Abstract
We investigated the epidemiology and etiology of encephalitis at four tertiary hospitals in Bangladesh during 2003–2005. Patients who met a clinical case definition for acute encephalitis and had cerebrospinal fluid (CSF) pleocytosis were eligible for enrollment; a standardized sampling pattern was used to enroll eligible patients. Recent Japanese encephalitis virus (JEV) infection was defined by presence of IgM antibodies against JEV in CSF or serum. Twenty (4%) of 492 cases had laboratory evidence of recent JEV infection; two died. All JE cases occurred during May–December, and cases were identified among all age groups. All cases resided in rural areas. Fifteen patients were re-assessed 4–6 weeks after hospitalization; 5 (33%) patients had physical disabilities and 7 (47%) reported cognitive difficulties. Infection with JEV is clearly an etiology of encephalitis in Bangladesh. Population-based studies to quantify burden of disease could assess options for targeted immunization programs.
Introduction
Japanese encephalitis virus (JEV), a mosquito-borne flavivirus, is a leading cause of viral encephalitis in Asia.1–3 Over the past three decades, the incidence of Japanese encephalitis (JE) has increased in parts of India, Nepal, and southeast Asia, with outbreaks of JE occurring in several areas that were previously not endemic for this disease.2,4–9 The reasons for this increased geographic distribution are uncertain, but may include population shifts, and changes in agricultural practices, animal husbandry, migratory bird patterns, and movement of vector mosquitoes to wider areas.1 It is estimated that JEV causes at least 50,000 cases of encephalitis each year in Asia, resulting in approximately 10,000 deaths with 15,000 survivors developing neurological and psychiatric sequelae.6 There is no effective antiviral treatment. Importantly, however, JE is a vaccine-preventable disease.2,10–12
Japanese encephalitis has been reported in India and Myanmar, the two countries that border Bangladesh.5,8,13,14 India has introduced a cost effective live-attenuated JE vaccine (SA 14-14-2) in a area hyperendemic for this disease area in 2006 after the JE epidemic in 2005.8,15 More than 44 million children were immunized during 2006–2008. By 2010, 102 million children are targeted to be immunized in 111 districts endemic for JE in 11 states in India.16 However, except for an outbreak in 1977 in the central part of Bangladesh,17 JE has not been recognized and no systematic assessments of disease occurrence have been carried out since that outbreak. We conducted a hospital-based study during June 2003–July 2005 in Bangladesh to assess the etiologies of encephalitis including JEV, and report the results of JE assessment.
Methods
Study site.
The study was performed at four tertiary care hospitals in different geographic areas (Figure 1). The study began in June 2003 at three sites (Dhaka, Mymensingh, and Rajshahi) and in December 2004 at a fourth site (Sylhet) (Figure 1). The study continued at all sites until July 2005.
Figure 1.
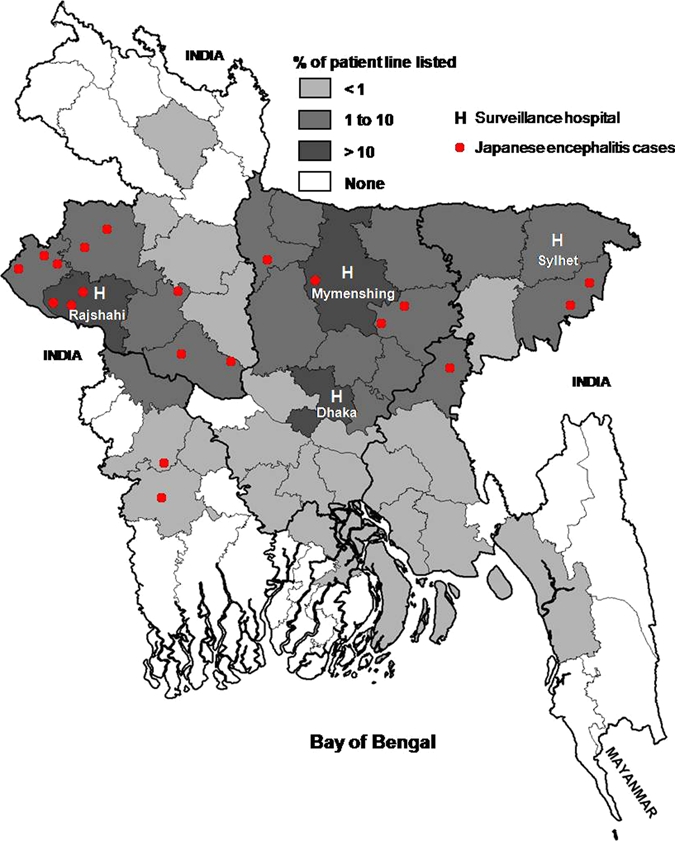
Districts of Bangladesh showing percentage of all line-listed acute encephalitis cases and distribution of Japanese encephalitis cases. This figure appears in color at www.ajtmh.org.
Patient enrollment.
Study physicians visited the hospital wards daily to review the admission logbooks and identify patients meeting our clinical case definition of acute encephalitis with indication for lumbar puncture, based on the judgment of the patient's attending physician. The clinical case definition of acute encephalitis included new onset of fever (temperature ≥ 38°C) or history of fever during the present illness along with altered mental status, (e.g., confusion, disorientation, coma) and/or a neurological deficit (i.e., focal or diffuse neurological dysfunction or new onset of seizures) with onset of the neurological symptoms within five days prior to hospitalization.
Enrollment in the study required that the patient met the clinical case definition and that he or she had cerebrospinal fluid (CSF) pleocytosis (defined as > 4 leukocytes/mm3 for patients > 6 weeks of age and > 14 leukocytes/mm3 for the patients ≤ 6 weeks of age). Lumbar punctures were performed by attending physicians as a part of routine clinical care. However, patients or their guardians had to provide consent for collection of an additional amount of CSF during the lumbar puncture to be included in the study. Because of high patient volume, patients were assessed for pleocytosis using a sampling scheme. Beginning with the first patient on the line list, patients were assessed for pleocytosis until a patient demonstrated pleocytosis and was enrolled. The next three patients irrespective of CSF cell counts on the list were skipped and the next patient was selected for assessment. This process continued through the line list until a patient with pleocytosis was found and enrolled. Patients meeting the clinical case definition and whose CSF demonstrated pleocytosis were eligible for JEV testing (Figure 2).
Figure 2.
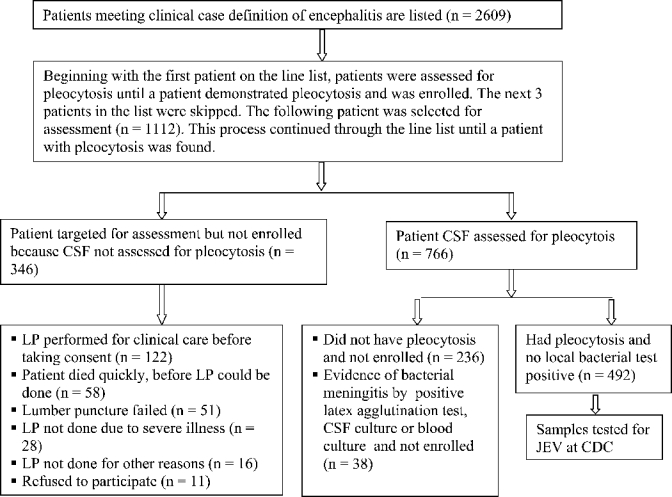
Flowchart for acute encephalitis cases, Bangladesh, June 2003–July 2005.
From October 2004 until the end of the study, patients who had a positive CSF or blood culture, or a positive CSF latex agglutination test result18 for Streptococcus pneumoniae; Neisseria meningitidis serogroups A, B, or C; or Haemophilus influenzae type b were excluded from JEV testing (Figure 2).
Ethical considerations.
Study physicians obtained written informed consent before sample and data collection from either patients or their guardians. Assent was obtained for patients 7–17 years of age. The study protocol was reviewed and approved by institutional review boards at International Center for Diarrheal Disease Research, Bangladesh (ICDDR,B) and the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia.
Data collection.
Using a standardized questionnaire, the study physician collected data on sociodemographic status, exposure to wild and domestic animals, mosquitoes, and other insect bites, travel (outside home district or outside Bangladesh) within one month before the onset of illness, clinical symptoms and signs including Glasgow coma score, results of local laboratory tests, type and duration of treatment, duration of hospitalization, and outcome of illness including mortality, and neurological and functional level of activity at time of hospital discharge.
Patient follow-up.
All surviving patients with JE were asked to return to the hospital and complete a follow-up interview 4–6 weeks after enrollment. During this visit, the study physician completed a follow-up questionnaire for documentation of short-term outcomes, residual neurological problems since discharge, the subject's overall quality of life, and current cognitive status. If the patient failed to return for the follow-up visit, the study physician or health worker attempted to visit the patient's home to evaluate the status of the patient and encourage the patient to return for the follow-up visit.
Specimen collection, local laboratory testing, storage, and shipment.
The study physicians collected CSF during a lumbar puncture performed for clinical assessment and serum samples during enrollment and the follow-up interview for JEV testing at CDC. The serum and CSF samples were aliquoted at study sites. The specimens were stored at −20°C in the study hospitals for no longer than one week and then transported to ICDDR,B in a cold box and frozen at −70°C. Aliquots were sent to CDC in dry ice.
Laboratory testing for JEV and case criteria.
Serum and CSF samples from patients enrolled in the study were tested at the Arboviral Diseases Branch, CDC (Fort Collins, CO). Specimens were tested using JEV and dengue virus (DENV) IgM-capture enzyme-linked immunosorbent assays.19 Specimens positive for IgM against JEV or DENV in serum or CSF were tested for JEV-and DENV-neutralizing antibodies by plaque reduction neutralization tests using a 90% cut-off value (PRNT90).20 Laboratory confirmation of recent JEV infection was defined as the presence of 1) IgM against JEV in CSF, or 2) IgM against JEV in serum with a JEV PRNT90 titer ≥ 20 and a JEV PRNT90 to DENV PRNT90 titer ratio ≥ 4.
Statistical analysis.
The data were entered in Epi-Info version 6 software (CDC, Atlanta, GA) and analyzed by using STATA/SE 10 statistical software.21 We calculated medians (range) for continuous data. Proportions were measured for categorical data.
Results
During the study period, 2,609 patients met the clinical case definition of acute encephalitis, had indication for lumbar puncture, and were included on the line list (Figure 2). Among the line listed cases, 808 (31%) were from Dhaka, 878 (34%) were from Mymensingh, 768 (29%) were from Rajshahi, and 155 (6%) were from Sylhet.
A total of 1,112 patients from the line listing were assessed for pleocytosis. Of these, 492 (44%) patients had pleocytosis, were enrolled, and had samples tested for JEV (Figure 2). Twenty (4%) of the 492 patients evaluated had laboratory evidence of recent JEV infection.
Cases of JE were identified in 3 (2%) of 131 of study patients in Dhaka, 4 (3%) of 156 study patients in Mymensingh, 11 (6%) of 173 study patients in Rajshahi, and 2 (6%) of 32 study patients in Sylhet.
Overall, the median age of JE cases was 18 years (range = 1.5 months–55 years). Cases were identified among all age groups with 11 (55%) cases greater than 15 years of age (Table 1). Eleven (55%) cases were males. All cases lived in rural areas and 12 (61%) resided in the northwestern part of the country (Figure 1). None of the cases traveled outside Bangladesh within the month before illness. Eighteen (90%) of the cases occurred during May–October, and no cases were identified during January–April (Figure 3). Fourteen (70%) JE cases were identified in the first year, and six (30%) JE cases were identified in the second year (Figure 3). In the first year, 14 (5%) of 285 encephalitis patients were diagnosed with JE. In the second year, 6 (3%) of 207 encephalitis patients were positive for JEV.
Table 1.
Demographic characteristics of 20 cases of Japanese encephalitis, Bangladesh
| Characteristic | Value |
|---|---|
| Median age, years, (range) | 18 (1.5 months–55 years) |
| Age group, years, no. (%) | |
| £ 5 | 2 (10) |
| 6–10 | 4 (20) |
| 11–15 | 3 (15) |
| 16–20 | 4 (20) |
| 21–40 | 5 (25) |
| > 40 | 2 (10) |
| Sex, no. (%) | |
| M | 11 (55) |
| F | 9 (45) |
| Occupation, no. (%) | |
| Farmer | 2 (10) |
| Student | 9 (45) |
| Housewife | 4 (20) |
| Children less than school age, no. (%) | 2 (10) |
| Day laborer/petty business/unemployed, no. (%) | 3 (15) |
Figure 3.

Number of Japanese encephalitis cases, by month of symptom onset, Bangladesh, June 2003–July 2005.
Patients with JE had fever (100%), altered consciousness (100%), convulsion (85%), headache (79%), severe weakness or lethargy (57%), stiff neck (55%), and vomiting (35%) (Table 2). The median (range) of their Glasgow coma scores was 8 (4–15), and 11 (55%) patients had a Glasgow coma score ≤ 7 during clinical evaluation at the time of recruitment. Six (30%) patients left the hospital with neurological sequelae that included focal weakness (n = 4) and paralysis (n = 1).
Table 2.
Clinical features of 20 cases of Japanese encephalitis, Bangladesh
| Features | Value |
|---|---|
| Symptoms, no. (%) | |
| Fever | 20 (100) |
| Altered consciousness | 20 (100) |
| Convulsion | 17 (85) |
| Headache | 15 (75) |
| Severe weakness or lethargy | 13 (65) |
| Stiff neck | 11 (55) |
| Vomiting | 7 (35) |
| Nausea | 6 (30) |
| Muscle pain | 3 (15) |
| Limb weakness | 3 (15) |
| Signs, no. (%) | |
| Temperature > 37.8°C | 8 (40) |
| Neck rigidity | 13 (65) |
| Babinski's sign (extensor plantar response) | 8 (42) |
| Abnormal pupillary light reflex (sluggish/absent) | 7 (35) |
| Kernig's sign | 6 (30) |
| Tremor/rigidity/myoclonus | 5 (25) |
| Glasgow coma score, median (range) | 8 (4–15) |
| Grade | |
| 15 | 0 (0) |
| 13–14 | 1 (5) |
| 8–12 | 8 (40) |
| 3–7 | 11 (55) |
| Discharged with complication (focal weakness, paralysis and cognitive function impairment),* no. (%) | 6 (30) |
| Duration of hospitalization in days, median (range) | 9 (4–26) |
| Death, no (%) | 2 (10) |
Focal weakness (n = 4), paralysis (n = 1), and cognitive function impairment (n = 3).
Fifteen patients, including five of the six patients who had residual neurological findings upon hospital discharge, were evaluated 4–6 weeks after recruitment. At that time, five (33%) patients had residual neurological problems (e.g., dysarthria, limb weakness, hearing impairment, and urinary incontinence). Seven (47%) reported difficulties with thinking/reasoning skills (Table 3). Two patients died; one at the hospital and one at home.
Table 3.
Follow-up of 15 cases of Japanese encephalitis 4–6 weeks after enrollment in the study, Bangladesh
| Finding | No. (%) |
|---|---|
| Patient unable to resume his/her previous activities | 7 (47) |
| Reported deficits in thinking and reasoning since illness | 7 (47) |
| Depression | 4 (27) |
| Dysarthria | 4 (27) |
| Limb weakness | 2 (13) |
| Hearing impairment | 2 (13) |
| Personality change | 2 (13) |
| Urinary incontinence | 1 (7) |
The mean CSF leukocyte count 109 cells/mm3 (range = 7–980 cells/mm3) with a predominance of lymphocytes (mean = 75%, range = 30–100%). The CSF glucose concentrations ranged from 46 to 191 mg/dL (mean = 66 mg/dL); CSF protein levels were generally mildly elevated (mean = 58 mg/dL, range = 24–102 mg/dL).
Discussion
This is the first study to document human JE viral infection in Bangladesh since 1977. We cannot determine if the virus has re-emerged or if it has been present, but not recognized, since the 1977 outbreak because of the limited diagnostic capacity for JE in Bangladesh.22 Our study to assess etiologies of encephalitis in Bangladesh demonstrated evidence of acute JEV infection in 4% of enrolled cases. The cases were identified at all of the four participating study hospitals. However, 11 (6%) of encephalitis patients had JE at Rajshahi, but only three (2%) were identified in Dhaka, the site with the largest urban catchment area. All age groups were represented in the study, but more than 50% of the JE cases were greater than 15 years of age. Most JE cases occurred during May–October during monsoon and post-monsoon seasons.
The total number of JE cases who came to the four hospitals during our study was likely higher than the 20 cases identified by laboratory testing through our surveillance. In our study, 492 (64%) of the 766 patients evaluated had evidence of pleocytosis and had no bacterial infection. If we applied this same proportion to the other 1,843 patients meeting the clinical case definition for encephalitis but who were not evaluated for pleocytosis, we would have expected to identify an additional 1,183 patients or a total of 1,675 patients with encephalitis and pleocytosis. Of the 492 patients tested for JEV infection, 20 (4%) were positive. If this same proportion were applied to the projected number of patients with encephalitis and pleoctyosis, we would have identified a total of 68 cases of JE at the study sites.
Our study hospitals were located in urban areas, but all the JE cases were from rural agricultural areas, with 55% from areas around the Rajshahi Medical College Hospital in the northwestern part of Bangladesh. The high prevalence of JE in rural areas could be because the requisite conditions (i.e., vector, reservoir host and virus) are all present.1 This finding is consistent with the finding that JE is more common in rural rice-cultivating areas throughout Asia.23,24 The low-lying and/or rice-producing rural areas of Bangladesh are conducive to breeding the mosquito vectors (Culex vishnui, Cx. triaeniorhynchus, and Cx. gelidus) that transmit JEV.17,25 Common aquatic birds, such as egrets and herons, and pigs are reservoirs for JEV. Although pigs are less common than cows and goats in this predominantly Muslim country, the pigs found in Bangladesh often forage over wide-ranging areas.
Case of JE identified in this study represented all age groups. In JE-endemic areas of southeast Asia, most people are infected with JEV before 15 years of age.2,3,6,26 The occurrence of cases in Bangladesh among somewhat older persons might reflect a recent introduction of JEV, or may be caused by less intense transmission, leading to a larger population of susceptible adults than in other countries endemic for JE. In Nepal, the age group affected also included young adults and adults and may have been caused by migration of working people from non-endemic areas to endemic areas.27
All JE infections were identified during May–December, with 90% identified during May–October. The assessment of JE prevalence in Sylhet in northeastern Bangladesh was limited because patients were only enrolled from December 2004 through July 2005 and this period was only three months of a single JE transmission season. These findings are consistent with seasonality noted in other areas of southeast Asia.2,6,8,28,29 This finding may be related to seasonal increases in the Culex mosquito population, the main vector for transmission of JEV, during and just after the rainy season (June–September).2,6,8,28–30
Similar to other studies, almost one-third of our patients with JE had neurological sequelae and almost half had subjective cognitive function impairment at the six-week follow-up interview.6,31 This finding suggests that JE may represent a substantial burden in an already impoverished country because of the resultant lack of productivity and caregiver activities.
It is likely that our assessment captured only a small proportion of JE cases in Bangladesh. Many sick persons may not have come to a health facility for care or may have sought care in hospitals other than the tertiary care centers included in our study. The overall case-fatality rate of 10% was also lower than that reported in other studies,4 which may be caused by late care-seeking behavior, resulting in patients dying before reaching the hospital. Additionally, some patients might have died in a hospital before a lumbar puncture could be performed or a lumbar puncture may not have been performed because of severe illness. It is likely that some of these patients had JE, which further contributes to an underestimate of case fatality.
The evaluation of other etiologies of encephalitis is ongoing. The findings of this study indicate that JEV infection is clearly an etiology of encephalitis in Bangladesh and is worthy of closer inspection as a potentially important cause of encephalitis. Better assessment of disease incidence and the financial and functional impact of both acute illness with JE and the long-term sequelae of illness on cases and family units will aid in clarifying the overall public health burden and societal impact of JE in this population. The findings from these efforts would impact decision-making on policies regarding prevention, especially for the potential introduction of JE vaccine in Bangladesh and what populations should be targeted for vaccine programs.
Acknowledgments
We thank Drs. Abu Taher Azad, Sultana Monira Hossain, Rahima Afroza, Farah Naz Shoma, Nahida Zafrin Tuly, Mohammed Monirul Islam Khan, Tarana Tanjima Azad Lucky, Enamul Haque, Mahidul Alam, Syed Mortaza Ali, and Bidith Ranjan for help with patient recruitment, enrollment, and data collection; Christine Ellis, Roselyn Hochbein, Janeen, Olga Kosoy, Robert Lanciotti, Barbara Johnson, Grant Campbell, and John Roehrig for laboratory testing and data interpretation; Nihar Roy for laboratory support at ICDDR,B; the study participants and their relatives for participation, and Milton Quiah for administrative support; and Dorothy Southern for critically reviewing the manuscript. The ICDDR,B acknowledges with gratitude the commitment of CDC to its research efforts.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention.
Authors’ addresses: M. Jahangir Hossain, Emily S. Gurley, and Robert F. Breiman, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mails: jhossain@icddrb.org, egurley@icddrb.org, and rbreiman@ke.cdc.gov. Susan Montgomery, Lyle Petersen, James Sejvar, Marc Fischer, Amanda Panella, and Ann M. Powers, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: zqu6@cdc.gov, lxp2@cdc.gov, zea3@cdc.gov, mxf2@cdc.gov, ahf6@cdc.gov, and akp7@cdc.gov. Nazmun Nahar and A. K. M. Rafique Uddin, Dhaka Medical College Hospital, Dhaka, Bangladesh, E-mails: nahar@dab-bd.org and rudduin-bsm@yahoo.com. M. Ekhlasur Rahman, Mymensingh Medical College Hospital, Myemsingh, Bangladesh and MAG Osmani Medical College Hospital, Sylhet, Bangladesh, E-mail: pediatrics_03@yahoo.com. A. R. M. Saifuddin Ekram, Rajshahi Medical College Hospital, Rajshahi, Bangladesh, E-mail: armsekram@yahoo.com. Stephen P. Luby, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh and Centers for Disease Control and Prevention, Atlanta, GA, E-mail: sluby@icddrb.org.
References
- 1.Halstead SB, Jacobson J. Japanese encephalitis. Adv Virus Res. 2003;61:103–138. doi: 10.1016/s0065-3527(03)61003-1. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB, Tsai TF. In: Vaccines. Fourth edition. Plotkin SA, Orenstein WA, editors. Philadelphia: Saunders; 2004. pp. 919–958. (Japanese encephalitis vaccines). [Google Scholar]
- 3.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 4.Report of the Bi-Regional Meeting on Japanese Encephalitis (WHO SEA/WPR AND /PATH's JE Project) Bangkok, Thailand: 2005. March 30–April 1, 2005. [Google Scholar]
- 5.World Health Organization . The World Health Report, 1996: Fighting Disease, Fostering Development. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 6.World Health Organization Japanese encephalitis vaccines. Wkly Epidemiol Rec. 2006;81:331–340. [PubMed] [Google Scholar]
- 7.Fischer M, Campbell G. In: Health Information for International Travel, 2008. Arguin PM, Kozarsky PE, Reed C, editors. Atlanta: US Department of Health and Human Services, Public Health Services; 2007. pp. 190–201. (Japanese Encephalitis). [Google Scholar]
- 8.Parida M, Dash PK, Tripathi NK, Ambuj, Sannarangaiah S, Saxena P, Agarwal S, Sahni AK, Singh SP, Rathi AK, Bhargava R, Abhyankar A, Verma SK, Rao PV, Sekhar K. Japanese encephalitis outbreak, India, 2005. Emerg Infect Dis. 2006;12:1427–1430. doi: 10.3201/eid1209.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partridge J, Ghimire P, Sedai T, Bista MB, Banerjee M. Endemic Japanese encephalitis in the Kathmandu Valley, Nepal. Am J Trop Med Hyg. 2007;77:1146–1149. [PubMed] [Google Scholar]
- 10.Hennessy S, Liu Z, Tsai TF, Strom BL, Wan CM, Liu HL, Wu TX, Yu HJ, Liu QM, Karabatsos N, Bilker WB, Halstead SB. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347:1583–1586. doi: 10.1016/s0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- 11.Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, Kotchasenee S, Gingrich JB, Latendresse J, Fukai K. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608–614. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 12.Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine. 2000;18((Suppl 2)):1–25. doi: 10.1016/s0264-410x(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 13.Myint L, Hlaing W, Thu HM, Than SM, Aye KT, Ha T, Moe K, Thein S. Investigation of Japanese encephalitis virus infection in Bogalay Township, Myanmar in 1999. Trop Med. 2000;42:47–52. [Google Scholar]
- 14.Kaur R, Agarwal CS, Das D. An investigation into the JE epidemic of 2000 in Upper Assam: a perspective study. J Commun Dis. 2002;34:135–145. [PubMed] [Google Scholar]
- 15.Liu W, Clemens JD, Kari K, Xu ZY. Cost-effectiveness of Japanese encephalitis (JE) immunization in Bali, Indonesia. Vaccine. 2008;26:4456–4460. doi: 10.1016/j.vaccine.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Raina VK, Khaparde SD. India: JE immunization in Routine Immunization-Opportunities and Challenges. Ministry of Health and Family Welfare. Government of India. Presented at Fourth Biregional Meeting on the Control of Japanese Encephalitis; Bangkok, Thailand: 2009. June 8–9, 2009. [Google Scholar]
- 17.Khan AM, Khan AQ, Dobrzynski L, Joshi GP, Myat A. A Japanese encephalitis focus in Bangladesh. J Trop Med Hyg. 1981;84:41–44. [PubMed] [Google Scholar]
- 18.Slidex Meningite-Kit 5. REF 58 803 . Marcy l’Etoile. France: bioMerieux; http://www.biomerieux.com Available at. [Google Scholar]
- 19.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 21.STATA . Data Analysis and Statistical Software, Version 10. College Station, TX: Stata Corporation; http://www.stata.com Available at. [Google Scholar]
- 22.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 23.Marfin AA, Eidex RS, Kozarsky PE, Cetron MS. Yellow fever and Japanese encephalitis vaccines: indications and complications. Infect Dis Clin North Am. 2005;19:151–168 ix. doi: 10.1016/j.idc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Sunish IP, Reuben R. Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India. I. Abiotic. Med Vet Entomol. 2001;15:381–392. doi: 10.1046/j.0269-283x.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Renshaw M, Elias M, Maheswary NP, Hassan MM, Silver JB, Birley MH. A survey of larval and adult mosquitoes on the flood plains of Bangladesh, in relation to flood-control activities. Ann Trop Med Parasitol. 1996;90:621–634. doi: 10.1080/00034983.1996.11813092. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organiztion Japanese encephalitis vaccines. Wkly Epidemiol Rec. 1998;73:337–344. [PubMed] [Google Scholar]
- 27.Wierzba TF, Ghimire P, Malla S, Banerjee MK, Shrestha S, Khanal B, Sedai TR, Gibbons RV. Laboratory-based Japanese encephalitis surveillance in Nepal and the implications for a national immunization strategy. Am J Trop Med Hyg. 2008;78:1002–1006. [PubMed] [Google Scholar]
- 28.Kar NJ, Bora D, Sharma RC, Bhattacharjee J, Datta KK, Sharma RS. Epidemiological profile of Japanese encephalitis in Gorakhpur district, Uttar Pradesh, 1982–1988. J Commun Dis. 1992;24:145–149. [PubMed] [Google Scholar]
- 29.Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, White N. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ. 2008;86:178–186. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur AR, Agarwal CS, Das D. An investigation into the JE epidemic of 2000 in Upper Assam: a perspective study. J Commun Dis. 2002;34:135–145. [PubMed] [Google Scholar]
- 31.Kari K, Liu W, Gautama K, Mammen MP, Jr, Clemens JD, Nisalak A, Subrata K, Kim HK, Xu ZY. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Med. 2006;4:8. doi: 10.1186/1741-7015-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


