Abstract
Advances in stem cell biology have afforded promising results for the generation of various cell types for therapies against devastating diseases. However, a prerequisite for realizing the therapeutic potential of stem cells is the development of bioprocesses for the production of stem cell progeny in quantities that satisfy clinical demands. Recent reports on the expansion and directed differentiation of human embryonic stem cells (hESCs) in scalable stirred-suspension bioreactors (SSBs) demonstrated that large-scale production of therapeutically useful hESC progeny is feasible with current state-of-the-art culture technologies. Stem cells have been cultured in SSBs as aggregates, in microcarrier suspension and after encapsulation. The various modes in which SSBs can be employed for the cultivation of hESCs and human induced pluripotent stem cells (hiPSCs) are described. To that end, this is the first account of hiPSC cultivation in a microcarrier stirred-suspension system. Given that cultured stem cells and their differentiated progeny are the actual products used in tissue engineering and cell therapies, the impact of bioreactor's operating conditions on stem cell self-renewal and commitment should be considered. The effects of variables specific to SSB operation on stem cell physiology are discussed. Finally, major challenges are presented which remain to be addressed before the mainstream use of SSBs for the large-scale culture of hESCs and hiPSCs.
Introduction
Recent advances in stem cell biology and biotechnology have sparked hope that stem/progenitor-based therapies will soon be available for devastating maladies such as Parkinson, cardiovascular diseases, and diabetes. Two distinctive attributes underlying the stem cells' therapeutic potential are their ability for multilineage differentiation and their extensive proliferative capacity. Taking advantage of these attributes will require the elucidation of mechanisms underlying the processes of stem cell self-renewal and commitment. Equally important is the development of bioprocesses for the robust production of stem cells and their progeny in clinically relevant quantities.
The number of cells utilized in cell therapy protocols including those involving the use of engineered tissues, falls in the range of a few tens of millions to a few billion.1 For example, 1 × 109 to 2 × 109 cardiomyocytes are required to replace damaged cardiac tissue after myocardial infarction.2 Moreover, ∼9000 islets/kg weight3 or 1.3 × 109 insulin-producing β-cells per 70-kg patient4 are needed for insulin independence after islet transplantation. A bioartificial liver device with ∼1010 hepatocytes (10–20% of native liver cells) can support a patient with fulminant hepatic failure.5
The production of such quantities of cells can be achieved with the use of bioreactors. Different designs have been employed for the culture of stem/progenitor cells, including bioreactors with fibrous matrices,6 flat-bed chambers with grooves,7 and fixed-bed culture vessels.8 Other features (e.g., electromechanical stimulation9) may also be incorporated in bioreactors for the culture of tissue constructs.
A broader overview of issues pertaining to bioprocess fundamentals for the production of stem/progenitor cells and their derivatives has been provided in recent reports.10–12 Here, we concentrated on the use of stirred-suspension bioreactors (SSBs) which offer distinct advantages for the expansion and directed differentiation of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) (i.e., human pluripotent stem cells) in clinically relevant amounts although the majority of the issues discussed pertain to most types of progenitor cells (e.g., hematopoietic, neuronal). In conventional stirred-suspension vessels, concentrations of 106–107 mammalian cells/mL are common. The production of 1 × 109 to 10 × 109 stem cell-derived cells for clinical use would require SSBs with working volumes of a few hundred milliliters to a few liters, although issues related to the respective efficiencies of differentiation and downstream processing (e.g., selection of a specific cell type) should be considered as well. SSBs also have a simple design, can be scaled-up, and allow for online monitoring and control of the culture variables affecting the self-renewal and directed differentiation of stem cells. Further, these bioreactors provide the operator with the flexibility of various modes including the culture of cells as aggregates, on microcarriers, or in scaffolds. Most importantly, SSBs are heavily utilized in the biotechnology industry. Hence, stem cell systems developed around this bioreactor type may be easier to translate to a commercial production setting than entirely novel designs.
In current embodiments of the SSB culture technology, cells are the means for the synthesis of products such as antibodies, enzymes, vaccines, and viruses. In the context of stem cell cultivation, the cells are the actual product.13 This spurs additional considerations regarding the selection of culture conditions and their effects on the self-renewal and differentiation state of cultured stem cells. In this article, we review advances in the use of SSBs for the scalable expansion and commitment of ESCs. Most importantly, our recent findings on the expansion of hESCs and hiPSCs in SSBs are presented. We further discuss challenges that must be overcome before such systems find widespread application in the generation of stem cell derivatives. Our results and recent reports from other groups on the propagation and differentiation of ESCs in SSBs point to the important role that this culture modality will play in the development of bioprocesses for the generation of therapeutically useful stem cells including hESCs, hiPSCs, adult progenitor cells, and their derivatives.
Modes of Human Pluripotent Stem Cell Culture in SSBs
Human ESCs are propagated as colonies on flat substrata, whereas the culture of hESCs in a three-dimensional (3D) configuration (e.g., embryoid bodies [EBs]) is typically associated with their differentiation. Depending on the desired progeny, stem cells may be cultured as monolayers (e.g., for obtaining pancreatic islet precursors14) or aggregates (e.g., cardiomyocyte differentiation15), or in scaffolds (e.g., hematopoietic commitment16). As discussed below, SSBs accommodate various such configurations while allowing for the dynamic adjustment of the culture microenvironment.
SSB culture of pluripotent stem cells as aggregates
Early attempts to grow ESCs in scalable suspension cultures capitalized on the tendency of undifferentiated stem cells to form aggregates or EBs. We and others17–19 have reported the expansion of mouse ESCs (mESCs) as aggregates in stirred-suspension vessels. In the presence of leukemia inhibitory factor (LIF), mESCs proliferate as aggregates without significant loss of viability (typically >85%) and with doubling times comparable to those of mESCs cultured in dishes. More importantly, the cells maintain their expression of pluripotency markers such as SSEA-1, Oct3/4A, and Nanog, even after multiple, successive passages.17,20 Besides propagation, SSBs have been used for differentiating mESCs to a particular progeny. Aggregates of mESCs carrying a neomycin phosphotransferase gene (conferring resistance to G418) flanked by the α-myosin heavy chain (α-MHC) promoter were coaxed to become cardiomyocytes in 250-mL spinner flasks equipped with a paddle-type impeller.21 The cells were cultured in fetal bovine serum-supplemented medium and 1 nM all-trans retinoic acid was added on day 9. At the same time, G418 was included for the selection of cardiomyocyte-like cells. By day 18, ∼1.9 × 107 cells were present and 71% of these was MF20+. A scaled-up version of the same system with a fully automated 2-L bioreactor featuring a pitched-blade impeller was reported later.22 Implementation of a similar differentiation/selection protocol led to the production of 1.28 × 109 ventricular- and atrial- or pacemaker-like cells after 18 days. The overall cardiomyocyte purity was greater than 99.99% as judged by immunostaining for α-MHC. Although these studies were performed with mESCs, they demonstrated that SSBs are promising vehicles for the large-scale expansion and differentiation of human pluripotent stem cells.
The culture of hESCs in suspension bioreactors was first reported by Gerecht-Nir et al.23 Preformed EBs of hESCs were maintained in slow-turning lateral vessels (STLVs) and high-aspect rotating vessels (HARVs) known for their low-shear environment. (These rotating culture vessels may not be considered strictly as SSBs.) Although massive agglomeration of EBs was observed in the HARV, the cells grew 70-fold in STLV, reaching 3.6 × 107 cells/mL after 28 days (Table 1). When compared with dish cultures of hESCs, the DNA concentration and total protein content were threefold higher in the STLV. The cells remained viable (despite an initial decrease in viability) and gave rise to cells of the three germ layers, suggesting that the bioreactor microenvironment did not alter the initial developmental events. The same group recently24 compared the cultivation of hESCs as EBs in STLVs, spinner flasks with either double glass ball bulb-shaped impeller or paddle impeller, Erlenmeyer flasks, and Petri dishes. The authors examined how shear at seeding, cell seeding concentration, and initial size of the hESC clumps affect the culture outcome in these vessels. Cells in glass bulb impeller-equipped spinner flasks (at 75 rpm) reached the highest concentration of 5 × 106 cells/mL or 6.4-fold expansion in 10 days compared with only 1.2-fold expansion for STLV cultures (rotated at 16 rpm). Human ESCs cultured in spinner flasks with paddle-type impeller (operated at 105 rpm) grew to 1.7 × 106 viable cells/mL (2.2-fold) over the same period. The resulting EBs contained cells of all germ layers, including beating cardiomyocyte-like cells, α-fetoprotein+ cells, CD31+ endothelial cells, and β3-tubulin+ neuroectoderm cells. Although it was unclear how the agitation rate (and corresponding shear levels) for each culture modality was selected, these findings illustrate the impact of bioreactor's operating conditions on the propagation of hESCs.
Table 1.
Expansion and Differentiation of Human Embryonic Stem Cells in Various Stirred-Suspension Bioreactors
| Culture mode | Expansion | Differentiation | Reference |
|---|---|---|---|
| Aggregate culture | 15-fold in 21 days (spinner flask with hanging magnetic stir bar) | CD34+CD31+ hematopoietic progenitors peaked (5–6%) at 14 days | Cameron et al.25 |
| 70-fold in 28 days (slow-turning lateral vessel) | EB formation—no specific cell lineage differentiation | Gerecht-Nir et al.23 | |
| 1.2-fold in 10 days (slow-turning lateral vessel) | EB formation—no specific cell lineage differentiation | Yirme et al.24 | |
| 6.4-fold in 10 days (spinner flask with glass ball impeller) | EB formation—no specific cell lineage differentiation | ||
| 2.2-fold in 10 days (spinner flask with paddle impeller) | EB formation—no specific cell lineage differentiation | ||
| 5.6-fold in 7 days (spinner flask with triangular impeller and glass-etched baffles) | Aggregate formation—no specific cell lineage differentiation | This study | |
| Microcarrier culture | 34- to 45-folda in 8 days | Differentiation to definitive endoderm (FOXA2+/SOX17+) cells with >80% efficiency | Lock et al.65 |
| 6.8-foldb in 14 days | – | Fernandes et al.67 | |
| 4.2-foldb in 7 days (5.8-foldb at day 5) | – | Oh et al.68 |
Based on initial hESCs attached onto microcarriers.
Based on hESCs inoculated for attachment to microcarriers. No information is available on the attachment efficiency of hESCs onto microcarriers.
hESCs, human embryonic stem cells; EB, embryoid body.
Cameron et al.25 also reported the culture of hESCs as EBs in spinner flasks with medium containing serum. The cells became mainly hematopoietic progenitors but cells that expressed ectoderm- and endoderm-specific genes were also present. A 15-fold expansion was measured over 21 days of bioreactor culture compared with only 4-fold in static cultures.
Unlike mESCs, hESCs differentiate extensively when cultured as aggregates in SSBs. The differences in the culture of hESCs and mESCs hamper the direct translation of earlier findings from mESC studies to the culture of hESCs in SSBs. For example, we have demonstrated17 that mESCs can be adapted to defined serum-free medium containing LIF for their expansion in dishes and spinner flasks. However, LIF is not sufficient for the maintenance of hESC self-renewal.26,27 Elucidation of the mechanisms preventing the differentiation of hESCs will clear the way for developing chemically defined media for hESCs. Even so, multiple efforts on the development of defined media for hESC culture have been reported as discussed elsewhere in this article.
Current hESC methods also require that hESC colonies are only partially dissociated into small clumps instead of single cells to remain viable and self-renewing. In contrast, mESCs can be passaged as single cells without loss of their pluripotent status. Seeding-dispersed mESCs in a stirred-suspension vessel favors a fairly narrow distribution of aggregate sizes which can be controlled through adjustments in the agitation rate.17,19 This in turn leads to a reduction in the uncontrolled differentiation of ESCs as observed in oversized aggregates in spinner flasks. Inoculation of hESCs as small clusters with variable size results in an uneven distribution, and size differences are further magnified by cell proliferation in the bioreactor.
Hence, devising methods for the seeding of hESCs as single cells may be advantageous for their propagation in suspension bioreactors. Watanabe et al.28 demonstrated that when hESCs are treated with the selective Rho-associated kinase (ROCK) inhibitor Y-27632, dissociation-induced apoptosis is markedly diminished even in serum-free suspension cultures. Cells treated with Y-27632 form floating aggregates in low-adhesion dishes without loss of their viability. Moreover, ectoderm differentiation of these cells was demonstrated. Others have also shown that exposure to Y-27632 promotes hESC self-renewal29 even after cryopreservation,30 suggesting that the observed effect is independent of the hESC line used. Inhibition of the Rho-associated kinase in hESCs may be combined with hESC colony dissociation methods which limit the risk for unprompted differentiation. Bajpai et al.31 showed that hESC colonies treated with accutase (a commercially available enzyme mix with proteolytic and DNAse activities) can be dissociated into single cells and passaged multiple times without differentiation. Our group (Fig. 1) and others are currently working toward adapting such protocols to the culture of hESCs in stirred-suspension vessels and interesting findings are anticipated in the near future.
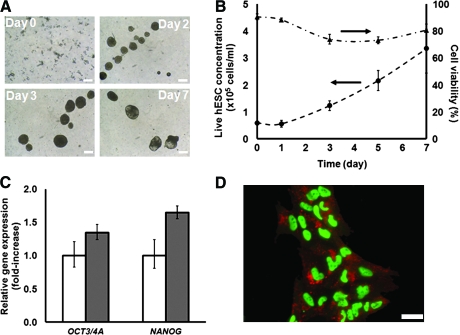
FIG. 1.
Human embryonic stem cells (hESCs) propagated as aggregates in stirred-suspension culture. (A) H1 hESC colonies were dissociated into single cells using accutase31 in the presence of the Rho-associated kinase (ROCK) inhibitor Y-27632. Single cells were incubated in mouse embryonic fibroblast (mEF)-conditioned medium with 10% Matrigel (to facilitate initial aggregation) and 10 μM Y-27632 for 30 min. Subsequently, the cell suspension was introduced into spinner flasks with mEF-conditioned medium and the agitation rate was set to 60 rpm. The cells formed stable aggregates and grew as shown in the micrographs from days 0, 2, 3, to 7. (B) Live cell concentration and viability of H1 hESCs cultured in a spinner flask with mEF-conditioned medium supplemented with Y-27632. The flask was seeded with 6 × 104 hESCs/mL. The upper arrow points indicates that cell viability values for the curve right under it should be read on the vertical axis on the right. Similarly, the lower arrow indicates that cell concentration values for the curve below this arrow are found on the vertical axis on the left. (C) Quantitative polymerase chain reaction was employed to probe the expression of pluripotency genes by hESCs cultured in the bioreactor (dark bars). White bars represent the expression of the corresponding genes in hESCs maintained in Matrigel-coated dishes (control). Values are shown as mean ± standard deviation (n = 3). (D) Cells from the bioreactor were plated and immunostained for OCT4 (green) and SSEA-4 (red). Scale bars: (A) 200 μm, (D) 50 μm. Color images available online at www.liebertonline.com/ten.
Culture of encapsulated stem cells in stirred suspension
The excessive agglomeration of ESC aggregates or EBs reduces cell yields32 and may be circumvented by cell encapsulation. Magyar et al.33 showed almost a decade ago that mESCs forming EBs can be encapsulated in alginate microbeads. The alginate concentration affected the 3D organization of cells because cysts were observed within EBs in 1.1% (w/v) but not in 1.6% (w/v) alginate capsules. When the encapsulated cells were seeded in gelatin-coated dishes, spontaneously beating cardiomyocyte-like cells and smooth muscle cells (in the presence of retinoic acid) emerged. More recently, mESCs encapsulated in alginate beads were cultured in 50 mL HARV bioreactors while being coaxed toward osteogenic lineages.34
Human ESC aggregates have also been encapsulated in 1.1% (w/v) alginate beads and cultured for up to 260 days without any passaging or enzymatic/mechanical manipulation.35 Approximately 5–7 hESC aggregates were encapsulated in each alginate bead and grew to a maximum size of 400–500 μm without outgrowing the alginate matrix. The cultured hESCs expressed pluripotency markers such as OCT-4, SSEA-4, TRA-1-60, and TRA-1-81. After their prolonged culture, these cells gave rise to cells of the three germ layers, that is, chondrocyte-, neuronal-, and pneumocyte-like cells. In addition to alginate, poly(lactic-co-glycolic acid)/poly(l-lactic acid) scaffolds,36 hydrogels of agarose,37 synthetic semiinterpenetrating polymers,38 and hyaluronic acid39 have been used for the encapsulation and culture of hESCs.
Most of these matrices have been shown to support self-renewal and/or differentiation of hESCs in static cultures. In principle, stem cells can be cultured in SSBs when seeded in scaffolds with particular biochemical and mechanical features. Scaffolds have been used in suspension bioreactor cultures to enhance the formation of 3D tissues and the differentiation of stem/progenitor cells to myocardium,40 bone,41 cartilage,42,43 pancreatic islets,44 hematopoietic cells,16 and vascular grafts.45 The scaffold environment can be customized by embedding primordial tissue,46 growth factors,47 and small functional groups48 including moieties for the binding of cells or controlled degradation of the matrix49 (e.g., see Ref.50). Thus, microenvironments can be created within SSBs that are suitable for the self-renewal of stem cells or for guiding their differentiation along with promoting the organization of cells in 3D structures akin to those of native tissues. Such encapsulated tissue constructs are less susceptible to immunorejection by the host and their delivery is better targeted as opposed to suspensions of stem cell-derived cells.2 Moreover, the in vivo degradation kinetics of the scaffold can be tuned permitting sufficient time for the vascularization of the engineered tissue and its functional integration with the host organ.
Microcarrier culture of pluripotent stem cells
Microcarrier bioreactors have been used for the culture of several cell types,51–54 including adult progenitor cells.55,56 Several attributes of the microcarrier SSB make it attractive for hESC expansion and directed differentiation. First, microcarrier systems afford the flexibility of culturing cells either within macroporous or on compact microcarriers. For example, mESCs cultured on macroporous beads can be coaxed to cardiomyocytes.40 Conversely, cells on the surface of compact beads assume a configuration akin to that in monolayers—unlike in EBs—and have direct exposure to the bulk medium. This is important for preserving the self-renewal capacity of hESCs or when efficient differentiation (e.g., to endoderm14) is hindered by the formation of EBs. Second, microcarrier cultures are characterized by high surface-to-volume ratio, thereby accommodating higher cell densities compared with those in static cultures. More importantly, the available area for cell growth can be adjusted easily by changing the amount of microcarriers. Culture of hESCs on microcarriers—effectively at a lower local density—may limit the unregulated loss of pluripotency observed in EBs. Third, high cell concentrations are also facilitated by the tight control and monitoring of the SSB microenvironment. Such control of the culture is very important not only for the propagation of uncommitted hESCs but also for their directed differentiation upon treatment with multiple soluble agents added to/removed from the medium in a particular sequence.
mESCs proliferate on microporous collagen-coated dextran beads (Cytodex 3), glass microcarriers, and macroporous gelatin-based beads (Cultispher S) in spinner flasks.19,57,58 Under different inoculated cell densities and microcarrier concentrations, mESCs on microcarriers increased from ∼70-fold (8 days) to ∼190-fold (15 days). The cultured mESCs expressed Oct4, Nanog, and SSEA-1, and when dissociated from the beads, they formed EBs yielding cells with markers such as Flk-1, CD34 and α-MHC (mesoderm), HNF-3β19 (endoderm), and β3-tubulin57 (ectoderm).
The use of multiple bead types in the above reports illustrates another advantage of the microcarrier culture of ESCs. Microcarriers can be customized,59 for example, by attaching various synthetic peptides60 or extracellular matrix molecules, to accommodate the adhesion needs of diverse cell types or hESC lines. This is important considering that different extracellular matrix molecules support in varying degrees the proliferation of pluripotent hESCs. Beads coated with molecules such as collagen (a component of Matrigel) or fibronectin (which also supports hESC growth61) are commercially available. In fact, different types of microcarriers were recently tested with hESCs in low-adherence plates62,63 and mixed results were shown. For example, Nie et al.63 reported that Cytodex 3 beads appeared to promote better attachment and viability of hESCs, whereas others62 observed that hESCs on these beads exhibited the poorest growth with little or no recovery of viable cells after 48–72 h. Such discrepancies may be due to the different hESC lines used. Nonetheless, the adhesion efficiency of hESCs increased when the beads were coated with Matrigel or seeded with mouse embryonic fibroblasts (mEFs). Cells grew on beads at a comparable rate to hESCs expanded in dishes, remained positive for pluripotency markers (e.g., OCT4, TRA-1-81), and when induced they differentiated to cells of the three germ layers. In fact, hESCs cultured on microcarriers for multiple passages and cryopreserved displayed a better recovery after thawing compared with freely suspended hESCs.63
We demonstrated for the first time64,65 that hESCs can be cultured on microcarriers in a SSB. Human ESCs grew at lower agitation rates (45 and 60 rpm) with a doubling time comparable to that of hESCs maintained in static cultures (∼36 h), whereas growth was severely retarded at a higher stirring speed (80 rpm). Moreover, hESCs seeded at 5 × 104 to 20 × 104 cells/mL populated the Matrigel-coated beads with 23–30% efficiency compared with only ∼7% observed in dish cultures.66 The cells were positive for OCT3/4A, NANOG, REX1, SSEA-4, and TRA-1-81 during their expansion, whereas the expression of lineage-specific genes was either minimal or absent. These findings show that propagation of undifferentiated hESCs in microcarrier cultures is feasible under appropriately selected operating conditions. Accordingly, more recently, others67,68 have also reported the expansion of hESCs on microcarriers in spinner flasks. Interestingly enough, we have applied successfully the same methodology for the culture of B12-3 hiPSCs69 on microcarriers in SSBs (Fig. 2).
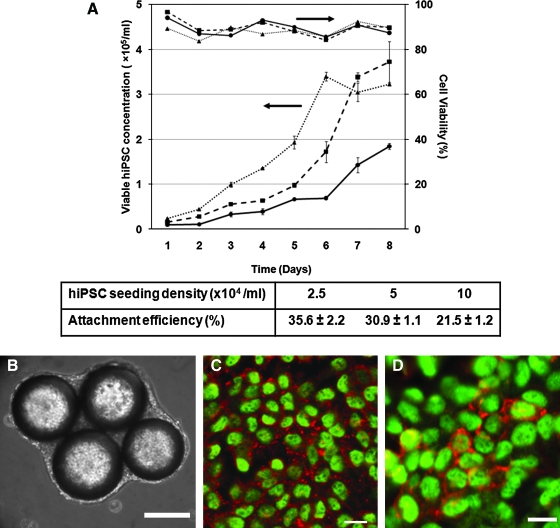
FIG. 2.
Expansion of human induced pluripotent stem cells (hiPSCs) in a microcarrier bioreactor. Human iPSCs (B12-3, see Ref.69) were seeded on Matrigel-coated beads and cultured in a spinner flask as described previously for the culture of hESCs on microcarriers.65 (A) Temporal profiles of hiPSC concentration and viability for different seeding cell densities: solid line with circles: 0.25 × 105 hiPSCs/mL; dashed line with squares: 0.5 × 105 hiPSCs/mL; dotted line with triangles: 1 × 105 hiPSCs/mL. The cells were cultured at 45 rpm. The efficiencies of initial attachment of hiPSCs on the beads for the above seeding densities are shown in the table as mean ± standard deviation. The upper arrow points indicates that cell viability values for the curve right under it should be read on the vertical axis on the right. Similarly, the lower arrow indicates that cell concentration values for the curve below this arrow are found on the vertical axis on the left. (B) hiPSCs on microcarriers on day 6. (C) hiPSCs dissociated from beads on day 8 were plated and stained for OCT3/4A (green) and SSEA4 (red), or (D) OCT3/4A (green) and TRA-1-60 (red). Scale bars: (B) 200 μm, (C, D) 20 μm. Color images available online at www.liebertonline.com/ten.
In the same study,65 we directed hESCs in a microcarrier bioreactor to definitive endoderm cells, which are precursors of islets and hepatocytes. For this purpose, H1 and H9 hESCs expanded on microbeads were treated with activin and Wnt3a in a medium with 0–2% serum. Cells coexpressing FOXA2 and SOX17 (signifying definitive endoderm cells) emerged with efficiencies >80% (compared with ∼63% in dishes). The differentiating cells transitioned through a mesendoderm stage as reported previously for mESCs70 and hESCs.14 Subsequent treatments of these cells with appropriate factors (e.g., KGF) yielded cells expressing posterior foregut markers. Our results support the use of microcarrier SSBs for the directed differentiation of hESCs en masse.
Even so, several issues should be addressed before microcarrier systems can be used for commercial hESC-based applications. For example, the hESC subcloning efficiency on beads is low (although better than in static cultures). Coating the microcarriers with Matrigel has proven effective in improving the poor adhesion of hESCs but the compound's animal origin makes it unsuitable for the culture of cells for therapies. The same is true about the use of mEFs to coat beads63 for hESC culture although clinical-grade human fibroblasts could be employed.71 The initial attachment of cells to beads may be improved by functionalizing the beads with animal product-free molecules, which enhances hESC adhesion. The observed low subcloning efficiency of hESCs in microcarrier cultures is also due to the seeding of hESCs as clumps instead as single cells on beads. Methods for the dispersion of hESC colonies (see SSB culture of pluripotent stem cells as aggregates) into single cells prior to seeding onto microcarriers may alleviate this issue. In addition, we observed a decline in the hESC viability during switching from expansion to differentiation conditions in the bioreactor. This coincided with the withdrawal of serum replacement supplements used during the expansion step. Cell death was also noted during differentiation of ESCs in static cultures, suggesting that the bioreactor environment per se is not the cause of this decline in viability. However, alternative formulations of defined media for optimal preservation of the viability of propagating/differentiating stem cell populations will play a central role in future implementations of such scalable culture systems (see also Chemically defined media for stem cell culture).
Lastly, dissociating and separating large quantities of cells from beads after expansion/differentiation and between passages is challenging. Different separation methods have been described, including low-speed density centrifugation72 and tangential flow (but not dead-end) filtration.73 Effective deployment of microcarrier-based bioprocesses for the expansion/directed differentiation of hESCs will rely upon the engineering of high-throughput strategies for efficient cell–bead separation and cell retention.
Major Variables in the Culture of Stem Cells in SSBs
The physiology of stem cells in conventional static cultures is affected largely by the global (bulk concentration) and local availabilities (concentration gradients) of oxygen, nutrients, and growth factors. The outcome of ESC culture depends on factors such as medium composition,17 pH,74 and cell inoculation density.18 However, a distinct feature of the SSB culture is stirring, which creates a homogeneous but dynamic environment. The agitation-induced shear and the availability of dissolved oxygen are discussed in more detail here. Besides their effects on viability, shear and dissolved oxygen may alter the gene expression of ESCs and thus their fate decision processes.
Agitation
Agitation is essential for ensuring that all cells in stirred tank cultures are exposed to a medium with same composition, and for keeping the aggregates or microcarriers suspended. Maintenance of stem cell self-renewal may require a homogeneous environment considering that differentiation is associated with the presence of concentration gradients of (e.g., growth) factors.75 Stirring, however, creates shear which may aid in controlling the size of aggregates by avoiding the formation of oversized clusters (as shown for mESCs17,18 and other cell types76,77 cultured in spinner flasks) or the agglomeration of cell-laden beads. The maximum mean aggregate size 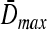 is related to adjustable SSB culture variables such as the kinematic viscosity ν of the medium and the viscous energy dissipation per unit fluid mass ε (see Eq. 9) as follows:
is related to adjustable SSB culture variables such as the kinematic viscosity ν of the medium and the viscous energy dissipation per unit fluid mass ε (see Eq. 9) as follows:
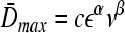 |
(1) |
Values for the parameters c, α, and β can be estimated from experimental data as shown for BHK cells78 and neural stem cells79 cultured in spinner flasks with ball and paddle impellers.
However, it is the damage which shear can cause on the cultured cells that makes essential the selection of an appropriate agitation rate N. In addition, shear depends on the geometric characteristics of the vessel and the impeller, the presence of baffles, and the physical properties of the medium. An estimate of the shear stress in an SSB is the integrated shear factor80,81:
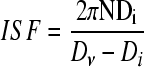 |
(2) |
where Di and Dν are the diameters of the impeller and the vessel, respectively. Alternatively, a time-averaged shear rate 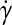 was proposed by Croughan et al.82:
was proposed by Croughan et al.82:
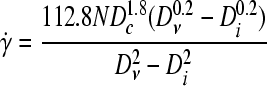 |
(3) |
The diameter of the vortex zone Dc is given as
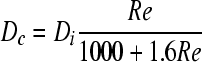 |
(4) |
and the Reynolds number as
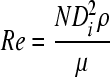 |
(5) |
with ρ and μ being the density and dynamic viscosity of the medium, respectively. The maximum time-averaged shear rate
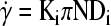 |
(6) |
occurs in the jet off the impeller and depends on the impeller type (Ki ∼ 0.4 cm−1 for flat-blade impeller). Then, the corresponding maximum shear stress is given as
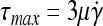 |
(7) |
Another expression for τmax was later developed based on a model flow-field assuming isotropic turbulence.83 Fluid perturbations at the impeller region create a cascade of eddies with decreasing size down to the viscous dissipation range. The size η of the smallest (known as Kolmogorov scale84) eddies can be calculated as
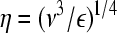 |
(8) |
and the viscous energy dissipation per unit fluid mass is
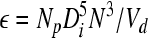 |
(9) |
with Np and Vd being the dimensionless power number and the dissipation volume, respectively. The eddy size decreases as the agitation speed increases. Kolmogorov-eddies with sizes similar to those of microcarriers or aggregates are detrimental for cultured cells.82,85,86 Bead-to-bead collisions can also be a major source of cell damage although their effects are more difficult to model.84 Single cells in suspension are also damaged by similar-size eddies in the absence of bubbles87,88 (see also Dissolved oxygen) but the formation of such small eddies requires impractically intense agitation. Thus, suspended single cells are damaged at much higher agitation rates compared with cells in aggregates or on microcarriers. Under this model, the maximum shear stress89 which the cells experience can be estimated from
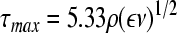 |
(10) |
Most reports on the effects of shear on cells focus on cell damage and death. Shear stress at ∼0.65 Pa is sufficient to remove human embryonic kidney cells cultured on surfaces,90 whereas significant damage ensues at 26 dyn/cm2. Others89 have suggested that shear stress at a range of 15–30 dyn/cm2 reduces cell viability. Obviously, these thresholds for shear stress are cell type-dependent and the conditions can be chosen to lower the shear in the SSB to a level that the cells can tolerate well. Cormier et al.18 successfully expanded mESCs as aggregates in spinner flasks at 80–100 rpm which corresponds to a maximum shear stress ranging between 4.5 and 6.1 dyn/cm2. However, extensive mESC damage and no proliferation were noted when stirring was increased to 120 rpm or a maximum shear stress of 7.8 dyn/cm2. For comparison, STLVs which have also been used for ESC culture exhibit very low shear stress levels91 (∼10−2 dyn/cm2).
The damaging effects of shear on cells cultured in SSBs may be reduced with the use of additives (shear protectants). Various additives have been studied for protecting animal cells against agitation (and aeration) damage, including serum, pluronic polyols, celluloses, and dextrans. These compounds exert their effects by making cells more resistant to shear (e.g., physical incorporation in the cell membrane) or by decreasing the level of transmitted shear forces to the cells (e.g., by varying the viscosity of the culture medium).92 Protein-containing additives are less desirable not only because of their higher cost but also because of the complications they introduce in downstream processing and their potential effects on stem cell physiological state. The impact of various shear protectants on growth kinetics and viability has been studied for different types of cells cultured in SSBs. Recently, dextran (up to 50 g/L) or carboxymethylcellulose (up to 10 g/L) was used to modulate the viscosity of medium for the culture of mouse neural progenitor cells as aggregates in SSBs,79 and a correlation (using Eq. 1) was derived for controlling the average size of mouse neural progenitor cell aggregates. A similar methodology as described previously for suspended cells (see Ref.92 and citations therein) and microcarriers93 can be implemented for assessing the protective effects of a candidate additive on ESCs cultured in SSBs. To our knowledge, however, a detailed analysis of the effects of shear-protection additives on ESC proliferation, viability, and pluripotency has not been carried out to date.
Even when the operating conditions are such so that the maximum shear is kept well below that causing cell damage, shear can have a significant impact on stem cell self-renewal. The role of mechanical force transduction on ESC physiology has not been fully elucidated. Human ESCs cultured under cyclic strain retain their pluripotency94 most likely because of the observed induction of transforming growth factor-β/activin/nodal expression.95 mESCs exposed to 10 dyn/cm2 raise their histone acetylase activity, resulting in remodeling of their chromatin structure with concomitant increase in cardiac- and vascular-specific markers.96 Even mESC-derived Flk-1+ cells subjected to a shear stress of 1.5–10 dyn/cm2 exhibit increased proliferation and expression of endothelial proteins (Flt-1, VE-cadherin, and PECAM-1).97 In addition, preferential differentiation toward cardiac cells has been noted for mESCs cultured in rotary orbital suspension and spinner flasks.20,98 Further research is warranted into the signaling mechanisms triggered by shear and regulating the fate decisions in stem cells.99 Notably, efforts in this direction have been intensified with the design and fabrication of microfluidic devices which allow for close monitoring and control of the stem cell microenvironment.100–102
Dissolved oxygen
Stem cells in the developing embryo experience low oxygen levels. Oxygen tension in the mammalian reproductive tract is ∼60 mmHg in hamsters and rabbits and decreases to 24 mmHg during blastocyst formation and implantation.103 The uterus of rhesus monkeys is also characterized by low oxygen levels of 11 mmHg. Although the reasons for such low oxygen tension are unclear, this environment is hypothesized to protect the ESCs from oxygen toxicity while inducing the upregulation of an array of genes orchestrating the earliest steps of development.
Studies on mESCs have suggested that hypoxia promotes differentiation through negative regulation of the LIF-STAT3 signaling by the hypoxia-inducible factor-1α gene.104 This is in line with the observed increase in the efficiency of cardiogenic differentiation of mESCs105 and hESCs106 cultured under hypoxic conditions (4% oxygen) in bioreactors. Moreover, fewer spontaneous chromosomal aberrations have been observed66 when hESCs are cultured at 2% versus 21% oxygen. Comparison of gene expression between hESCs maintained under 4% or 20% reveals the differential regulation of (mainly hypoxia-inducible factor-controlled) genes encoding enzymes for carbohydrate metabolism and cellular redox state, although pluripotency markers such as OCT4 and NANOG remained largely unaffected.107 Moreover, hESCs cultured at 20% oxygen also show upregulation of genes associated with lineage specification, suggesting that hypoxic conditions favor the maintenance of pluripotency. Nevertheless, the effects of hypoxia on ESCs remain unsettled. For example, Chen et al.108 showed that the culture of hESCs at 5% oxygen is not advantageous for keeping the cells undifferentiated compared with their culture at normoxia.
These observations point to the impact of oxygen regulation on the outcome of ESC cultures in SSBs. Cells residing within clusters experience oxygen transfer limitations with increasing aggregate size. Oxygen diffusion models developed for multicellular spheroids or tumors109–111 can be applied to ESC clusters.112 However, one should also take into account the typically diverse ultrastructural attributes of ESC aggregates. Stem cell clusters may undergo compaction as observed in other cell types,113 contain cysts, or form external layers of extracellular matrix with variable thickness.114 The transfer of oxygen from the bulk medium should be at least equal to the specific oxygen consumption rate (OCR) of ESCs. We have measured an OCR of 1.7 × 10−18 mol oxygen/(cell)(s) for H9 hESCs cultured as aggregates (maximum size of 400 μm) in mEF-conditioned medium. Because of its strong dependence on culture conditions, the OCR should be determined experimentally for a particular system.
Besides the profile of oxygen inside ESC aggregates, the transfer of oxygen in the bulk medium becomes particularly important as the size of the culture vessel increases. Larger vessels typically feature modules for forced oxygenation (sparging) because headspace aeration is not sufficient to maintain proper levels of dissolved oxygen. However, the resulting bubbles contribute to the shear stress (due to agitation) inside the bioreactor. The cell damage caused by cell-to-bubble interactions in the bulk is not as significant115 as that induced by bubble coalescence and breakup close to the air–medium interphase.84 This problem can be ameliorated by adjusting the sparging gas flow rate and using surface tension-lowering additives (e.g., pluronics). As already mentioned, a detailed analysis of the effects of such additives on ESC proliferation and pluripotency is not yet available. mESCs cultured as EBs in a 2-L stirred tank with sparging and in the presence of 0.0125% antifoam C retained their viability and adopted a cardiomyocyte-like phenotype.22 Whether expansion of undifferentiated stem cells in a bioreactor with active aeration (as opposed to oxygen transfer from the headspace) in the presence of surface active agents is feasible remains an open question.
Large-Scale Bioreactor Culture of Human Pluripotent Stem Cells: Challenges
Despite recent advances in the culture of human stem cells in SSBs, multiple issues remain to be addressed before these bioreactors can be utilized in clinical stem cell-based applications. Design rules and pertinent heuristics are well established for the scale-up of SSBs for animal cell culture but hESCs pose unique challenges. After extensive mitosis in the bioreactor, hESCs may develop karyotypic abnormalities associated with prolonged culture,116–118 which render them unfit for clinical use. Unfortunately, no methods exist for the real-time probing of cultured hESCs for chromosomal aberrations. Similarly, methods are lacking for online monitoring of the differentiation of hESCs to a particular progeny in bioreactors. Although reporter genes with lineage-specific promoters have been introduced to hESCs for optimization of differentiation protocols, such genetic manipulations should be avoided for stem cells intended for therapies. Moreover, differentiation of hESCs typically results in heterogeneous populations and the presence of undifferentiated cells can lead to tumor formation. Accordingly, new high-fidelity technologies will be necessary for the sorting of un/differentiated cells in medically relevant quantities based on specific markers.
The design of large-scale bioprocesses should conform to good manufacturing practices (GMPs) for the production of clinical-grade stem cell derivatives. GMPs go beyond the bioreactor and encompass automation for process monitoring and control, maintenance of sterile conditions, and efficient operation management. Stem cells used in the process should also conform to GMPs mainly from a safety standpoint. Unfortunately, most hESC lines which are available today have been exposed to animal cells or proteins rendering them unsuitable for therapies. For example, nonhuman immunogenic molecules have been detected in hESCs cultured on mEFs.119 Human ESCs exhibit low immunogenicity,120,121 but the changes in immunocompatibility brought about by their culture should be further elucidated. Addressing these concerns will require the development of new GMP-compliant hESC lines. Beyond the significant cost of deriving and maintaining new hESC lines, nontrivial ethical issues stem from the use of human oocytes for the generation of these cells. However, alternative methods for deriving hESCs122,123 and the recent generation of hiPSCs124,125 may alleviate such concerns. The generation of hiPSCs from the adult cells of a patient may also abolish the need for costly generation and maintenance of numerous hESC lines with diverse human leukocyte antigen (HLA) profiles.
As the bioreactor size for stem cell products increases, the cost per unit product reduces (economy of scale). Yet, small-scale production may be necessary to generate patient-specific cells. If so, many of the scale-up issues for SSBs are put at ease. As already mentioned, bioreactors with working volumes of a few hundred milliliters to a few liters may be sufficient to produce the cell quantities needed per patient. However, the development of defined media for the culture of human pluripotent stem cells and the modeling of stem cell populations are deemed significant irrespective of the scale of the process and are discussed below.
Chemically defined media for stem cell culture
Human ESCs were first derived and cultured on layers of mEFs or feeder cells126 isolated from the nonvisceral tissue of day E13.5 embryos. The feeder cells are mitotically inactivated (chemically or by irradiation) to avoid the risk of fast-growing strains overtaking the hESC culture. The inactivation of feeder cells, however, does not eliminate the risk for transmission of mEF-borne pathogens to stem cells. Human fibroblasts127–129 including commercially available fibroblast lines have been used as feeder cells for the derivation and culture of hESCs for over 20–30 passages130 in media supplemented with a serum replacer.131 Even so, many of these mEF surrogates are generally maintained in fetal bovine serum, posing again an increased risk of xenopathogen contamination. Therefore, research has been spurred into alternative, feeder-free systems for the maintenance of hESCs or their derivatives. The issue of eliminating the use of feeder cells is accentuated if one considers the scalability of bioprocesses for stem cell production.
A common practice is the cultivation of hESCs on Matrigel-coated surfaces without mEFs but in mEF-conditioned medium supplemented with basic fibroblast growth factor132 and serum replacer. The bone morphogenetic protein suppressor Noggin appears to further limit the aberrant differentiation of cultured hESCs.133 Even basic fibroblast growth factor alone was shown to support the propagation of undifferentiated H9 and H7 hESCs for 15 passages without the use of conditioned medium.134 These have been major steps toward abolishing the need for coculture of hESCs with xenogeneic feeder cells.
Nonetheless, hESCs on Matrigel tend to differentiate over time despite the use of pro–self-renewal factors.135 With clinical applications of stem cells in mind, these approaches still do not address the possible zoonosis associated with the use of Matrigel (a mixture of ECM molecules extracted from mouse Engelbreth–Holm–Swarm tumors136) or feeder cell-conditioned medium. To that end, Ludwig and coworkers137 showed that hESCs can be sustained for up to 6 months without feeder cells in medium containing only recombinant or human proteins. Yao et al.138 also demonstrated the maintenance of hESCs and their directed differentiation in a chemically defined medium with the B27 and N2 supplements.
Ultimately, media for the cultivation of pluripotent hESCs and hiPSCs should be not only free of animal products but also inexpensive, thereby making future bioprocesses economically attractive. Given the high cost of growth factors, attempts have been made to minimize their use, for example, by including natural and synthetic small molecules that support the stem cell self-renewal or differentiation and can be isolated/synthesized economically. Small molecules have been shown to target signal transduction pathways such as the Wnt, Hedgehog, retinoid, and NF-κB, which either alone or in concert dictate the fate of stem cells (Table 2).139–147 Sato et al.148 demonstrated that mESCs and hESCs remain undifferentiated in culture when treated with 6-bromoindirubin-3′-oxime, which activates the canonical Wnt/β-catenin pathway by selectively inhibiting GSK-3β. Another small molecule, IQ-1, was shown to maintain mESC pluripotency by preventing β-catenin from switching between transcriptional coactivators CREB binding protein (CBP) and p300.149 Small molecules targeting the Wnt or Hedgehog pathway also promote differentiation of stem cells to cardiac,150 hematopoietic,151 neuronal,152 and bone153 cell phenotypes. With the advent of high-throughput screening technologies, small-molecule libraries154 are analyzed to identify molecular interactions leading to particular stem cell responses (see Refs.155,156). Structural and functional information for such small molecules can be found at the public database PubChem (http://pubchem.ncbi.nlm.nih.gov/) established as part of the Molecular Libraries Roadmap Initiative by the National Institutes of Health.
Table 2.
Small Molecules Supporting the Self-Renewal and/or Differentiation of Human Embryonic Stem Cells
| Molecule | Concentration | Reference |
|---|---|---|
| 6-Bromoindirubin-3′-oxime—a Glycogen synthase kinase 3 (GSK-3) inhibitor; maintains ESC self-renewal | 2 μM | Sato et al.148 |
| Stauprimide—downregulates the expression of c-Myc; increases the efficiency of directed differentiation | 1 μM | Zhu et al.146 |
| 5-Aza-2′-deoxycytidine—a DNA demethylation agent; promotes cardiac differentiation | 1–10 μM | Xu et al.15 |
| Ascorbic acid—promotes cardiac differentiation | 0.1 mM | Passier et al.143 |
| Dorsomorphin—a bone morphogenetic protein inhibitor; promotes neural differentiation | 200 nM | Wada et al.144 |
| Purmorphamine—an activator of Hedgehog pathway; induces neuronal differentiation | 0.5–2 μM | Li et al.142 |
| Rosiglitazone—a peroxisome proliferator-activated receptor gamma (PPARγ) agonist; promotes adipogenic differentiation | 1 μM | Xiong et al.145 |
| SAG—an agonist of Hedgehog signaling; enhances spinal neuron differentiation | 0.01–1 μM | Wada et al.144 |
| SB203580—a p38 mitogen-activated protein kinase (MAPK) inhibitor; promotes cardiac differentiation | <10 μM | Graichen et al.147 |
Predictive models for stem cell populations
An important aspect of a stem cell bioprocess is the use of models for predicting the number and fate of stem cells with respect to adjustable culture variables. In turn, such models form the basis for process control and optimization strategies toward a desired outcome. Various deterministic, stochastic, and hybrid quantitative frameworks have been reported for stem cell populations and have been reviewed elsewhere.157–160 Here, we discuss population balance equation (PBE)-based approaches which have been applied to cells cultured in bioreactors and can be extended to include features pertinent to stem cells.
The physiological state of cultured stem cells is affected by multiple factors (e.g., cytokines, serum) including bioreactor-specific parameters (e.g., agitation rate). Segregated PBE models can be used to describe the diversity (e.g., stem cell mass, age, and differentiation status) of stem cell ensembles through frequency functions representing the probability that a cell, picked at random, is in a particular state. Population balance models have been employed in a wide range of processes including aerosol dynamics, particle aggregation, crystallization, protein precipitation, and microbial and animal cell population dynamics (in culture or in vivo).161–166 PBE-based approaches have been utilized to predict and control the property distributions of cells cultured in bioreactors.167,168 The number distribution function 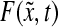 varies with time t as described by a general cell PBE:
varies with time t as described by a general cell PBE:
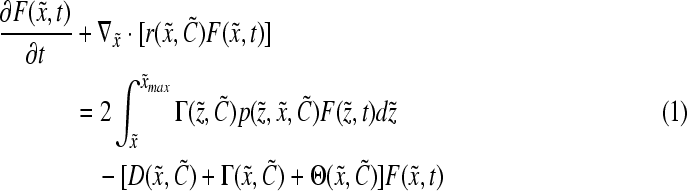 |
(11) |
where 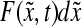 represents the number of cells per unit of culture volume that at time t have physiological state representation between
represents the number of cells per unit of culture volume that at time t have physiological state representation between 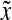 and
and 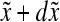 . Each cell is characterized by a set of variables (e.g., mass, protein) contained in the vector
. Each cell is characterized by a set of variables (e.g., mass, protein) contained in the vector 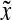 , and
, and 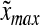 corresponds to the maximum values of these variables. The vector
corresponds to the maximum values of these variables. The vector 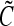 encompasses the concentration of nutrients, cytokines, and other factors that affect cell proliferation, differentiation, and death. The distribution of birth state,
encompasses the concentration of nutrients, cytokines, and other factors that affect cell proliferation, differentiation, and death. The distribution of birth state, 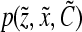 , describes how the cellular material is partitioned among daughter cells. The quantity
, describes how the cellular material is partitioned among daughter cells. The quantity 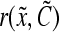 is the single-cell growth rate167 and the functions
is the single-cell growth rate167 and the functions 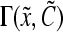 and
and 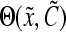 are the cell division and death intensities, respectively. Cell differentiation is represented by
are the cell division and death intensities, respectively. Cell differentiation is represented by 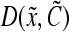 . Additional terms can be incorporated for conditions specific to each culture system (e.g., dilution rate for bioreactors with continuous operation). The PBEs can be coupled to expressions describing the variation of the elements of
. Additional terms can be incorporated for conditions specific to each culture system (e.g., dilution rate for bioreactors with continuous operation). The PBEs can be coupled to expressions describing the variation of the elements of 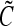 in the culture.
in the culture.
For systems of stem cells undergoing differentiation, PBEs can be written for distinct cell types. Pisu et al.169 presented a mass-structured PBE-based model for the differentiation of mesenchymal stem cells into bone cells. The model included equations for three density functions corresponding to undifferentiated mesenchymal cells, chondrocytes, and osteoblasts. These equations were coupled to temporal profiles of extracellular matrix components (glycosaminoglycans, collagen) and growth factors (bone morphogenetic proteins and other transforming growth factor-β ligands). The choice of mass as an independent variable of the PBE is advantageous because the first moment of the population density function is the biomass. In principle, the distribution of masses in a cell population can be determined by flow cytometry. Nonetheless, as Pisu et al.169 pointed out, there are no published data pertinent to PBE modeling expressed in terms of stem cell size (or mass) distribution in the literature to date. This may be because the cell mass provides only limited information regarding the differentiation state of stem cells or their progeny. In addition, there are challenges in acquiring reliable mass-based distributions of stem/progenitor cell populations. For example, hESCs tend to form clusters, making difficult to distinguish mitotic/dividing cells based on their size by flow cytometry.
For stem cells cultured in bioreactors as aggregates, PBEs can be used to describe the aggregate number distribution (Fig. 3). Similar approaches have been implemented for the aggregation of platelets and other cell types.170–172 We have applied a mass-structured PBE model for the distribution of mESC aggregate sizes in SSB cultures:
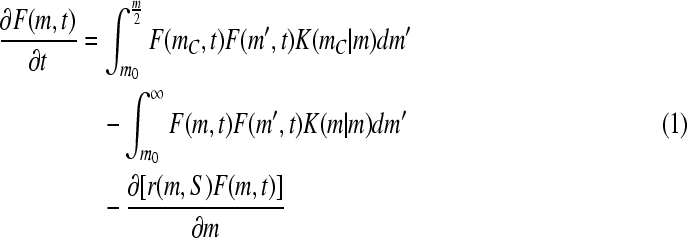 |
(12) |
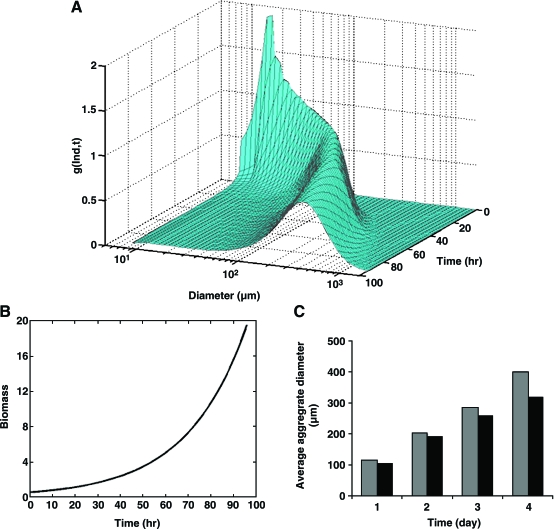
FIG. 3.
Solution of a population balance equation for mouse ESCs (mESCs) cultured as aggregates in a 100 mL-spinner flask. Approximately 2 × 104 mESCs/mL were seeded and cultured for 4 days at 60 rpm as described.17 Equation 12 was recast in terms of the logarithm of the mESC aggregate diameter d (i.e.,  ) instead of mass, assuming a spherical shape for single cells and aggregates. The von Smoluchowski kernel,
) instead of mass, assuming a spherical shape for single cells and aggregates. The von Smoluchowski kernel, 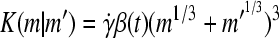 , was used and the equation was solved as described before.170
, was used and the equation was solved as described before.170 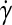 is an average shear rate (Eq. 3) in the culture vessel. The collision efficiency
is an average shear rate (Eq. 3) in the culture vessel. The collision efficiency 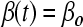 was estimated in 12-h intervals based on distributions of mESC aggregate sizes determined by image analysis of micrographs. (A) The first moment
was estimated in 12-h intervals based on distributions of mESC aggregate sizes determined by image analysis of micrographs. (A) The first moment 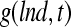 of the density function
of the density function 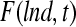 normalized to the total biomass (i.e.,
normalized to the total biomass (i.e., 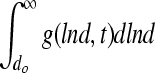 , where
, where 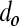 is the diameter of a single mESC) is shown for mESC aggregates. (B) Temporal profile of the total biomass. (C) Values for the average diameter of mESC aggregates at different times. Gray bars: simulation; black bars: experimental data. Color images available online at www.liebertonline.com/ten.
is the diameter of a single mESC) is shown for mESC aggregates. (B) Temporal profile of the total biomass. (C) Values for the average diameter of mESC aggregates at different times. Gray bars: simulation; black bars: experimental data. Color images available online at www.liebertonline.com/ten.
where F(m,t)dm is the mean number of aggregates with mass between m and m + dm in a unit volume of the culture at time t. The first integral describes the formation of ESC aggregates with mass m (m-aggregates) due to aggregation of clusters with masses m′ < m and mc = m − m′ (mo refers to the mass of a single cell). The last two terms represent the “loss” of ESC m-clusters due to their aggregation with m′-clusters to form larger aggregates and due to cell proliferation (r(m, S)), which depends on the concentration of substrate S. The PBE can be expanded to include other phenomena influencing the process (e.g., aggregate breakage173,174). The kernel K(m|m′), which captures the probability of adhesion between aggregates with masses m and m′, was originally reported for spherical body collisions in laminar shear175 and various forms considered for aggregation processes are reviewed elsewhere.176,177 In general, the aggregation kernel is in the form:
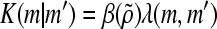 |
(13) |
where 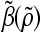 encompasses the efficiency of collisions dependent on multiple parameters described by the vector
encompasses the efficiency of collisions dependent on multiple parameters described by the vector 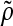 (e.g., the masses m and m′, time t, shear rate). The function λ(m,m′) is the kernel of orthokinetic aggregation of the m and m′ clusters. Moreover, the effect of nutrient/factor concentration S and supplementation on the process is introduced via the growth rate r(m, S).
(e.g., the masses m and m′, time t, shear rate). The function λ(m,m′) is the kernel of orthokinetic aggregation of the m and m′ clusters. Moreover, the effect of nutrient/factor concentration S and supplementation on the process is introduced via the growth rate r(m, S).
Constraints on the aggregate size of stem cells cultured in a SSB can be applied based, for example, on the transport of oxygen or factors important for stem cell self-renewal (e.g., LIF for mESCs) or directed differentiation. Then, the above PBE model can be used to predict an optimal distribution of stem cell aggregate sizes dependent on the operating variables and time of SSBs.
To date, application of PBE models to bioreactor culture systems for animal cells has been limited. First, the algorithmic complexity increases substantially as the vector of state variables, 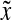 , is expanded. This adds significantly to the computational requirements, although this concern is alleviated considering the rapid increase in available computing power. Second, there are no known general forms of the various single-cell functions involved in PBEs (e.g., for differentiation
, is expanded. This adds significantly to the computational requirements, although this concern is alleviated considering the rapid increase in available computing power. Second, there are no known general forms of the various single-cell functions involved in PBEs (e.g., for differentiation 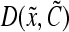 or partition
or partition 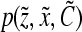 ). Identification of such functions requires measurements at a single-cell level. Rapid estimation of parameters in distributions of single-cell properties178 can be achieved by flow cytometry. Thus far, flow cytometric analysis in this context has yielded information about the position of cells in the cell cycle in relation to their size (or mass).179,180 However, multiple-state variables besides the cell mass will be necessary to describe the populations of stem cells in either culture or tissues. For example, subpopulations of cells may be ascribed state variables representing the expression of markers typical of pluripotency (e.g., OCT3/4A, NANOG) or of specific lineages. Assessing the distribution of these state variables will necessitate the development of highly specific antibodies and the engineering of stem cell lines carrying reporter genes coupled to lineage-specific gene promoters. As these tools become widely available, PBE-based models will become more tractable for stem cell applications.
). Identification of such functions requires measurements at a single-cell level. Rapid estimation of parameters in distributions of single-cell properties178 can be achieved by flow cytometry. Thus far, flow cytometric analysis in this context has yielded information about the position of cells in the cell cycle in relation to their size (or mass).179,180 However, multiple-state variables besides the cell mass will be necessary to describe the populations of stem cells in either culture or tissues. For example, subpopulations of cells may be ascribed state variables representing the expression of markers typical of pluripotency (e.g., OCT3/4A, NANOG) or of specific lineages. Assessing the distribution of these state variables will necessitate the development of highly specific antibodies and the engineering of stem cell lines carrying reporter genes coupled to lineage-specific gene promoters. As these tools become widely available, PBE-based models will become more tractable for stem cell applications.
Acknowledgments
E.S.T. expresses gratitude for the invitation and the support provided by the AMRITA Vishwa Vidyapeetham University for his participation in the NANOBIO 2009 conference. The hospitality and help from numerous faculty, staff, and students of the AMRITA Centre for Nanosciences are gratefully acknowledged. The hiPSC line B12-3 was a kind gift from Dr. Douglas A. Melton (Department of Stem Cell and Regenerative Biology, Howard Hughes Medical Institute, Harvard Stem Cell Institute, Harvard University). D.E.K. and D.J. are recipients of a Mark Diamond Research Fund grant. This work was funded by a J.D. Watson Award from the New York State Office of Science, Technology, and Academic Research and by a National Institutes of Health grant (HL092398) to E.S.T.
Disclosure Statement
No competing financial interests exist.
References
- 1.Palsson B.O. Bhatia S.N. Tissue Engineering. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- 2.Jing D. Parikh A. Canty J.M. Tzanakakis E.S. Stem cells for heart cell therapies. Tissue Eng Part B Rev. 2008;14:393. doi: 10.1089/ten.teb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan E.A. Lakey J.R. Paty B.W. Imes S. Korbutt G.S. Kneteman N.M. Bigam D. Rajotte R.V. Shapiro A.M. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 4.Lock L.T. Tzanakakis E.S. Stem/progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng. 2007;13:1399. doi: 10.1089/ten.2007.0047. [DOI] [PubMed] [Google Scholar]
- 5.Tzanakakis E.S. Hess D.J. Sielaff T.D. Hu W.S. Extracorporeal tissue engineered liver-assist devices. Annu Rev Biomed Eng. 2000;2:607. doi: 10.1146/annurev.bioeng.2.1.607. [DOI] [PubMed] [Google Scholar]
- 6.Li Y. Kniss D.A. Lasky L.C. Yang S.T. Culturing and differentiation of murine embryonic stem cells in a three-dimensional fibrous matrix. Cytotechnology. 2003;41:23. doi: 10.1023/A:1024283521966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandstrom C.E. Bender J.G. Miller W.M. Papoutsakis E.T. Development of novel perfusion chamber to retain nonadherent cells and its use for comparison of human “mobilized” peripheral blood mononuclear cell cultures with and without irradiated bone marrow stroma. Biotechnol Bioeng. 1996;50:493. doi: 10.1002/(SICI)1097-0290(19960605)50:5<493::AID-BIT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Meissner P. Schroder B. Herfurth C. Biselli M. Development of a fixed bed bioreactor for the expansion of human hematopoietic progenitor cells. Cytotechnology. 1999;30:227. doi: 10.1023/A:1008085932764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandon N. Cannizzaro C. Chao P.H. Maidhof R. Marsano A. Au H.T. Radisic M. Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirouac D.C. Zandstra P.W. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Placzek M.R. Chung I.M. Macedo H.M. Ismail S. Mortera Blanco T. Lim M. Cha J.M. Fauzi I. Kang Y. Yeo D.C. Ma C.Y. Polak J.M. Panoskaltsis N. Mantalaris A. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6:209. doi: 10.1098/rsif.2008.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmins N.E. Nielsen L.K. Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 2009;27:415. doi: 10.1016/j.tibtech.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Kunas K.T. Papoutsakis E.T. Damage mechanisms of suspended animal cells in agitated bioreactors with and without bubble entrainment. Biotechnol Bioeng. Biotechnol Bioeng. 1990;2009;36102:476. 980. doi: 10.1002/bit.260360507. discussion 977. [DOI] [PubMed] [Google Scholar]
- 14.D'Amour K.A. Bang A.G. Eliazer S. Kelly O.G. Agulnick A.D. Smart N.G. Moorman M.A. Kroon E. Carpenter M.K. Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 15.Xu C. Police S. Rao N. Carpenter M.K. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 16.Liu H. Roy K. Biomimetic three-dimensional cultures significantly increase hematopoietic differentiation efficacy of embryonic stem cells. Tissue Eng. 2005;11:319. doi: 10.1089/ten.2005.11.319. [DOI] [PubMed] [Google Scholar]
- 17.Kehoe D.E. Lock L.T. Parikh A. Tzanakakis E.S. Propagation of embryonic stem cells in stirred suspension without serum. Biotechnol Prog. 2008;24:1342. doi: 10.1002/btpr.57. [DOI] [PubMed] [Google Scholar]
- 18.Cormier J.T. Nieden N.I. Rancourt D.E. Kallos M.S. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 19.Fok E.Y. Zandstra P.W. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 20.zur Nieden N.I. Cormier J.T. Rancourt D.E. Kallos M.S. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol. 2007;129:421. doi: 10.1016/j.jbiotec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Zandstra P.W. Bauwens C. Yin T. Liu Q. Schiller H. Zweigerdt R. Pasumarthi K.B. Field L.J. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder M. Niebruegge S. Werner A. Willbold E. Burg M. Ruediger M. Field L.J. Lehmann J. Zweigerdt R. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 23.Gerecht-Nir S. Cohen S. Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng. 2004;86:493. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 24.Yirme G. Amit M. Laevsky I. Osenberg S. Itskovitz-Eldor J. Establishing a dynamic process for the formation, propagation, and differentiation of human embryoid bodies. Stem Cells Dev. 2008;17:1227. doi: 10.1089/scd.2007.0272. [DOI] [PubMed] [Google Scholar]
- 25.Cameron C.M. Hu W.S. Kaufman D.S. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94:938. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 26.Daheron L. Opitz S.L. Zaehres H. Lensch M.W. Andrews P.W. Itskovitz-Eldor J. Daley G.Q. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey R.K. Beattie G.M. Lopez A.D. Bucay N. King C.C. Firpo M.T. Rose-John S. Hayek A. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T. Takahashi J.B. Nishikawa S. Muguruma K. Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 29.Harb N. Archer T.K. Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Ibanez R. Unger C. Stromberg A. Baker D. Canals J.M. Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 31.Bajpai R. Lesperance J. Kim M. Terskikh A.V. Efficient propagation of single cells accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- 32.Dang S.M. Kyba M. Perlingeiro R. Daley G.Q. Zandstra P.W. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 33.Magyar J.P. Nemir M. Ehler E. Suter N. Perriard J.C. Eppenberger H.M. Mass production of embryoid bodies in microbeads. Ann NY Acad Sci. 2001;944:135. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang Y.S. Cho J. Tay F. Heng J.Y. Ho R. Kazarian S.G. Williams D.R. Boccaccini A.R. Polak J.M. Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Siti-Ismail N. Bishop A.E. Polak J.M. Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946. doi: 10.1016/j.biomaterials.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Levenberg S. Huang N.F. Lavik E. Rogers A.B. Itskovitz-Eldor J. Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang S.M. Gerecht-Nir S. Chen J. Itskovitz-Eldor J. Zandstra P.W. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 38.Li Y.J. Chung E.H. Rodriguez R.T. Firpo M.T. Healy K.E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 39.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akasha A.A. Sotiriadou I. Doss M.X. Halbach M. Winkler J. Baunach J.J. Katsen-Globa A. Zimmermann H. Choo Y. Hescheler J. Sachinidis A. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: preparation and characterization. Cell Physiol Biochem. 2008;22:665. doi: 10.1159/000185550. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein A.S. Juarez T.M. Helmke C.D. Gustin M.C. Mikos A.G. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 42.Kuo C.K. Li W.J. Mauck R.L. Tuan R.S. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 43.Freed L.E. Langer R. Martin I. Pellis N.R. Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc Natl Acad Sci USA. 1997;94:13885. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.H. Hao E. Savinov A.Y. Geron I. Strongin A.Y. Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. 2009;87:983. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieponice A. Soletti L. Guan J. Deasy B.M. Huard J. Wagner W.R. Vorp D.A. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 2008;29:825. doi: 10.1016/j.biomaterials.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anjomshoa M. Karbalaie K. Mardani M. Razavi S. Tanhaei S. Nasr-Esfahani M.H. Baharvand H. Generation of motor neurons by coculture of retinoic acid-pretreated embryonic stem cells with chicken notochords. Stem Cells Dev. 2009;18:259. doi: 10.1089/scd.2008.0049. [DOI] [PubMed] [Google Scholar]
- 47.Seliktar D. Zisch A.H. Lutolf M.P. Wrana J.L. Hubbell J.A. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 48.Benoit D.S. Schwartz M.P. Durney A.R. Anseth K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutolf M.P. Lauer-Fields J.L. Schmoekel H.G. Metters A.T. Weber F.E. Fields G.B. Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 51.van Wezel A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216:64. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- 52.Hu W.S. Giard D.J. Wang D.I. Serial propagation of mammalian cells on microcarriers. Biotechnol Bioeng. 1985;27:1466. doi: 10.1002/bit.260271011. [DOI] [PubMed] [Google Scholar]
- 53.Kuriyama S. Nakano T. Yoshimura N. Ohuchi T. Moritera T. Honda Y. Mass cultivation of human retinal pigment epithelial cells with microcarrier. Ophthalmologica. 1992;205:89. doi: 10.1159/000310319. [DOI] [PubMed] [Google Scholar]
- 54.Borys M.C. Papoutsakis E.T. Formation of bridges and large cellular clumps in CHO-cell microcarrier cultures: effects of agitation, dimethyl sulfoxide and calf serum. Cytotechnology. 1992;8:237. doi: 10.1007/BF02522041. [DOI] [PubMed] [Google Scholar]
- 55.Frauenschuh S. Reichmann E. Ibold Y. Goetz P.M. Sittinger M. Ringe J. A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol Prog. 2007;23:187. doi: 10.1021/bp060155w. [DOI] [PubMed] [Google Scholar]
- 56.Schop D. Janssen F.W. Borgart E. de Bruijn J.D. van Dijkhuizen-Radersma R. Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med. 2008;2:126. doi: 10.1002/term.73. [DOI] [PubMed] [Google Scholar]
- 57.Abranches E. Bekman E. Henrique D. Cabral J.M. Expansion of mouse embryonic stem cells on microcarriers. Biotechnol Bioeng. 2007;96:1211. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes A.M. Fernandes T.G. Diogo M.M. da Silva C.L. Henrique D. Cabral J.M. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 59.Kato D. Takeuchi M. Sakurai T. Furukawa S. Mizokami H. Sakata M. Hirayama C. Kunitake M. The design of polymer microcarrier surfaces for enhanced cell growth. Biomaterials. 2003;24:4253. doi: 10.1016/s0142-9612(03)00319-3. [DOI] [PubMed] [Google Scholar]
- 60.Varani J. Inman D.R. Fligiel S.E. Hillegas W.J. Use of recombinant and synthetic peptides as attachment factors for cells on microcarriers. Cytotechnology. 1993;13:89. doi: 10.1007/BF00749935. [DOI] [PubMed] [Google Scholar]
- 61.Amit M. Shariki C. Margulets V. Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 62.Phillips B.W. Horne R. Lay T.S. Rust W.L. Teck T.T. Crook J.M. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138:24. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 63.Nie Y. Bergendahl V. Hei D.J. Jones J.M. Palecek S.P. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lock L.T. Tzanakakis E.S. Scalable production of pancreatic islet progenitors for diabetes cell therapies. Med J Malaysia. 2008;63 Suppl A:5. [PubMed] [Google Scholar]
- 65.Lock L.T. Tzanakakis E.S. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15:2051. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forsyth N.R. Musio A. Vezzoni P. Simpson A.H. Noble B.S. McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. 2006;8:16. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes A.M. Marinho P.A. Sartore R.C. Paulsen B.S. Mariante R.M. Castilho L.R. Rehen S.K. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz J Med Biol Res. 2009;42:515. doi: 10.1590/s0100-879x2009000600007. [DOI] [PubMed] [Google Scholar]
- 68.Oh S.K. Chen A.K. Mok Y. Chen X. Lim U.M. Chin A. Choo A.B. Reuveny S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2:219. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Huangfu D. Osafune K. Maehr R. Guo W. Eijkelenboom A. Chen S. Muhlestein W. Melton D.A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 70.Gadue P. Huber T.L. Paddison P.J. Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips B.W. Lim R.Y. Tan T.T. Rust W.L. Crook J.M. Efficient expansion of clinical-grade human fibroblasts on microcarriers: cells suitable for ex vivo expansion of clinical-grade hESCs. J Biotechnol. 2008;134:79. doi: 10.1016/j.jbiotec.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Billig D. Clark J.M. Ewell A.J. Carter C.M. Gebb C. The separation of harvested cells from microcarriers: a comparison of methods. Dev Biol Stand. 1983;55:67. [PubMed] [Google Scholar]
- 73.Tao T.-Y. Ji G.-Y. Hu W.S. Serial propagation of mammalian cells on gelatin-coated microcarriers. Biotechnol Bioeng. 1988;32:1037. doi: 10.1002/bit.260320811. [DOI] [PubMed] [Google Scholar]
- 74.Chaudhry M.A. Bowen B.D. Piret J.M. Culture pH and osmolality influence proliferation and embryoid body yields of murine embryonic stem cells. Biochem Eng J. 2009;45:126. [Google Scholar]
- 75.Gurdon J.B. Harger P. Mitchell A. Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- 76.Moreira J.L. Alves P.M. Rodrigues J.M. Cruz P.E. Aunins J.G. Carrondo M.J. Studies of baby hamster kidney natural cell aggregation in suspended batch cultures. Ann NY Acad Sci. 1994;745:122. doi: 10.1111/j.1749-6632.1994.tb44368.x. [DOI] [PubMed] [Google Scholar]
- 77.Sen A. Kallos M.S. Behie L.A. Effects of hydrodynamics on cultures of mammalian neural stem cell aggregates in suspension bioreactors. Ind Eng Chem Res. 2001;40:5350. [Google Scholar]
- 78.Moreira J.L. Santana P.C. Feliciano A.S. Cruz P.E. Racher A.J. Griffiths J.B. Carrondo M.J. Effect of viscosity upon hydrodynamically controlled natural aggregates of animal cells grown in stirred vessels. Biotechnol Prog. 1995;11:575. doi: 10.1021/bp00035a012. [DOI] [PubMed] [Google Scholar]
- 79.Sen A. Kallos M.S. Behie L.A. Expansion of mammalian neural stem cells in bioreactors: effect of power input and medium viscosity. Brain Res Dev Brain Res. 2002;134:103. doi: 10.1016/s0165-3806(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 80.Hu W.S. Quantitative and Mechanistic Analysis of Mammalian Cell Cultivation on Microcarriers. Cambridge, MA: Department of Applied Biological Science, Massachusetts Institute of Technology; 1983. [Google Scholar]
- 81.Sinskey A.J. Fleischaker R.J. Tyo M.A. Giard D.J. Wang D.I. Production of cell-derived products: virus and interferon. Ann NY Acad Sci. 1981;369:47. doi: 10.1111/j.1749-6632.1981.tb14176.x. [DOI] [PubMed] [Google Scholar]
- 82.Croughan M.S. Hamel J.F. Wang D.I. Hydrodynamic effects on animal cells grown in microcarrier cultures. Biotechnol Bioeng. 1987;29:130. doi: 10.1002/bit.260290117. [DOI] [PubMed] [Google Scholar]
- 83.Hinze J.O. Turbulence. New York: McGraw-Hill; 1975. [Google Scholar]
- 84.Papoutsakis E.T. Fluid-mechanical damage of animal cells in bioreactors. Trends Biotechnol. 1991;9:427. doi: 10.1016/0167-7799(91)90145-8. [DOI] [PubMed] [Google Scholar]
- 85.Cherry R.S. Papoutsakis E.T. Physical mechanisms of cell damage in microcarrier cell culture bioreactors. Biotechnol Bioeng. 1988;32:1001. doi: 10.1002/bit.260320808. [DOI] [PubMed] [Google Scholar]
- 86.Cherry R.S. Papoutsakis E.T. Growth and death rates of bovine embryonic kidney cells in turbulent microcarrier bioreactors. Bioprocess Biosyst Eng. 1989;4:81. [Google Scholar]
- 87.Kunas K.T. Papoutsakis E.T. Damage mechanisms of suspended animal cells in agitated bioreactors with and without bubble entrainment. Biotechnol Bioeng. 1990;36:476. doi: 10.1002/bit.260360507. [DOI] [PubMed] [Google Scholar]
- 88.Mcqueen A. Meilhoc E. Bailey J.E. Flow effects on the viability and lysis of suspended mammalian-cells. Biotechnol Lett. 1987;9:831. doi: 10.1007/BF01026191. [DOI] [PubMed] [Google Scholar]
- 89.Cherry R.S. Kwon K.Y. Transient shear stresses on a suspension cell in turbulence. Biotechnol Bioeng. 1990;36:563. doi: 10.1002/bit.260360603. [DOI] [PubMed] [Google Scholar]
- 90.Stathopoulos N.A. Hellums J.D. Shear stress effects on human embryonic kidney cells in vitro. Biotechnol Bioeng. 1985;27:1021. doi: 10.1002/bit.260270713. [DOI] [PubMed] [Google Scholar]
- 91.Begley C.M. Kleis S.J. The fluid dynamic and shear environment in the NASA/JSC rotating-wall perfused-vessel bioreactor. Biotechnol Bioeng. 2000;70:32. doi: 10.1002/1097-0290(20001005)70:1<32::aid-bit5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 92.Papoutsakis E.T. Media additives for protecting freely suspended animal cells against agitation and aeration damage. Trends Biotechnol. 1991;9:316. doi: 10.1016/0167-7799(91)90102-n. [DOI] [PubMed] [Google Scholar]
- 93.Lakhotia S. Papoutsakis E.T. Agitation induced cell injury in microcarrier cultures. Protective effect of viscosity is agitation intensity dependent: experiments and modeling. Biotechnol Bioeng. 1992;39:95. doi: 10.1002/bit.260390114. [DOI] [PubMed] [Google Scholar]
- 94.Saha S. Ji L. de Pablo J.J. Palecek S.P. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 95.Saha S. Ji L. de Pablo J.J. Palecek S.P. TGFbeta/activin/nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Illi B. Scopece A. Nanni S. Farsetti A. Morgante L. Biglioli P. Capogrossi M.C. Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 97.Yamamoto K. Sokabe T. Watabe T. Miyazono K. Yamashita J.K. Obi S. Ohura N. Matsushita A. Kamiya A. Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 98.Sargent C.Y. Berguig G.Y. McDevitt T.C. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng Part A. 2009;15:331. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- 99.Wang J.H. Thampatty B.P. Mechanobiology of adult and stem cells. Int Rev Cell Mol Biol. 2008;271:301. doi: 10.1016/S1937-6448(08)01207-0. [DOI] [PubMed] [Google Scholar]
- 100.Kim L. Vahey M.D. Lee H.Y. Voldman J. Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip. 2006;6:394. doi: 10.1039/b511718f. [DOI] [PubMed] [Google Scholar]
- 101.Kamei K. Guo S. Yu Z.T. Takahashi H. Gschweng E. Suh C. Wang X. Tang J. McLaughlin J. Witte O.N. Lee K.B. Tseng H.R. An integrated microfluidic culture device for quantitative analysis of human embryonic stem cells. Lab Chip. 2009;9:555. doi: 10.1039/b809105f. [DOI] [PubMed] [Google Scholar]
- 102.Figallo E. Cannizzaro C. Gerecht S. Burdick J.A. Langer R. Elvassore N. Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular microenvironments. Lab Chip. 2007;7:710. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 103.Fischer B. Bavister B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 104.Jeong C.H. Lee H.J. Cha J.H. Kim J.H. Kim K.R. Yoon D.K. Kim K.W. Hypoxia-inducible factor-1 alpha inhibits self-renewal of mouse embryonic stem cells in vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. J Biol Chem. 2007;282:13672. doi: 10.1074/jbc.M700534200. [DOI] [PubMed] [Google Scholar]
- 105.Bauwens C. Yin T. Dang S. Peerani R. Zandstra P.W. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 106.Niebruegge S. Bauwens C.L. Peerani R. Thavandiran N. Masse S. Sevaptisidis E. Nanthakumar K. Woodhouse K. Husain M. Kumacheva E. Zandstra P.W. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 107.Westfall S.D. Sachdev S. Das P. Hearne L.B. Hannink M. Roberts R.M. Ezashi T. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008;17:869. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H.F. Kuo H.C. Chen W. Wu F.C. Yang Y.S. Ho H.N. A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Hum Reprod. 2009;24:71. doi: 10.1093/humrep/den345. [DOI] [PubMed] [Google Scholar]
- 109.Adam J. Maggelakis S. Diffusion regulated growth characteristics of a spherical prevascular carcinoma. Bull Math Biol. 1990;52:549. doi: 10.1007/BF02462267. [DOI] [PubMed] [Google Scholar]
- 110.Casciari J.J. Sotirchos S.V. Sutherland R.M. Mathematical modelling of microenvironment and growth in EMT6/Ro multicellular tumour spheroids. Cell Prolif. 1992;25:1. doi: 10.1111/j.1365-2184.1992.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 111.Grossmann U. Profiles of oxygen partial pressure and oxygen consumption inside multicellular spheroids. Recent Results Cancer Res. 1984;95:150. doi: 10.1007/978-3-642-82340-4_9. [DOI] [PubMed] [Google Scholar]
- 112.Gassmann M. Fandrey J. Bichet S. Wartenberg M. Marti H.H. Bauer C. Wenger R.H. Acker H. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:2867. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tzanakakis E.S. Hansen L.K. Hu W.S. The role of actin filaments and microtubules in hepatocyte spheroid self-assembly. Cell Motil Cytoskeleton. 2001;48:175. doi: 10.1002/1097-0169(200103)48:3<175::AID-CM1007>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 114.Sachlos E. Auguste D.T. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29:4471. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Michaels J.D. Mallik A.K. Papoutsakis E.T. Sparging and agitation-induced injury of cultured animals cells: do cell-to-bubble interactions in the bulk liquid injure cells? Biotechnol Bioeng. 1996;51:399. doi: 10.1002/(SICI)1097-0290(19960820)51:4<399::AID-BIT3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 116.Buzzard J.J. Gough N.M. Crook J.M. Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 22:381. doi: 10.1038/nbt0404-381. author reply 82, 2004. [DOI] [PubMed] [Google Scholar]
- 117.Draper J.S. Smith K. Gokhale P. Moore H.D. Maltby E. Johnson J. Meisner L. Zwaka T.P. Thomson J.A. Andrews P.W. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 118.Maitra A. Arking D.E. Shivapurkar N. Ikeda M. Stastny V. Kassauei K. Sui G. Cutler D.J. Liu Y. Brimble S.N. Noaksson K. Hyllner J. Schulz T.C. Zeng X. Freed W.J. Crook J. Abraham S. Colman A. Sartipy P. Matsui S. Carpenter M. Gazdar A.F. Rao M. Chakravarti A. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 119.Martin M.J. Muotri A. Gage F. Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 120.Drukker M. Katchman H. Katz G. Even-Tov Friedman S. Shezen E. Hornstein E. Mandelboim O. Reisner Y. Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 121.Li L. Baroja M.L. Majumdar A. Chadwick K. Rouleau A. Gallacher L. Ferber I. Lebkowski J. Martin T. Madrenas J. Bhatia M. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 122.Cowan C.A. Atienza J. Melton D.A. Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 123.Cibelli J.B. Grant K.A. Chapman K.B. Cunniff K. Worst T. Green H.L. Walker S.J. Gutin P.H. Vilner L. Tabar V. Dominko T. Kane J. Wettstein P.J. Lanza R.P. Studer L. Vrana K.E. West M.D. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295:819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 125.Yu J. Vodyanik M.A. Smuga-Otto K. Antosiewicz-Bourget J. Frane J.L. Tian S. Nie J. Jonsdottir G.A. Ruotti V. Stewart R. Slukvin I.I. Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 126.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 127.Choo A.B. Padmanabhan J. Chin A.C. Oh S.K. Expansion of pluripotent human embryonic stem cells on human feeders. Biotechnol Bioeng. 2004;88:321. doi: 10.1002/bit.20247. [DOI] [PubMed] [Google Scholar]
- 128.Genbacev O. Krtolica A. Zdravkovic T. Brunette E. Powell S. Nath A. Caceres E. McMaster M. McDonagh S. Li Y. Mandalam R. Lebkowski J. Fisher S.J. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril. 2005;83:1517. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 129.Richards M. Fong C.Y. Chan W.K. Wong P.C. Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 130.Cheng L. Hammond H. Ye Z. Zhan X. Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 131.Price P.J. Goldsborough M.D. Tilkins M.L. Embryonic stem cell serum replacement. U.S. Patent. 1998. Patent version number: WO/1998/030679. Application number: PCT/US1998/000467.
- 132.Rosler E.S. Fisk G.J. Ares X. Irving J. Miura T. Rao M.S. Carpenter M.K. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev Dyn. 2004;229:259. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- 133.Xu R.H. Peck R.M. Li D.S. Feng X. Ludwig T. Thomson J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 134.Xu C. Rosler E. Jiang J. Lebkowski J.S. Gold J.D. O'Sullivan C. Delavan-Boorsma K. Mok M. Bronstein A. Carpenter M.K. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 135.Ullmann U. In't Veld P. Gilles C. Sermon K. De Rycke M. Van de Velde H. Van Steirteghem A. Liebaers I. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13:21. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- 136.Kleinman H.K. McGarvey M.L. Liotta L.A. Robey P.G. Tryggvason K. Martin G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 137.Levenstein M.E. Ludwig T.E. Xu R.H. Llanas R.A. VanDenHeuvel-Kramer K. Manning D. Thomson J.A. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao S. Chen S. Clark J. Hao E. Beattie G.M. Hayek A. Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fraichard A. Chassande O. Bilbaut G. Dehay C. Savatier P. Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci. 1995;108 (Pt 10):3181. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 140.Wobus A.M. Kaomei G. Shan J. Wellner M.C. Rohwedel J. Ji G. Fleischmann B. Katus H.A. Hescheler J. Franz W.M. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997;29:1525. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 141.Pevsner-Fischer M. Morad V. Cohen-Sfady M. Rousso-Noori L. Zanin-Zhorov A. Cohen S. Cohen I.R. Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 142.Li X.J. Hu B.Y. Jones S.A. Zhang Y.S. Lavaute T. Du Z.W. Zhang S.C. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Passier R. Oostwaard D.W. Snapper J. Kloots J. Hassink R.J. Kuijk E. Roelen B. de la Riviere A.B. Mummery C. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005;23:772. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 144.Wada T. Honda M. Minami I. Tooi N. Amagai Y. Nakatsuji N. Aiba K. Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells. PLoS One. 2009;4:e6722. doi: 10.1371/journal.pone.0006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiong C. Xie C.Q. Zhang L. Zhang J. Xu K. Fu M. Thompson W.E. Yang L.J. Chen Y.E. Derivation of adipocytes from human embryonic stem cells. Stem Cells Dev. 2005;14:671. doi: 10.1089/scd.2005.14.671. [DOI] [PubMed] [Google Scholar]
- 146.Zhu S. Wurdak H. Wang J. Lyssiotis C.A. Peters E.C. Cho C.Y. Wu X. Schultz P.G. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 147.Graichen R. Xu X. Braam S.R. Balakrishnan T. Norfiza S. Sieh S. Soo S.Y. Tham S.C. Mummery C. Colman A. Zweigerdt R. Davidson B.P. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 148.Sato N. Meijer L. Skaltsounis L. Greengard P. Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 149.Miyabayashi T. Teo J.L. Yamamoto M. McMillan M. Nguyen C. Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 2007;104:5668. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tseng A.S. Engel F.B. Keating M.T. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 151.Naito A.T. Shiojima I. Akazawa H. Hidaka K. Morisaki T. Kikuchi A. Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ding S. Wu T.Y. Brinker A. Peters E.C. Hur W. Gray N.S. Schultz P.G. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci USA. 2003;100:7632. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu X. Walker J. Zhang J. Ding S. Schultz P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 2004;11:1229. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 154.Peterson R.T. Link B.A. Dowling J.E. Schreiber S.L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McNeish J.D. Stem cells as screening tools in drug discovery. Curr Opin Pharmacol. 2007;7:515. doi: 10.1016/j.coph.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 156.Ding S. Schultz P.G. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 157.Viswanathan S. Zandstra P.W. Towards predictive models of stem cell fate. Cytotechnology. 2003;41:75. doi: 10.1023/A:1024866504538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O'Neill A. Schaffer D.V. The biology and engineering of stem-cell control. Biotechnol Appl Biochem. 2004;40:5. doi: 10.1042/BA20030195. [DOI] [PubMed] [Google Scholar]
- 159.Yener B. Acar E. Aguis P. Bennett K. Vandenberg S.L. Plopper G.E. Multiway modeling and analysis in stem cell systems biology. BMC Syst Biol. 2008;2:63. doi: 10.1186/1752-0509-2-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loeffler M. Roeder I. Conceptual models to understand tissue stem cell organization. Curr Opin Hematol. 2004;11:81. doi: 10.1097/01.moh.0000133648.83991.af. [DOI] [PubMed] [Google Scholar]
- 161.Koch A.L. Schaechter M. A Model for statistics of the cell division process. J Gen Microbiol. 1962;29:435. doi: 10.1099/00221287-29-3-435. [DOI] [PubMed] [Google Scholar]
- 162.Fredrickson A.G. Tsuchiya H.M. Continuous propagation of microorganisms. AIChE J. 1963;9:459. [Google Scholar]
- 163.Fredrickson A.G. Ramkrishna D. Tsuchiya H.M. Statistics and dynamics of procaryotic cell populations. Math Biosci. 1967;1:327. [Google Scholar]
- 164.Nielsen L.K. Bender J.G. Miller W.M. Papoutsakis E.T. Population balance model of in vivo neutrophil formation following bone marrow rescue therapy. Cytotechnology. 1998;28:157. doi: 10.1023/A:1008098118491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sherer E. Tocce E. Hannemann R.E. Rundell A.E. Ramkrishna D. Identification of age-structured models: cell cycle phase transitions. Biotechnol Bioeng. 2008;99:960. doi: 10.1002/bit.21633. [DOI] [PubMed] [Google Scholar]
- 166.Nielsen L.K. Papoutsakis E.T. Miller W.M. Modeling ex vivo hematopoiesis using chemical engineering metaphors. Chem Eng Sci. 1998;53:1913. [Google Scholar]
- 167.Mantzaris N.V. Daoutidis P. Cell population balance modeling and control in continuous bioreactors. J Process Control. 2004;14:775. [Google Scholar]
- 168.Daoutidis P. Henson M.A. Dynamics and control of cell populations in continuous bioreactors. AIChE Symp Ser. 2002;326:274. [Google Scholar]
- 169.Pisu M. Concas A. Cao G. A novel simulation model for stem cells differentiation. J Biotechnol. 2007;130:171. doi: 10.1016/j.jbiotec.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 170.Belval T.K. Hellums J.D. Analysis of shear-induced platelet aggregation with population balance mathematics. Biophys J. 1986;50:479. doi: 10.1016/S0006-3495(86)83485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Laurenzi I.J. Diamond S.L. Monte Carlo simulation of the heterotypic aggregation kinetics of platelets and neutrophils. Biophys J. 1999;77:1733. doi: 10.1016/S0006-3495(99)77019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anderson E.C. Petersen D.F. Cell growth and division. II. Experimental studies of cell volume distributions in mammalian suspension cultures. Biophys J. 1967;7:353. doi: 10.1016/S0006-3495(67)86593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang P.Y. Hellums J.D. Aggregation and disaggregation kinetics of human blood platelets. Part II. Shear-induced platelet aggregation. Biophys J. 1993;65:344. doi: 10.1016/S0006-3495(93)81079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ramkrishna D. Population Balances: Theory and Applications to Particulate Systems in Engineering. London: Academic Press; 2000. [Google Scholar]
- 175.von Smoluchowski M. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z Phys Chem. 1917;92:129. [Google Scholar]
- 176.Aldous D.J. Deterministic and stochastic models for coalescence (aggregation and coagulation): a review of the mean-field theory for probabilists. Bernoulli. 1999;5:3. [Google Scholar]
- 177.Smit D.J. Hounslow M.J. Paterson W.R. Aggregation and gelation. I. Analytical solutions for CST and batch operation. Chem Eng Sci. 1994;49:1025. [Google Scholar]
- 178.Srienc F. Cytometric data as the basis for rigorous models of cell population dynamics. J Biotechnol. 1999;71:233. [Google Scholar]
- 179.Darzynkiewicz Z. Crissman H. Jacobberger J.W. Cytometry of the cell cycle: cycling through history. Cytometry A. 2004;58:21. doi: 10.1002/cyto.a.20003. [DOI] [PubMed] [Google Scholar]
- 180.Kacmar J. Srienc F. Dynamics of single cell property distributions in Chinese hamster ovary cell cultures monitored and controlled with automated flow cytometry. J Biotechnol. 2005;120:410. doi: 10.1016/j.jbiotec.2005.06.031. [DOI] [PubMed] [Google Scholar]