Abstract
A promising method to fabricate tissue-engineered blood vessels is to have cells synthesize the supportive extracellular matrix scaffold of the tissue-engineered blood vessel; however, a shortcoming of this method has been limited elastogenesis. Previously, we found that arterial smooth muscle cells (ASMCs) produced significant quantities of elastin when transduced with splice variant 3 of the proteoglycan versican (V3). In this study, we assessed whether elastogenesis and the structural properties of entirely cell-derived engineered vascular constructs could be improved by the incorporation of V3-transduced rat ASMCs. After 18 weeks of culture, V3 constructs had more tropoelastin, more elastin crosslinks, higher burst strengths, greater elasticity, and thicker collagen fiber bundles compared with empty-vector controls. The expression of elastin and elastin-associated proteins was increased in V3 and control ASMC monolayer cultures when ascorbic acid, which promotes collagen synthesis and inhibits elastogenesis, was removed from the medium. Engineered vascular constructs with ascorbate withdrawn for 14 weeks, after an initial 4-week exposure to ascorbate, exhibited increased elastin, desmosine content, elasticity, and burst strength compared with constructs exposed continuously to ascorbate. Our results show that V3 coupled with limited exposure to ascorbate promotes elastogenesis and improves the structural and functional properties of engineered vascular constructs.
Introduction
Arteriosclerosis and peripheral arterial disease affect more than 4 million patients in the United States, with approximately 600,000 vascular bypass procedures performed each year.1 In both cardiac and peripheral bypass surgery, autologous veins or arteries are the gold standard for replacement blood vessels; however, a substantial number of patients lack suitable vessels for grafting. Accordingly, a significant effort has been made to develop engineered vascular grafts, in particular, grafts made from synthetic materials. Synthetic grafts, however, have not been completely satisfactory, as they are prone to thrombosis, foreign body response, stenosis, calcification, and bacterial contamination.2
An alternative to synthetic vascular grafts involves the production of tissue-engineered blood vessels (TEBVs) from cells seeded into prefabricated supportive scaffolds made from natural extracellular matrix (ECM) proteins. Currently, this approach is problematic, in that no available prefabricated scaffold closely matches the structure of native vascular ECM. In this context, recent approaches have explored entirely biogenic TEBVs in which the supportive ECM scaffold is synthesized by the cells themselves.3 With optimization of the ECM scaffold and the use of autologous cells, biogenic TEBVs may provide the best possible host integration, homeostasis, self-renewal, and vasomodulatory response.
The mechanical properties of the walls of native arteries are determined in large part by the resilient ECM protein elastin, which is deposited by resident arterial smooth muscle cells (ASMCs) in the form of elastic fibers.4 The elastic fibers are arranged in concentric, fenestrated lamellae in the media of the vessel wall.5 In addition to its mechanical contribution, elastin regulates ASMC growth—elastin knockout mice die from vascular stenosis as a consequence of excessive ASMC proliferation and synthesis of an occlusive neointimal matrix.6
Since elastic fibers are critical to the performance of native blood vessels, there is substantial interest in developing TEBVs enriched in elastin.5,7–9 Notably, elastin synthesis is prominent in late fetal and perinatal vasculature; however, adult ASMCs synthesize little or no elastin either in vivo or in vitro.10,11 Consequently, a major deficiency in TEBVs that incorporate adult ASMCs is the lack of functional elastic fibers and lamellae,12–14 which can lead to mechanical failure.13 Therefore, an important goal in TEBV fabrication is to induce adult ASMCs to produce a functional elastin architecture.
Our previous studies have shown that manipulating the expression of versican, a proteoglycan synthesized by ASMCs, promotes the synthesis and assembly of elastic fibers in vitro and in vivo.15 Full-length versican (V0) inhibits elastogenesis, which may be mediated by chondroitin sulfate (CS)–glycosaminoglycan (GAG) chains attached to two of its four domains.16 In contrast, the versican splice variant 3 (V3), which lacks CS-GAG chains, is elastogenic. The mechanism for the proelastogenic effect of V3 is unclear, but may involve displacement of full-length versican from the pericellular environment where elastic fibers first form.15
We have shown that adult rat ASMCs that expressed V3 following retroviral transduction produced tropoelastin and formed highly crosslinked elastic fibers in vitro and in vivo.15 Therefore, the induction of elastogenesis by adult ASMCs transduced with V3 could be a particularly useful strategy for making TEBVs with functional levels of elastin. Another strategy is to control the use of the media additive ascorbate that has been shown to inhibit elastin synthesis.17 In this study, we evaluate the effect of both V3 transduction and ascorbate on production of elastin and elastin-associated proteins by adult rat ASMCs cultured either in monolayers or incorporated into engineered constructs that simulate the vascular media. We also compare the histological and mechanical properties of the vascular constructs.
Materials and Methods
Cells and monolayer cultures
ASMCs, originally isolated from Fischer 344 rat aortas,18 were transduced with V3-packaged (LV3) or empty-retroviral control (LX) particles (courtesy of Dr. A.D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA). Several transductions were performed with ASMCs of different passages and, for each transduction, parallel pools of LX and LV3 cells were selected by means of the neomycin analogue G418 (800 μg/mL) and passaged in high-glucose (25 mM) Dulbecco's modified Eagle's medium (DMEM; Irvine Scientific, Santa Ana, CA; cat. no. 9024) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA; cat. no. S11150), sodium (Na)-pyruvate (Irvine Scientific; cat. no. 9334), nonessential amino acids (Irvine Scientific; cat. no. 9304), glutaMAX™ (Invitrogen, Carlsbad, CA; cat. no. 35050-061), and 50 U/mL penicillin/50 μg/mL streptomycin (Invitrogen; cat. no. 15070-063). LV3 ASMC populations were selected for high levels of V3 synthesis using northern blot assays.15 LV3 and LX ASMCs were used for experiments between five and nine passages after transduction. Transduced ASMCs were maintained routinely in high-glucose DMEM with 10% FBS, 1% nonessential amino acids, penicillin (100 U/mL), and streptomycin (100 μg/mL). For evaluation of monolayer cultures, LV3 and LX ASMCs were cultured at confluence for 30 days in the presence or absence of 500 μM Na ascorbate (Sigma Chemical, St. Louis, MO) and assayed, as described below, to determine (1) levels of expression of tropoelastin, lysyl oxidase (LOX), fibrillin-1, and fibulin-5 mRNAs and (2) levels of elastin crosslinking, as measured by quantitation of desmosine.
Construction of tubular vascular constructs
LV3 ASMCs overexpressing versican V3 (passages 3–5) were initially characterized by northern blot analysis as having high levels of tropoelastin mRNA versus the control LX ASMCs. The LV3 and LX ASMCs were grown to produce tissue sheets7 as follows: the ASMCs were seeded at 104 cells/cm2 on gelatin-coated T-75 flasks and cultured in a basal medium of DMEM supplemented with Ham's F12 (20%), FBS (FetalClone®; HyClone, Logan, UT) (20%), glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), and Na ascorbate (500 μM) (Sigma Chemical). After an initial culture period of 12 weeks, the resulting cell sheets were rolled around Teflon™-coated (DuPont, Wilmington, DE), stainless steel mandrel supports of 4.6 mm diameter and cultured on the mandrels in basal medium for an additional 4 weeks. Subsequently, the LV3 and LX constructs were separated into two groups that received either (1) basal medium (two LV3 and two LX constructs) or (2) basal medium lacking ascorbate (two LV3 and two LX constructs). The two groups were then cultured for 14 weeks in their respective media, which was changed three times a week. After a total of 18 weeks of culture on mandrels, the tubular constructs were cut into ring-shaped segments (Fig. 1) for histological, biochemical, mechanical, and physiological assays.
FIG. 1.
Engineered vascular construct on mandrel support. This specimen has been divided into ring-shaped segments (arrowheads) for analysis. Color images available online at www.liebertonline.com/ten.
Measurements of wall thickness and histological analysis of vascular constructs
Wall thicknesses of vascular constructs were measured from 3 mm long, ring-shaped segments fixed with 10% neutral-buffered formalin (NBF). Images of segment cross sections were acquired using a stereomicroscope equipped with a high-resolution CCD camera (Insight Color Mosaic; Diagnostic Instruments, Sterling Heights, MI). Digital images of 10 randomly selected locations of the wall of each construct were measured using digital calipers (SPOT Image Analysis software, Windows version 4.6; Diagnostic Instruments).
For histological analysis, ring-shaped segments from the vascular constructs were placed directly into NBF and kept at 4°C for up to 72 h, followed by dehydration in an alcohol series and embedment in paraffin. Paraffin sections of 5 μm thickness were stained with modified Movat's19 and picrosirius red. Modified Movat's demonstrated general histological structure and revealed proteoglycans, GAGs, collagen, and elastin, whereas picrosirius red showed collagen organization and structure under plane-polarized light.20
Immunohistochemical assays of vascular constructs
For immunohistochemical analyses of tropoelastin in the vascular constructs, ring-shaped segments of the constructs (and rat aortas, which served as positive controls) were fixed overnight in 10% NBF at 4°C, dehydrated, and embedded in paraffin. Sections of 5 μm thickness were deparaffinized, blocked 1 h in 2% goat serum in phosphate-buffered saline (PBS), incubated 2 h in a 1:1000 dilution of a rabbit polyclonal antibody to bovine tropoelastin (a kind gift from Dr. Robert Mecham, Washington University, St. Louis, MO), washed in PBS, and incubated 1 h in biotinylated goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA) (1:400 in PBS). Subsequently, sections were washed in PBS, exposed for 30 min to streptavidin–horseradish peroxidase (Invitrogen) (1:400 in PBS/0.1% bovine serum albumin), and developed for 10 min in 3,3′-diaminobenzidine (Sigma Chemical).
Measurement of mRNA
Elastin, collagen-I, fibulin-1, fibulin-5, fibrillin-1, and LOX mRNA levels in LV3 and LX ASMC monolayer cultures and in the tubular vascular constructs were determined from total RNA extracts (Agilent Total RNA kit; Agilent, Santa Clara, CA). An Applied Biosystems HT7900 analyzer (Foster City, CA) and validated stock probe sets were used to perform quantitative real-time reverse-transcription polymerase chain reaction analyses. For each experimental condition, three independent RNA extractions were made, and each extract was analyzed in triplicate.
Desmosine analyses
ASMC monolayer cultures and isolated, ring-shaped segments from tubular vascular constructs were assayed for levels of desmosine (to establish elastin crosslink density) as follows: medium was aspirated from the ASMC monolayers, and the monolayers were rinsed once in PBS, then removed from the culture dishes with a cell scraper, and placed in Eppendorf tubes. The cell monolayer material and individual segments from the vascular constructs were lyophilized, weighed, placed in locking microfuge tubes, and hydrolyzed 24 h in 500 μL of 6 M HCl at 105°C. The hydrolysates were evaporated completely, redissolved in 1 mL of water, and microfuged. Five microliters of the hydrolysate was assayed by radioimmunoassay as described previously.21 Desmosine levels were expressed per milligram total protein in the hydrolysate, as established by a ninhydrin-based method.22
Ultrastructural analyses
Cross sections of the tubular vascular constructs were fixed in standard Karnovsky's fixative, postfixed in OsO4, dehydrated, embedded in Epon resin, ultrathin sectioned, and stained with uranyl acetate/lead citrate with or without ruthenium red. Stained sections were imaged by transmission electron microscopy (TEM), and the digitized electron micrographs were analyzed with SPOT Image Analysis software to establish the diameters of individual collagen fibrils and collagen fiber bundles.
Mechanical testing of vascular constructs
Biomechanical properties (stress–stain profile and burst pressure) of tubular vascular constructs formed from LV3 and LX ASMCs were tested on a pressure-strain test bed. Vascular constructs (four LV3 and four LX) with lengths of approximately 3.3 cm were bathed in 37°C media and first underwent stress preconditioning by cycling the intraluminal pressure between 0 and 120 mmHg three times. Stress–strain measurements were then performed by raising the intraluminal pressure of each construct in 1–5 mmHg increments and measuring their external diameters with a stereomicroscope and high-resolution CCD camera (Insight Color Mosaic; Diagnostic Instruments). For each vascular construct, the pressure–strain relation was tested at least four times between 0 and 120 mmHg. After these measurements were taken, the constructs were pressurized to destruction, and the burst pressure was recorded. Calculation of vessel circumferential compliance was made assuming incompressibility of the construct wall using the relation C = (ΔD/D0)/ΔP, where ΔD is the change in vessel diameter for a given change in pressure (ΔP), from an initial diameter D0.23
Viability and vasoresponsiveness of vascular constructs
Ring-shaped segments (ringlets) (each 3 mm in length) isolated from LV3 and LX tubular vascular constructs (Fig. 1) were evaluated in a tissue myograph equipped with a PowerLab® data acquisition system (AD Instruments, Colorado Springs, CO). The ringlets were mounted in jacketed tissue baths containing physiological saline solution (HEPES; pH 7.4) prewarmed to 37°C and oxygenated with 100% O2. Preload of approximately 0.4 mN was applied to each ringlet before testing. The viability of the ringlets was assessed by measuring the response to depolarization (80 mM K+), and then repolarization once the K+ was washed out. Both vasoconstriction (U46619, a nonhydrolysable analog of thromboxane A2) and vasodilation (Na nitroprusside, a nitric oxide donor) modes of vasoresponse were tested.24
Statistical analysis
Results, where applicable, were expressed as mean ±standard deviation. Student's t-test was applied to determine statistical significance between experimentals and controls, with a p-value of < 0.05 deemed significant.
Results
V3 promotes elastogenesis by ASMC monolayer cultures
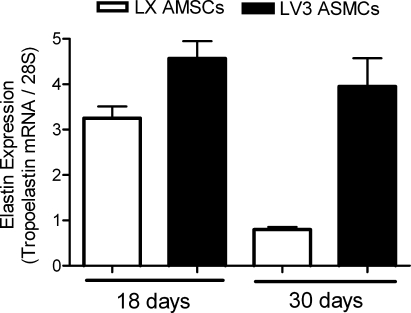
Using northern blot assays, we measured the mRNA expression levels of tropoelastin in versican V3-overexpressing (LV3) ASMCs and in LX empty-vector controls grown in monolayer cultures. LV3 ASMCs expressed a significantly greater amount of tropoelastin mRNA after 18 days in vitro in comparison to the LX controls (Fig. 2). The difference in tropoelastin mRNA expression between LV3 and LX ASMCs was even more pronounced at 30 days of culture, in that tropoelastin mRNA levels were sustained by the LV3 ASMCs, but were significantly reduced in the LX ASMCs (Fig. 2).
FIG. 2.
Tropoelastin levels are sustained in V3-overexpressing ASMCs. Bar graph represents elastin mRNA levels from northern blots of both control (white columns) and V3-overexpressing (black columns) rat ASMC monolayer cultures after 18 or 30 days in vitro (n = 3). Expression of tropoelastin mRNA by LX ASMCs diminishes significantly after 30 days (p < 0.01), but is maintained by LV3 ASMCs. ASMCs, arterial smooth muscle cells. V3, versican splice variant 3.
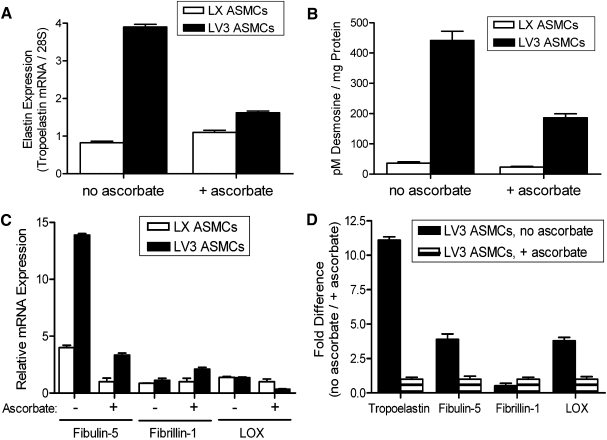
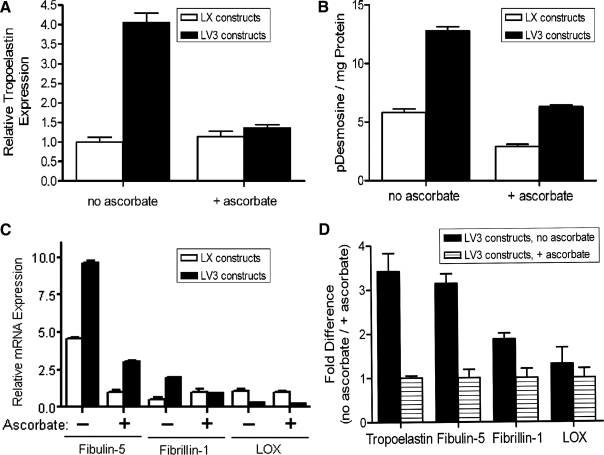
In subsequent in vitro experiments, using 30 days as an endpoint, the addition of 500 μM of ascorbate significantly inhibited the expression of tropoelastin mRNA by LV3 ASMCs (Fig. 3A). The relatively low expression of tropoelastin mRNA by LX ASMCs was not significantly affected by addition of ascorbate (Fig. 3A). In agreement with the expression of tropoelastin mRNA, 30-day cultures of LV3 ASMCs synthesized significantly more desmosine (indicative of mature, crosslinked elastin) than did LX ASMCs (Fig. 3B). Moreover, desmosine levels by LV3 ASMCs were significantly reduced by ascorbate (Fig. 3B). Levels of mRNA for the elastin-associated proteins fibulin-5 and fibrillin-1 were consistently higher in LV3 ASMCs than in LX ASMCs (Fig. 3C). Among LV3 ASMCs, not only did ascorbate substantially reduce the expression of tropoelastin mRNA but also significantly reduced the expression of fibulin-5 and LOX mRNAs (both of which have been shown to be essential for elastin fibrillogenesis and organization in vivo) (Fig. 3D). Ascorbate modestly increased fibrillin-1 mRNA in LV3 ASMCs (Fig. 3D). Collectively, the data indicated that elastogenesis by adult rat ASMCs in monolayer culture could be significantly increased by transducing the cells with V3 while limiting exposure of the cells to ascorbate.
FIG. 3.
Differential expression of elastin and elastin-associated proteins by ASMCs is influenced by ascorbate. (A) After 30 days of culture in the presence of 500 μM ascorbate, LV3 ASMCs exhibit a significantly reduced expression of tropoelastin mRNA relative to LV3 ASMCs cultured for the same period of time in the absence of ascorbate. The low levels of tropoelastin mRNA expressed by LX ASMCs are little affected by the presence or absence of ascorbate (n = 3), p < 0.05. (B) Desmosine levels, a measure of mature, crosslinked elastin, are very much higher in LV3 ASMCs compared with LX ASMCs and are significantly reduced in the presence of ascorbate (n = 3), p < 0.05. (C) mRNAs for the elastin-associated proteins fibulin-5 and fibrillin-1 are elevated in LV3 ASMCs compared with LX ASMCs, but LOX mRNA is similar or lower. For both LV3 and LX ASMCs, ascorbate reduced the levels of fibulin-5 and LOX mRNA, while increasing the levels of fibrillin-1 mRNA (p < 0.05). For each mRNA, values were normalized to the LX + ascorbate expression level. (D) In correspondence with levels of tropoelastin mRNA, ascorbate significantly reduces the mRNAs for the elastin-associated proteins fibulin-5 and LOX expressed by LV3 ASMCs (n = 3), p < 0.05. For each mRNA, values were normalized to the LV3 + ascorbate expression level. LOX, lysyl oxidase.
Transduction of ASMCs with V3 and limited exposure to ascorbate promote elastogenesis in engineered vascular constructs
After removal from the mandrel, the tubular vascular constructs were divided into sections (Fig. 1) for subsequent histological, biochemical, and biomechanical analyses, and measurement of vasoresponse. Macroscopically, constructs made from LV3 ASMCs had thicker walls than constructs made from LX ASMCs, regardless of whether ascorbate was present for the entire 18 weeks of culture or withdrawn after 4 weeks of culture (Table 1). Under conditions of continuous ascorbate, the LX constructs had slightly thicker walls than the LX constructs that had ascorbate withdrawn, but this difference was not statistically significant.
Table 1.
Effect of V3 Expression and Presence of Ascorbate on Wall Thickness of Tubular Vascular Constructs
| |
Wall thickness (μm) |
|
|---|---|---|
| Treatment | LX | V3 |
| Continuous ascorbate | 384 ± 43 (4) | 447a ± 46 (4) |
| Removal of ascorbate | 324 ± 17 (4) | 483 ± 21 (4) |
LV3 versus LX controls (n values are shown in parentheses for the number of ringlets measured). The values represent mean ± standard deviation.
p < 0.05.
V3, versican splice variant 3.
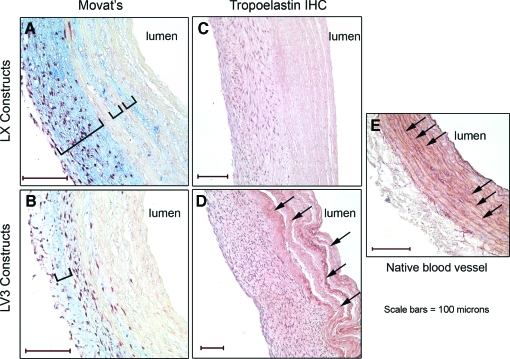
Histochemical assays with Movat's stain showed that the LX constructs (Fig. 4A) had greater quantities of proteoglycan/GAG-rich ECM (as indicated by a greater intensity of blue coloration) than did the LV3 constructs (Fig. 4B). Immunohistochemical staining for elastin showed that relatively little elastin was present in the walls of the LX constructs (Fig. 4C), whereas significant quantities of elastin were present in the walls of the LV3 constructs (Fig. 4D, arrows), especially in adluminal regions, as is typical of many native blood vessels (Fig. 4E, arrows). The differences in proteoglycan/GAG expression and elastin levels of LV3 and LX constructs exposed continuously to ascorbate were similar to corresponding LV3 and LX constructs that had ascorbate withdrawn (data not shown).
FIG. 4.
LV3 vascular constructs have less proteoglycan/glycosaminoglycan staining, and more elastin than LX vascular constructs. Paraffin sections of LV3 and LX vascular constructs were analyzed histologically. Movat's stain shows significantly more proteoglycan/glycosaminoglycan (blue/brackets) in LX constructs (A) versus LV3 constructs (B). Immunohistochemistry (IHC) for tropoelastin reveals that LV3 constructs (D) have quantitatively more staining than LX constructs (C) with relatively dense deposits of elastin arranged in lamellae (D, arrows) that resemble the elastic lamellae present in a native rat aorta immunolabeled for tropoelastin (E, arrows). Scale bars = 100 microns. Color images available online at www.liebertonline.com/ten.
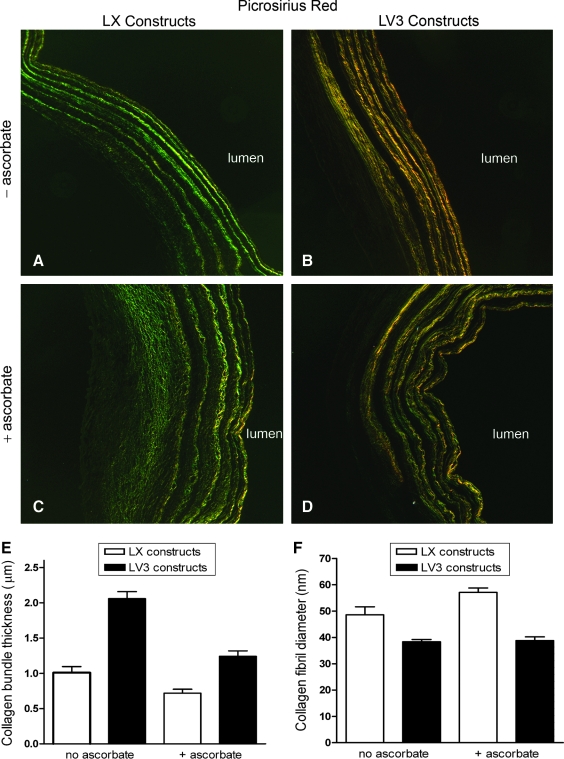
Picrosirius red staining and imaging under polarized light indicated that LV3 vascular constructs had thicker collagen fiber bundles. The LX constructs had relatively fine (green) bundles of collagen fibers (Fig. 5A, C), whereas LV3 constructs had thicker (yellow-red) collagen fiber bundles (Fig. 5B, D). Among the LV3 and LX constructs, the presence or absence of ascorbate made no observable difference in the appearance of collagen fibers after picrosirius red staining.
FIG. 5.
LV3 vascular constructs have thicker collagen fiber bundles. Picrosirius red staining and imaging under polarized light shows that LX constructs have relatively fine (green) bundles of collagen fibers (A, C), whereas LV3 constructs have thicker (yellow-red) collagen fiber bundles (B, D)—a result supported by transmission electron microscopy assays (E). In contrast, the average collagen fibril diameter of LV3 constructs was slightly less than that of LX constructs (F). Among LV3 and LX constructs, exposure to ascorbate reduced collagen bundle thickness (E), but had little effect on collagen fibril diameter (F).
The results obtained by picrosirius red staining were supported by TEM assays, which indicated that LV3 vascular constructs had significantly thicker collagen fiber bundles than did LX constructs (Fig. 5E). TEM also revealed that for both LV3 and LX constructs, exposure to ascorbate reduced collagen bundle thicknesses. Interestingly, the average diameter of individual collagen fibrils was slightly lower in LV3 constructs than in LX constructs (Fig. 5F). Collagen fibril diameters in LV3 and LX constructs were not significantly different in the presence or absence of ascorbate. Consistent with the ASMC monolayer data, mRNA for tropoelastin was elevated in LV3 constructs relative to LX constructs (although there was little difference between LV3 and LX constructs in the presence of ascorbate) (Fig. 6A). Compared with constructs that had ascorbate withdrawn, continuous exposure to ascorbate sharply reduced tropoelastin mRNA in LV3 constructs, but had little effect on the relatively low levels of tropoelastin mRNA in LX constructs (Fig. 6A). In addition to elevated tropoelastin mRNA (and increased immunohistochemical staining for tropoelastin; Fig. 4D), LV3 constructs contained higher levels of desmosine than did LX constructs (Fig. 6B), indicating that the LV3 constructs were enriched for crosslinked, insoluble elastin relative to the LX constructs. Further, addition of ascorbate to LV3 and LX constructs resulted in a substantial decrease in desmosine levels relative to constructs that had ascorbate withdrawn (Fig. 6B). This finding suggested that ascorbate was suppressing the deposition of mature, insoluble elastin within the constructs, just as it did in the ASMC monolayer cultures (Fig. 3B). Also consistent with our ASMC monolayer culture data was the finding that fibulin-5 and fibrillin-1 mRNAs were generally higher in LV3 constructs than in LX constructs, with LOX mRNA lower (Fig. 6C). Among LV3 constructs, the continuous presence of ascorbate significantly reduced mRNAs for tropoelastin, fibulin-5, fibrillin-1, and LOX (Fig. 6D).
FIG. 6.
Incorporation of LV3 ASMCs into engineered vascular constructs increases tropoelastin, elastin-associated proteins, and desmosine content, all of which are decreased in the presence of ascorbate. (A) Expression of tropoelastin mRNA was significantly greater in LV3 constructs than in LX constructs and was substantially decreased for both LV3 and LX constructs in the presence of ascorbate. (n = 4). (B) LV3 constructs had increased desmosine content compared with LX constructs. Removal of ascorbate significantly increased the amount of desmosine in both LX (p < 0.024) and LV3 (p < 0.0003) constructs (n = 6). (C) Elastin-associated proteins fibulin-5 and fibrillin-1 were elevated in LV3 constructs compared with the LX controls. Ascorbate reduced the levels of fibulin-5, fibrillin-1, and LOX for the LV3 constructs, while increasing the levels of fibrillin-1 for the LX constructs. For each mRNA, values were normalized to the LX + ascorbate expression level. (D) In LV3 constructs, expression of mRNAs for tropoelastin and the elastin-associated proteins fibulin-5, fibrillin-1, and LOX was significantly reduced in the presence of ascorbate (p < 0.05). In (D), mRNA levels were normalized to the LV3 + ascorbate expression level.
Expression of V3 and withdrawal of ascorbate increase the elasticity and strength of engineered vascular constructs
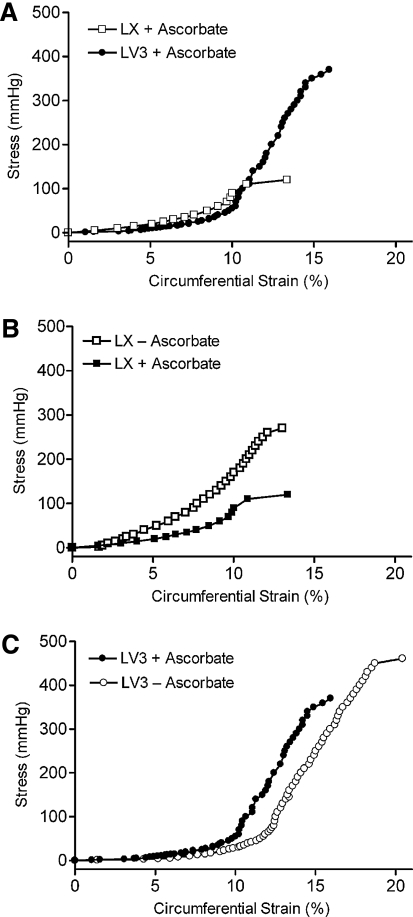
The stress–strain plot of LV3 constructs cultured continuously in ascorbate (Fig. 7A) indicated a high elasticity at low strains (0–12%) and a significant reduction in elasticity at higher strains (12–18%), similar to stress–strain responses of native blood vessels.25 At low strains, the LX constructs were slightly less elastic than the LV3 constructs, as indicated by a higher modulus (slope). In the presence of ascorbate, the LX constructs also burst at much lower pressures (Fig. 7A). Withdrawal of ascorbate from the LX constructs increased the burst pressure of the constructs tested, but decreased their elasticity relative to LX constructs exposed continuously to ascorbate (Fig. 7B and Table 2), whereas the withdrawal of ascorbate from the LV3 constructs led to an increase in both burst pressure and elasticity (the latter indicated by a lower modulus) relative to LV3 constructs exposed continuously to ascorbate (Fig. 7C). Destructive testing indicated that collectively the LV3 constructs failed at higher pressures (397 ± 55 mmHg; n = 3) than did the LX constructs (206 ± 60 mmHg; n = 3), p < 0.007. Compliance calculations of multiple vessels indicated that the LV3 constructs with the ascorbate removed were the most compliant followed by, in descending order, LV3 + ascorbate, LX + ascorbate, and LX − ascorbate (Table 2).
FIG. 7.
Pressure versus strain analysis of representative LV3 and LX vascular constructs. (A) LV3 constructs are slightly more elastic at low strains (0–12%) and fail at much higher pressures than LX constructs cultured under similar conditions. (B) LX constructs cultured in the absence of ascorbate had significantly decreased elasticity and increased burst pressure compared with LX constructs cultured in the presence of ascorbate. (C) LV3 constructs cultured in the absence of ascorbate are slightly more elastic at low strains and have higher burst pressures than LV3 constructs exposed to ascorbate. In (A–C), the standard deviations of the measurements were very small (2% or less); therefore, error bars are not shown for the sake of clarity.
Table 2.
Compliance
| Compliance (%/100 mmHg) | |
|---|---|
| LV3 − ascorbate | 14.4 ± 0.1 (8) |
| LV3 + ascorbate | 13.1 ± 0.3 (7) |
| LX + ascorbate | 12.2 ± 0.1 (6) |
| LX − ascorbate | 8.5 ± 0.2 (7) |
Compliance calculations indicated that the LV3 constructs with the ascorbate removed were the most compliant followed by, in descending order, LV3 + ascorbate, LX + ascorbate, and LX −ascorbate. The values represent mean ± standard deviation (n values are shown in parentheses).
LX and LV3 vascular constructs were vasoresponsive
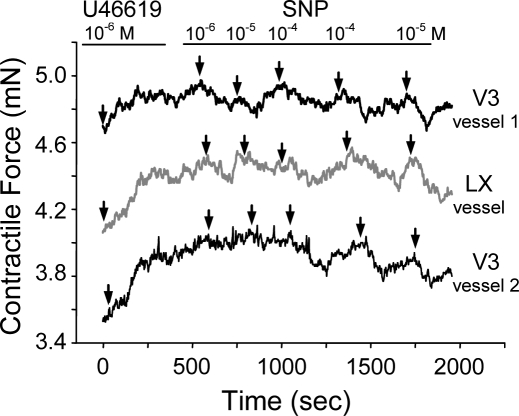
When ringlet segments 3 mm in length from the vascular constructs were evaluated in a tissue myograph, all the ringlets depolarized and then repolarized in response to a potassium bolus (data not shown). The vasoresponsiveness of the ringlets was determined by using agents that induced either vasoconstriction (U46619) or vasodilation (Na nitroprusside). Administration of U46619 induced a contractile response of approximately 0.5 mN, whereas Na nitroprusside induced relaxation responses that were as much as −0.4 mN (Fig. 8). These results showed that both the LX and LV3 constructs contained viable cells and were vasoresponsive.
FIG. 8.
Engineered vascular constructs are viable and vasoresponsive. Ringlets from LX and LV3 vascular constructs were evaluated for vasoresponse in a tissue myograph. Both contractile (U46619-induced) and relaxation (Na nitroprusside [SNP]-induced) modes of vasoresponse were tested at the indicated concentrations (M). Arrows indicate the times at which U46619 and SNP were added to the test chamber.
Discussion
In this study, we report on the results of a novel strategy for the construction of TEBVs that have improved structural and functional properties. The strategy involves the use of cell-mediated overexpression of the versican variant V3 combined with ascorbate deprivation, both of which enhanced the production of elastin. Engineered vascular constructs formed by the V3-overexpressing ASMCs were enriched in crosslinked elastin, had thicker collagen fiber bundles, increased compliance, and higher burst strengths compared with control vessels that lacked V3. In addition, these constructs were both viable and vasoresponsive, factors paramount for their application as in vivo grafts.
V3-overexpressing adult ASMCs are elastogenic,15 which is uncharacteristic of native adult cells.26 Not only do LV3 ASMCs show increased expression of tropoelastin in vitro but also the expression is maintained over time in comparison to empty-vector (LX) ASMC controls. Previous studies have shown that elastin synthesis by LV3 ASMCs is accompanied by organization of the elastin into functional elastic fibers and lamellae. For example, V3-transduced rat ASMCs introduced into balloon-injured rat carotid arteries in vivo produce neointimas with highly structured elastic lamellae similar to lamellae in the media of healthy arteries.15 In this study, use of V3 ASMCs conferred similar structural and functional benefits to engineered vascular constructs.
The use of SMCs in the construction of a functional TEBV has been attempted previously.27,28 Success, however, has been limited for a number of reasons. One reason is the high expression by ASMCs of GAGs or GAG-bearing ECM proteoglycans, such as versican, which lead to looser, less structurally cohesive ECM.14,29 This is a particular problem in TEBVs that incorporate human ASMCs, which have a characteristically high expression of proteoglycans and GAGs in comparison to rat or mouse ASMCs.30 Notably, GAGs inhibit elastin expression (particularly, CS-GAGs)15,16,31 and also have a limiting effect on collagen fibril thickness.29 There is recent evidence that the inhibiting effect of GAGs on collagen fibril thickness is mediated by proteoglycan-bound GAG (e.g., decorin) and not by free GAG.32 GAGs and CS-proteoglycans may also increase matrix metalloproteinase activity.33 Correspondingly, the increased concentration of GAGs that we observed in the control LX vascular constructs relative to the LV3 constructs was associated with thinner collagen fibril bundles and a lower content of elastin and elastin-associated proteins which may have contributed to the LX constructs being structurally weaker than the LV3 constructs. Collectively, our results and those of others underscore the importance of developing methods to limit GAG content (such as the CS of specific proteoglycans) in TEBVs.
In vascular tissue engineering, ascorbate has often been added to culture media to promote collagen synthesis.7,27,28 Ascorbate, however, inhibits the synthesis of both elastin and LOX (which mediates elastin crosslinking),17,34 and upregulates proteoglycans that are thought to inhibit elastin synthesis.35 This is consistent with our findings that the synthesis of elastin and a number of other molecules critical to elastic fiber assembly was increased when ascorbate was removed from the culture media. With respect to collagen synthesis and organization, we found that exposure of LV3 and LX vascular constructs to ascorbate was associated with reductions in the thickness of collagen fiber bundles. The reason for this effect of ascorbate is unclear; however, it may be linked to suppressed elastin synthesis, since we found that expression of V3 in vascular constructs was associated with increases both in elastin deposition and the thickness of collagen fiber bundles.
The formation of mature elastic fibers and lamellae is dependent on a series of coordinated events. Microfibrillar ECM scaffolds are thought to be necessary to properly orient tropoelastin monomers for optimal crosslinking.36 Once oriented, tropoelastin monomers are crosslinked at lysine residues (forming desmosine crosslinks) by members of the LOX family of enzymes, which are localized to the ECM microfibrillar scaffold. Important microfibrillar scaffold proteins include members of the fibrillin and fibulin families. The importance of fibrillin and fibulin in elastogenesis has been demonstrated by mouse knockout studies and in disease models such as Marfan,37 cutis laxa,38 and Costello39 syndromes. In this study, we found that increased elastogenesis induced by V3 in ASMC monolayer cultures and vascular constructs was associated not only with increases in tropoelastin mRNA and protein but also with mRNA for fibulin-5 and fibrillin-1. Interestingly, LOX mRNA was either unaffected or reduced in LV3 monolayer cultures and vascular constructs when compared with their LX counterparts, whereas desmosine content was consistently elevated in LV3 ASMC monolayers and constructs compared with LX controls. This suggests that crosslink activity is independent of LOX expression, or the changes that we observed in LOX mRNA are insufficient to change the ability of LOX to catalyze crosslinks. Given the ability of V3 to change the structure of ECM and levels of microfibrillar proteins on which LOX is docked, V3 may influence LOX catalytic activity—an area worthy of further study.
There were significant histological differences between LV3 vascular constructs and their LX controls. LV3 constructs had lower levels of GAGs, thicker, more mature collagen bundles, and more elastic fibers than LX constructs. The decrease in GAG content and increase in elastin deposition in the LV3 constructs were expected, given the previously observed effects of V3 expression on neointimal organization in balloon-injured carotids of rats15 and rabbits.40 What was unexpected was our finding that V3 overexpression in our vascular constructs led to the apparent deposition of thicker collagen fiber bundles. The observed effect of V3 expression on collagen fibril organization in vivo may relate to the lower levels of GAGs seen in the LV3 vascular constructs. For example, it has been shown in mice that addition of high concentration of GAGs to sponge implants leads to the production of thinner/finer collagen fibers in granulation tissue.41 The increased thickness of collagen fiber bundles in the LV3 constructs may contribute to their strength and stability. Moreover, a robust collagen network7 along with large collagen fiber bundle size has been shown to limit collagen turnover, perhaps by limiting the susceptibility of the collagen to cleavage by collagenases.42 Further, Wells et al.25 determined that qualitative differences in the collagen network (e.g., crosslinks) affected the vessel performance more than levels of collagen production. Alternatively, the increased wall thickness we observed in LV3 constructs may in part explain the increased mechanical stability of LV3 constructs, but will require further study. These observations, in combination with the observations of Merrilees et al.15,40 in neointima and the findings of the present study, suggest that introduction of V3 in native or engineered tissue mediates the production of a more compact, organized ECM with improved strength and stability.
For optimal performance, it is generally accepted that TEBVs should closely reproduce the viscoelastic characteristics of native blood vessels. Numerous studies have shown that synthetic TEBVs and autologous vein grafts exhibit compliance and caliber mismatch with their recipient arteries, which elicits intimal hyperplasia leading to graft apatency.43–45 Therefore, achieving a close match of caliber and compliance between the graft and the recipient artery is of critical importance. Using the methods described here, TEBVs can be fabricated with diameters that match recipient vessels and with compliances within natural ranges. The constructs we produced exhibited an 8–14% compliance, which is similar to a 6–16% compliance range that others have determined for small caliber (<7 mm diameter) native arteries.46
With regard to stress–strain behavior, our engineered vascular constructs first responded, following pressurization of the lumen, with high elasticity to a point of inflection where the subsequent responses were much less elastic. This division of the stress–strain response into regions of distinctly different elasticity is characteristic of native blood vessels.25 Consistently, LX constructs were less elastic than LV3 constructs at low strains. At higher strains (i.e., beyond the inflection point of the plot), where, in native vessels, the load is transferred primarily to collagen fibrils,25 the LV3 constructs failed at much higher pressures than the LX constructs. Notably, removal of ascorbate from LV3 constructs increased their elasticity and burst pressure, which may reflect our finding that limiting exposure to ascorbate increases elastin-associated protein deposition, elastin and elastin crosslinking, and also the size of collagen fiber bundles.
Curiously, the LX constructs cultured in the absence of ascorbate did not show a significant change in elastin expression relative to LX constructs exposed to ascorbate (Fig. 6A), but were less elastic based on modulus at all strains (Fig. 7B), These results might, in part, be a consequence of the higher levels of desmosine crosslinks (Fig. 6B) and thicker collagen fiber bundles (Fig. 5E) found in the LX constructs cultured in the absence of ascorbate.
In conclusion, we have constructed viable and vasoresponsive engineered vascular constructs from V3-overexpressing ASMCs. Compared with the empty-vector controls, the LV3 constructs were enriched in elastin and elastin-associated proteins, had elevated levels of elastin crosslinking, exhibited increased structural integrity with higher burst strengths, and were more compliant. In addition, limitation of exposure to ascorbate significantly improved the structural and biomechanical properties of the constructs. While the constructs described here do not meet the threshold of burst strength considered desirable for safe implantation in vivo,47,48 the use of V3-overexpressing ASMCs and limitation of ascorbate may ultimately improve the performance of TEBVs through provision of a stronger, more elastic vascular media. Potentially, by combining engineered media populated with ASMCs with mechanically strong, fibroblast-based engineered adventitial layers (fibroblastic constructs have recently shown promising results as vascular grafts in humans3), a highly functional human TEBV could be produced.
Acknowledgments
The authors acknowledge the support from the University of Washington Engineered Biomaterials (UWEB) Program, National Science Foundation Engineering Research Center Grant #EEC-95929161 (T.N.W.); National Institutes of Health R21 EB005652 (R.B.V.); Center for Control of Inflammation and Tissue Repair, USAMRMC/Department of Defense #W81XWH-07-010246 (T.N.W./R.B.V.); and the American Heart Association Pre-Doctoral Fellowship #0310062Z (P.A.K.). Special thanks to Stephanie Lara and Kathleen Braun for technical assistance, and Dr. Virginia Green for careful editing and proofreading.
Disclosure Statement
No competing financial interests exist.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2009 Update. Dallas, TX: 2009. [Google Scholar]
- 2.Zhang W.J. Liu W. Cui L. Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAllister T.N. Maruszewski M. Garrido S.A. Wystrychowski W. Dusserre N. Marini A. Zagalski K. Fiorillo A. Avila H. Manglano X. Antonelli J. Kocher A. Zembala M. Cierpka L. de la Fuente L.M. L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 4.Wight T.N. The vascular extracelluar matrix. In: Fuster V., editor; Topol E.J., editor; Nabel E.G., editor. Atherothrombosis and Coronary Artery Disease. second edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. pp. 421–437. [Google Scholar]
- 5.Kielty C.M. Stephan S. Sherratt M.J. Williamson M. Shuttleworth C.A. Applying elastic fibre biology in vascular tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1293. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D.Y. Brooke B. Davis E.C. Mecham R.P. Sorensen L.K. Boak B.B. Eichwald E. Keating M.T. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 7.L'Heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T.N. Chronos N.A. Kyles A.E. Gregory C.R. Hoyt G. Robbins R.C. McAllister T.N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan S.W. Haller C.A. Sallach R.E. Apkarian R.P. Hanson S.R. Chaikof E.L. The effect of a recombinant elastin-mimetic coating of an ePTFE prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials. 2007;28:1191. doi: 10.1016/j.biomaterials.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Long J.L. Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 10.Parks W.C. Secrist H. Wu L.C. Mecham R.P. Developmental regulation of tropoelastin isoforms. J Biol Chem. 1988;263:4416. [PubMed] [Google Scholar]
- 11.Parks W.C. Kolodziej M.E. Pierce R.A. Phorbol ester-mediated downregulation of tropoelastin expression is controlled by a posttranscriptional mechanism. Biochemistry. 1992;31:6639. doi: 10.1021/bi00144a003. [DOI] [PubMed] [Google Scholar]
- 12.Niklason L.E. Abbott W. Gao J. Klagges B. Hirschi K.K. Ulubayram K. Conroy N. Jones R. Vasanawala A. Sanzgiri S. Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 13.Opitz F. Schenke-Layland K. Cohnert T.U. Starcher B. Halbhuber K.J. Martin D.P. Stock U.A. Tissue engineering of aortic tissue: dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc Res. 2004;63:719. doi: 10.1016/j.cardiores.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Dahl S.L. Rhim C. Song Y.C. Niklason L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrilees M.J. Lemire J.M. Fischer J.W. Kinsella M.G. Braun K.R. Clowes A.W. Wight T.N. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 16.Hinek A. Mecham R.P. Keeley F. Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. 1991;88:2083. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson J.M. LuValle P.A. Zoia O. Quaglino D., Jr. Giro M. Ascorbate differentially regulates elastin and collagen biosynthesis in vascular smooth muscle cells and skin fibroblasts by pretranslational mechanisms. J Biol Chem. 1997;272:345. doi: 10.1074/jbc.272.1.345. [DOI] [PubMed] [Google Scholar]
- 18.Lemire J.M. Merrilees M.J. Braun K.R. Wight T.N. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002;190:38. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt R. Wirtla J. Modification of movat pentachrome stain with improved reliability of elastin staining. J Histotechnol. 1996;19:325. [Google Scholar]
- 20.Junqueira L.C. Bignolas G. Brentani R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 21.Starcher B. James H. Evidence that genetic emphysema in tight-skin mice is not caused by neutrophil elastase. Am Rev Respir Dis. 1991;143:1365. doi: 10.1164/ajrccm/143.6.1365. [DOI] [PubMed] [Google Scholar]
- 22.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292:125. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 23.Salacinski H.J. Goldner S. Giudiceandrea A. Hamilton G. Seifalian A.M. Edwards A. Carson R.J. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001;15:241. doi: 10.1106/NA5T-J57A-JTDD-FD04. [DOI] [PubMed] [Google Scholar]
- 24.Okon E.B. Golbabaie A. van Breemen C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol. 2002;137:545. doi: 10.1038/sj.bjp.0704884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells S.M. Langille B.L. Lee J.M. Adamson S.L. Determinants of mechanical properties in the developing ovine thoracic aorta. Am J Physiol. 1999;277:H1385. doi: 10.1152/ajpheart.1999.277.4.H1385. [DOI] [PubMed] [Google Scholar]
- 26.Lemire J.M. Covin C.W. White S. Giachelli C.M. Schwartz S.M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994;144:1068. [PMC free article] [PubMed] [Google Scholar]
- 27.L'Heureux N. Paquet S. Labbe R. Germain L. Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Ogle B.M. Mooradian D.L. Manipulation of remodeling pathways to enhance the mechanical properties of a tissue engineered blood vessel. J Biomech Eng. 2002;124:724. doi: 10.1115/1.1519278. [DOI] [PubMed] [Google Scholar]
- 29.Merrilees M.J. Tiang K.M. Scott L. Changes in collagen fibril diameters across artery walls including a correlation with glycosaminoglycan content. Connect Tissue Res. 1987;16:237. doi: 10.3109/03008208709006979. [DOI] [PubMed] [Google Scholar]
- 30.Wight T.N. Merrilees M.J. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 31.Huang R. Merrilees M.J. Braun K. Beaumont B. Lemire J. Clowes A.W. Hinek A. Wight T.N. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 32.Bierbaum S. Douglas T. Hanke T. Scharnweber D. Tippelt S. Monsees T.K. Funk R.H. Worch H. Collageneous matrix coatings on titanium implants modified with decorin and chondroitin sulfate: characterization and influence on osteoblastic cells. J Biomed Mater Res A. 2006;77:551. doi: 10.1002/jbm.a.30572. [DOI] [PubMed] [Google Scholar]
- 33.Ra H.J. Parks W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faris B. Ferrera R. Toselli P. Nambu J. Gonnerman W.A. Franzblau C. Effect of varying amounts of ascorbate on collagen, elastin and lysyl oxidase synthesis in aortic smooth muscle cell cultures. Biochim Biophys Acta. 1984;797:71. doi: 10.1016/0304-4165(84)90383-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.R. Cha S.Y. Kim M.K. Kim J.C. Sung Y.K. Induction of versican by ascorbic acid 2-phosphate in dermal papilla cells. J Dermatol Sci. 2006;43:60. doi: 10.1016/j.jdermsci.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Bedell-Hogan D. Trackman P. Abrams W. Rosenbloom J. Kagan H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J Biol Chem. 1993;268:10345. [PubMed] [Google Scholar]
- 37.Ramirez F. Dietz H.C. Therapy insight: aortic aneurysm and dissection in Marfan's syndrome. Nat Clin Pract Cardiovasc Med. 2004;1:31. doi: 10.1038/ncpcardio0020. [DOI] [PubMed] [Google Scholar]
- 38.Hu Q. Loeys B.L. Coucke P.J. de Paepe A. Mecham R.P. Choi J. Davis E.C. Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15:3379. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 39.Hinek A. Braun K.R. Liu K. Wang Y. Wight T.N. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am J Pathol. 2004;164:119. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrilees M.J. Beaumont B. Braun K. Wight T. Use of the versican V3 gene to engineer an elastin-rich lipid-resistant neointima. Abstract FP59 presented at the 15th International Vascular Biology Meeting; Sydney, Australia. 2008. [Google Scholar]
- 41.Iocono J.A. Krummel T.M. Keefer K.A. Allison G.M. Paul H. Repeated additions of hyaluronan alters granulation tissue deposition in sponge implants in mice. Wound Repair Regen. 1998;6:442. doi: 10.1046/j.1524-475x.1998.60506.x. [DOI] [PubMed] [Google Scholar]
- 42.Perumal S. Antipova O. Orgel J.P. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci USA. 2008;105:2824. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teng Z.Z. Ji G.Y. Chu H.J. Li Z.Y. Zou L.J. Xu Z.Y. Huang S.D. Does PGA external stenting reduce compliance mismatch in venous grafts? Biomed Eng Online. 2007;6:12. doi: 10.1186/1475-925X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haruguchi H. Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs. 2003;6:227. doi: 10.1007/s10047-003-0232-x. [DOI] [PubMed] [Google Scholar]
- 45.Kute S.M. Vorp D.A. The effect of proximal artery flow on the hemodynamics at the distal anastomosis of a vascular bypass graft: computational study. J Biomech Eng. 2001;123:277. doi: 10.1115/1.1374203. [DOI] [PubMed] [Google Scholar]
- 46.Chamiot-Clerc P. Copie X. Renaud J.F. Safar M. Girerd X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc Res. 1998;37:811. doi: 10.1016/s0008-6363(97)00267-8. [DOI] [PubMed] [Google Scholar]
- 47.L'Heureux N. Dusserre N. Marini A. Garrido S. de la Fuente L. McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 48.Konig G. McAllister T.N. Dusserre N. Garrido S.A. Iyican C. Marini A. Fiorillo A. Avila H. Wystrychowski W. Zagalski K. Maruszewski M. Jones A.L. Cierpka L. de la Fuente L.M. L'Heureux N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]