology/dmennill/radiotelem.html Available at. Accessed November 9, 2009. [Google Scholar]
- 39.Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. New Haven, CT: The Connecticut Agricultural Experiment Station; 2005. [Google Scholar]
- 40.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams GM, Gingrich JB. Comparison of light traps, gravid traps, and resting boxes for West Nile virus surveillance. J Vector Ecol. 2007;32:285–291. doi: 10.3376/1081-1710(2007)32[285:coltgt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Biggerstaff BJ. PooledInfRate, Version 3.0: A Microsoft Excel Add-In to Compute Prevalence Estimates from Pooled Samples. 2006. http://www.cdc.gov/ncidod/dvbid/westnile/software.htm Available at. Accessed November 9, 2009.
- 43.Sallabanks R, Frances CJ. In: The Birds of North America Online. Poole A, editor. 1999. http://bna.birds.cornell.edu/bna/species/462/articles/introduction (American robin (Turdus migratorius)). Available at. Accessed November 9, 2009. [Google Scholar]
- 44.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond B Biol Sci. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostfeld R, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool. 2000;78:2061–2078. [Google Scholar]
- 46.Huang S, Hamer GL, Molaei G, Walker ED, Goldberg TL, Kitron UD, Andreadis TG. Genetic variation associated with mammalian feeding in Culex pipiens from a West Nile virus epidemic region in Chicago, Illinois. Vector Borne Zoonotic Dis. 2009;9:637–642. doi: 10.1089/vbz.2008.0146. [DOI] [PubMed] [Google Scholar]


