Abstract
Novel recombinant adeno-associated virus vectors pseudo-typed with serotype 8 capsid (rAAV2/8) have recently shown exciting promise as effective liver-directed gene transfer reagents. We have produced a novel liver-specific rAAV2/8 vector expressing the mouse phenylalanine hydroxylase (Pah) cDNA and have administered this vector to hyperphenylalaninemic PAH-deficient Pahenu2 mice, a model of human phenylketonuria (PKU). Our hypothesis was that this vector would produce sufficient hepatocyte transduction frequency and PAH activity to correct blood phenylalanine levels in murine PKU. Portal vein injection of recombinant AAV2/8 vector into five adult Pahenu2 mice yielded complete and stable (up to 17 weeks) correction of serum phenylalanine levels. Liver PAH activity was corrected to 11.5±2.4% of wild type liver activity and was associated with a significant increase in phenylalanine clearance following parenteral phenylalanine challenge. Although questions of long-term safety and stability of expression remain, recombinant AAV2/8-mediated, liver-directed gene therapy is a promising novel treatment approach for PKU and allied inborn errors of metabolism.
Keywords: phenylketonuria, phenylalanine, phenylalanine hydroxylase, adeno-associated virus, liver gene therapy, Mouse model
Phenylketonuria (PKU) is one of the most common inborn errors of metabolism with an incidence of approximately 1:16 000 births in North America. The most common cause of PKU is deficiency of phenylalanine hydroxylase (PAH; EC 1.14.16.1) due to recessively inherited mutations in the PAH gene.1 PAH is a homotetramer expressed primarily in the liver that catalyzes the irreversible hydroxylation of phenylalanine (Phe) to tyrosine.2 PAH deficiency is associated with impaired phenylalanine clearance and consequently hyperphenylalaninemia. Reduction of blood Phe levels through dietary protein restriction prevents the major manifestations of the disease (mental retardation, seizures, and growth failure), but shortcomings in this strategy exist, including the need to adhere consistently to an unpalatable and expensive diet, persistent mild cognitive deficits in some treated children,3 and severe teratogenic effects upon the fetuses of mothers who cannot maintain dietary control, the so-called maternal PKU syndrome.4 In order to prevent long-term complications of hyperphenylalaninemia, dietary therapy for PKU is recommended for the life of the affected individual.5 For these reasons, we and many other investigators have explored the development of permanent cell-directed treatments including gene therapy for PKU.6 All of these investigations have benefited significantly from the availability of a murine model of PAH deficiency, the Pahenu2 mouse.7 Published investigations of liver-directed gene therapy for murine PKU have utilized recombinant retrovirus based upon the Moloney murine leukemia virus (MoMLV),8 recombinant adenovirus, 9 or adeno-associated virus (AAV) vectors, either AAV serotype 2 (rAAV2)10,11 or pseudotyped with serotype 5 (rAAV2/5).12,13 Unfortunately, many of these attempts have been limited to varying degrees by either insufficient or temporary gene expression.
Novel recombinant AAV vectors pseudotyped with AAV serotype 8 capsid (rAAV2/8) have demonstrated improved liver transduction and increased levels of transgene expression14,15 in comparison to other AAV serotypes. Our hypothesis was that administration of a PAH-expressing rAAV2/8 vector would transduce sufficient numbers of hepatocytes and induce sufficient liver PAH activity to correct hyperphenylalaninemia in Pahenu2 mice. We produced a novel pseudotyped rAAV2/8 vector (LSPmPAH rAAV2/8) containing the murine Pah cDNA under the transcriptional control of a strong liver-specific promoter (LSP). The LSP is a combination of two copies of a human α1-microglobulin/bikunin enhancer and the promoter from the human thyroid hormone-binding globulin gene; this combination has been shown to direct high level liver specific gene expression, initially in hemophiliac dogs with Factor IX deficiency.16 The expression vector also contained a bovine growth hormone polyadenylation (pA) signal 3′ to the Pah cDNA but no other translation enhancer elements. Recombinant AAV particles pseudotyped with AAV serotype 8 capsid proteins were packaged by standard methods, purified, and dialyzed against Hanks buffer. A viral stock with a titer of approximately 2 × 1012 vector genomes/ml was produced. LSPmPAH rAAV2/8 vector in Hanks buffer was injected without further manipulation directly into hyperphenylalaninemic Pahenu2/Pahenu2 mice. These animals were homozygous for the same Pah mutation as described in the original BTBR-Pahenu2 strain7,17 but had been bred and back-crossed onto the C57Bl/6J background to increase breeding facility. Genotyping for the presence of the Pahenu2 mutation18 was performed by PCR analysis of tail biopsy DNA. All animals were fed standard mouse chow ad libitum providing approximately 23% of energy as protein.
In a preliminary trial, two C57Bl6-Pahenu2/Pahenu2 mice each received 2 × 1011 LSPmPAH rAAV2/8 vector genomes (vg) (1 × 1013 vg/kg body weight), injected via the tail vein or the portal vein (one animal each). Two additional mice each received 5 × 1011 vg (2.5 × 1013 vg/kg), again injected via the tail vein or the portal vein (one animal each). A fifth mouse received saline injection via the tail vein only as a control. Serum Phe levels were measured weekly for 8 weeks on 10 µl serum using a fluorometric procedure19 and then the animals were euthanized for tissue analysis. Serum Phe decreased to normal, and liver PAH activity was detected only in the single animal that had received 5 × 1011 vg LSPmPAH rAAV2/8 via portal vein injection. In contrast to recently published work with β-galactosidase expressing rAAV2/820 that showed equivalent transduction frequency following either portal or tail vein injection, portal vein injection of mouse PAH expressing rAAV2/8 was more efficacious than tail vein injection in this small trial, at least at these relatively low vector doses.
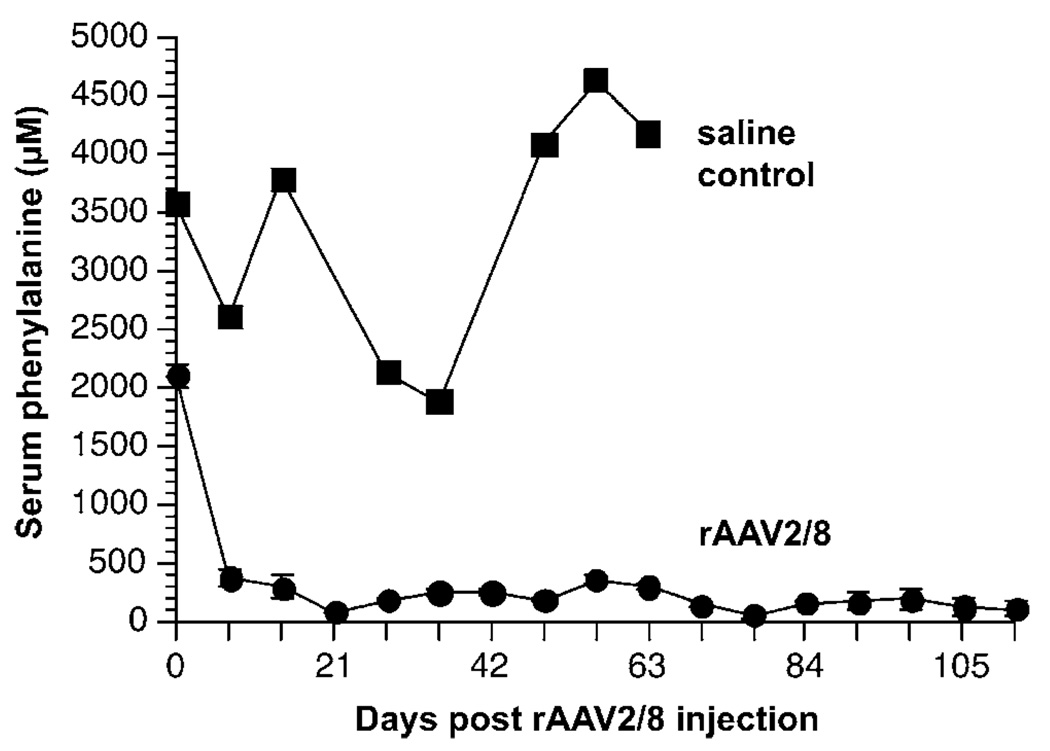
Subsequently, 5 × 1011 vg LSPmPAH rAAV2/8 (2.5 × 1013 vg/kg body weight) was administered to each of four additional adult C57Bl/6-Pahenu2/Pahenu2 mice via portal vein injection. The results of all five trials with 5 × 1011 vg via portal vein injection are summarized in Table 1. Serum Phe levels decreased to normal by 1 week following rAAV2/8 injection in all five animals (Figure 1), and this decrease from preinjection levels was statistically significant at all time points. At 8 weeks following rAAV2/8 injection (the final time point available for all five mice), the mean serum Phe had decreased from 2107 ± 102 µm (mean ± s.e.) to 148 ± 27.7 µm (P ⪡ 0.001). In the four mice that were allowed to survive beyond 8 weeks, this effect was sustained up to at least 17 weeks after injection, the final time point measured prior to euthanasia. Serum Phe concentration did not decrease following saline injection in the control animal.
Table 1.
Results following portal vein injection of 5 × 1011 LSPPAH rAAV2/8 vg/mouse
| Mouse | Gender | Serum phenylalanine (µM) | Phenylalanine tolerance (µM h) |
Vector genomes in liver (copies/haploid genome) |
Liver PAH activity (µmol/h/mg) |
% wild type PAH activity |
||
|---|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | Baseline | 8 weeks |
N = 3 assays per Mouse |
N = 3 assays per Mouse |
|||
| A | Male | 1839 | 81.1 | Not done | 2393 | 8.1 ± 3.4 | 18.6 ± 1.41 | 10.3† |
| B | Female | 2433 | 136 | 6347 | 2197 | 8.5 ± 0.7 | 17.9 ± 1.28 | 9.8* |
| C | Male | 2034 | 114 | 4171 | 1549 | 8.1 ± 0.6 | 27.3 ± 1.94 | 15.1* |
| D | Male | 2010 | 163 | 4983 | 1516 | 22.0 ± 2.5 | 19.0 ± 1.77 | 10.5† |
| E | Male | 2222 | 245 | 4736 | 1566 | 25.7 ± 2.4 | 19.8 ± 1.15 | 11.0† |
| Mean ± s.e. | 2107 ± 102 | 148 ± 27.8# | 5059 ± 462 | 1844 ± 187§ | 14.5 ± 8.5 | 20.8 ± 4.4 | 11.5 ± 2.4 | |
| Saline injected Pahenu2 | Female | 3579 | 4173 | Not done | 5638 | 0 | 1.2 ± 1.1 | 0.7 |
| Wild type | 85–175 | 181 ± 18.7 | 100 | |||||
Statistically significant decrease in serum Phe by one-tailed Student’s t-test (P = 1.4 × 10−5).
Statisically significant increase in Phe tolerance by one-tailed Student’s t-test (P = 0.0001).
Liver PAH activity was measured 8 weeks† or 17 week* after rAAV2/8 administration using a modified18 radiometric assay.33
Quantification of vector genome copy number. The number of vector genomes in liver was quantified by Southern blotting of 10 µg EcoR1-treated total DNA isolated from livers of rAAV2/8 treated mice. EcoR1 digestion excises the 2.0 kb mouse Pah cDNA from the rAAV2/8 vector and also a 6 kb genomic fragment that contains exon 6 from the murine Pah gene. Separately, Pah exon 6 was amplified by PCR from wild-type liver genomic DNA and radiolabeled by including 32P-α-dCTP in the PCR reaction. This radiolabeled 600 bp Pah exon 6 PCR product was used to detect both the murine Pah cDNA from the rAAV2/8 vector and the 6 kb murine Pah genomic fragment on the Southern blots. Southerns were imaged using a Biorad FX phosphoimager and densitometry performed using Biorad Quantity One software. Pah cDNA signals were normalized to the murine Pah genomic signal of that same animal then compared to the Pah cDNA signal from a standard series to calculate the vector copy number. The standard series was constructed using Pahenu2/Pahenu2 liver DNA spiked with increasing amounts of plasmid DNA (pCImPAH) containing the murine Pah cDNA and represented zero to 1000 cDNA copies per haploid genome. The Pah cDNA signals from this standard series were normalized against the genomic signal and plotted against the cDNA copy number to yield a linear standard curve with a correlation coefficient = 0.98.
Figure 1.
Time course of serum phenylalanine levels following LSPmPAH rAAV2/8 injection. Five Pahenu2/Pahenu2 mice received 5 × 1011 vg of LSPmPAH rAAV2/8 via portal vein injection and serum phenylalanine levels were measured weekly thereafter. A sixth mouse received a saline injection as a control. Mouse A and the saline control were sacrificed at eight weeks (56 days) for tissue analysis. The data are expressed as mean serum phenylalanine (µm) from all mice alive at that time point vs days postinjection with the error bars (for rAAV2/8 treated mice) representing one standard error (s.e.).
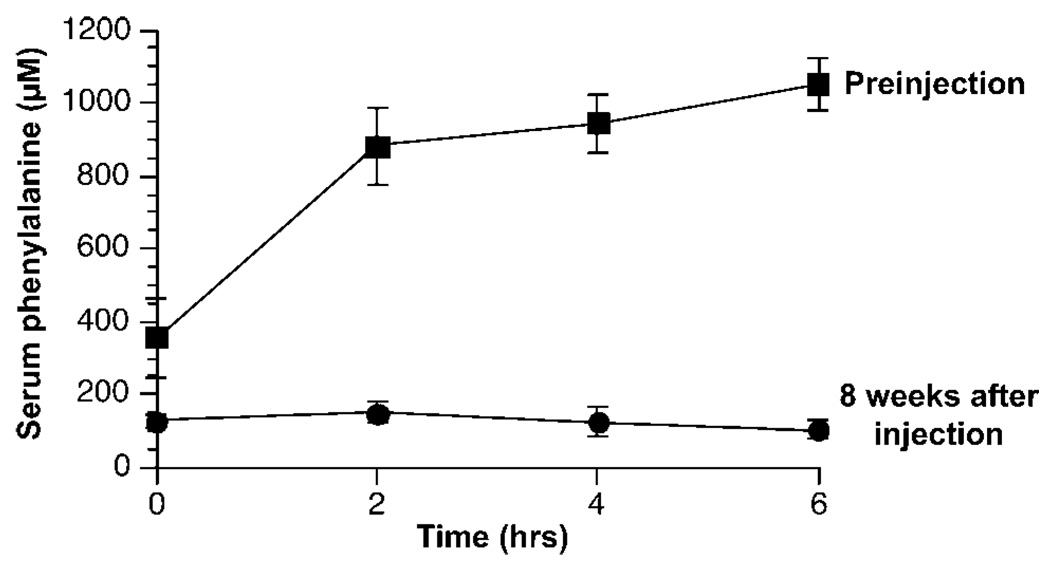
Prior to rAAV2/8 injection and again 8 weeks later, a parenteral Phe tolerance test was performed to evaluate Phe clearance capacity. The results of these Phe challenges are displayed in Figure 2. The mean Phe clearance as measured by the area under the curve (AUC) 8 weeks following rAAV2/8 administration was 1844 ± 187 µm h, a significant decrease from 5059 ± 462 preinjection (P = 0.0001); this result confirms that phenylalanine clearance had significantly improved in the five rAAV2/8-treated mice.
Figure 2.
Phenylalanine tolerance tests in Mice B–E. Phenylalanine tolerance tests were performed in Mice B–E 1 week prior to and again 8 weeks after LSPmPAH rAAV2/8 injection. The mice received Phe-free mouse chow (Harlan–Teklad) overnight prior to Phe challenge. The following morning, l-phenylalanine, 5 mg/ml in Hank’s buffered saline solution, was administered via intraperitoneal injection (dose = 0.5 mg/g body weight). Serum Phe was measured prior to and 2, 4, and 6 h after injection and was plotted against time. In these graphs, the area under the curve (AUC) is inversely proportional to the rate of Phe clearance, that is, as phenylalanine clearance improves following rAAV2/8 administration the area under the serum Phe vs time curve decreases. The data are expressed as mean serum phenylalanine (µm) ± s.e. vs time in hours post-Phe injection.
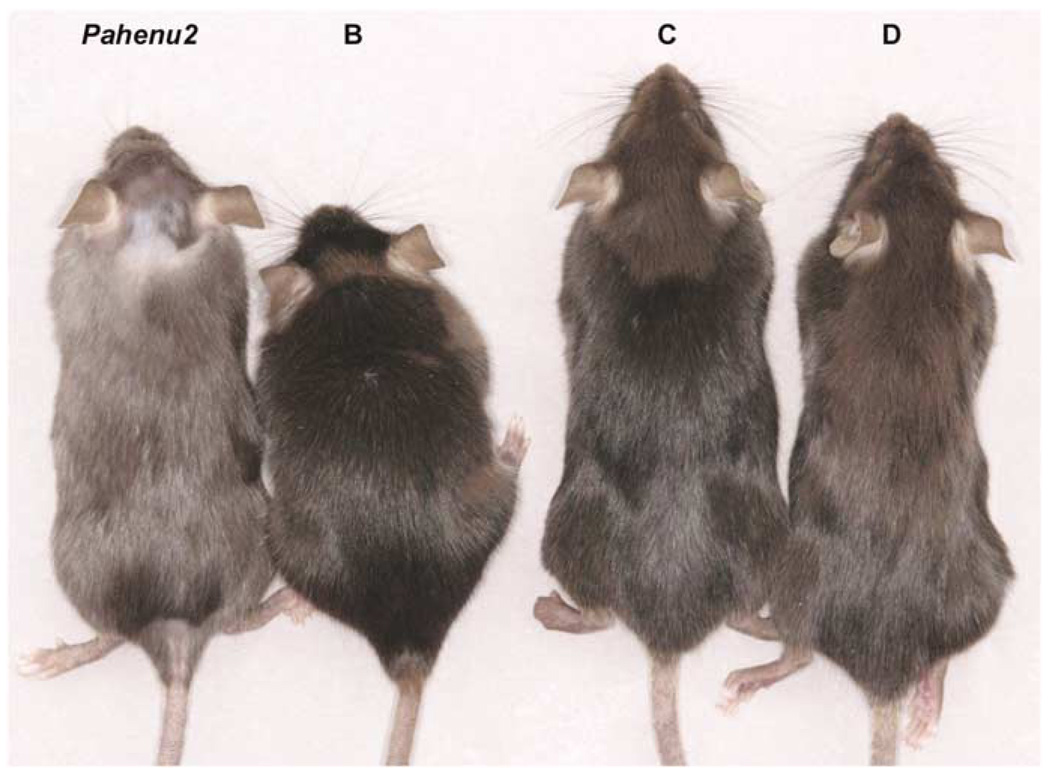
Hyperphenylalaninemia in C57Bl/6-Pahenu2/Pahenu2 mice is associated with relative hypopigmentation of coat color. This effect is likely due to the competitive inhibition of tyrosinase enzyme in melanocytes by phenylalanine.21 Following administration of LSPmPAH rAAV2/8 and normalization of serum Phe levels, coat color gradually darkened in the treated mice (Figure 3). The emergence of this effect was noticeable by 2 weeks following injection and complete by 8 weeks.
Figure 3.
Correction of coat color following LSPmPAH rAAV2/8 injection. Photograph of Mice B–D (on right) 8 weeks following LSPmPAH rAAV2/8 injection demonstrating nearly complete restoration of coat color pigmentation. The mouse on the left was an age-matched untreated female Pahenu2/Pahenu2 mouse with typical hyperphenylalaninemia-associated hypopigmentation.
Liver PAH activity was measured in whole-liver homogenate at 8–17 weeks following rAAV2/8 administration. Mouse A and the saline control were euthanized at 8 weeks while the remaining mice were killed 17 weeks following rAAV2/8 treatment. Liver PAH activity in the five mice (measured in triplicate) averaged 20.8 ± 4.4 nmol tyrosine produced/mg protein/h but ranged from 19.0 to 27.2 nmol/mg/h, equivalent to 11.0–15.1% wild-type C57Bl/6 liver PAH activity (wild-type liver PAH activity = 181 ± 18.7 nmol/mg/h, n = 4). In comparison, liver PAH activity measured only 1.23 ± 1.11 nmol/mg/h (0.7% wild type) in the control Pahenu2/Pahenu2 mouse 8 weeks following saline injection. No PAH activity was detected in the brain, heart, lung, spleen, kidney, skeletal muscle, or testis of Mouse A 8 weeks following rAAV2/8 administration.
The number of rAAV2/8 vector genomes in the liver was measured using Southern blotting to quantify accurately the relative amounts of rAAV2/8 vector-derived Pah cDNA in total liver DNA (Table 1). The number of vector genomes detected in the liver was 14.5 ± 8.5 vg/haploid genome, and ranged from 8.1 vg/haploid genome in Mice A and C to 25.7 vg/haploid genome in Mouse E. In this experiment, the frequency of hepatocyte transduction was not measured. Previously published data by other investigators suggest that portal vein injection of 3 × 1011 rAAV2/8 vg per mouse yields transduction in 15–25% of hepatocytes.20 If we assume 25% transduction frequency following portal vein injection of 5 × 1011 rAAV2/8 vg in this experiment, then each transduced cell would contain approximately 120 vector genomes and would express approximately 50% wild-type PAH activity per cell. Using PCR amplification of the Pah cDNA followed by Southern blotting to quantify the amount of PCR product present, no LSPmPAH rAAV2/8 vector genomes were detected in total DNA isolated from the brain, heart, lung, spleen, kidney, skeletal muscle, or testis of Mouse A; this assay is capable of detecting as few as 1 vg/haploid genome.
No adverse effects of rAAV2/8 vector administration were noted. Animals that underwent portal vein injection lost on average < 5% body weight postoperatively but had completely recovered by 1 week following injection. Histologic examination of formalin-fixed, H&E-stained liver sections following euthanasia revealed no abnormalities (data not shown). Lymphocytic infiltrates were absent from the livers of all mice examined.
Many other investigators have explored gene transfer as a potential therapy for PKU.6 Administration of recombinant adenovirus vector containing the human PAH cDNA to Pahenu2 mice resulted in complete correction of hyperphenylalaninemia but this effect lasted < 3 weeks due to immune-mediated rejection of adenovirus-infected hepatocytes.9 More recently, administration of PAH-expressing rAAV vectors (either rAAV211 or rAAV2/512,13) to Pahenu2 mice has resulted in greater stability of expression. Portal vein infusion of 1011 rAAV2 particles per male Pahenu2 mouse has yielded stable but only partial correction of serum phenylalanine levels (reported in abstract form only).10,22 No effect was seen in female mice, however. In the report of Oh et al.,11 portal vein injection of a higher dose (2 × 1012 vg/mouse) of rAAV2 (multiplicity of infection ≈ 4 × 104 vg/hepatocyte) yielded more complete correction with liver PAH activity of 17% of normal and a reduction in serum Phe to 360 ± 160 µm by 5 weeks following injection again in male mice only. The percentage of hepatocytes transduced was not reported. By 25 weeks postinjection, however, the number of vector genomes detectable in the liver decreased, the mean liver PAH activity had decreased to 9.5%, and serum Phe had increased to approximately 600 µm. Again, serum Phe in female Pahenu2 mice was unaltered following rAAV2 injection in this experiment. Several investigators have noted this difference in treatment effect between male and female mice following rAAV2 administration. Recombinant AAV vectors transduced the liver less efficiently in female mice due to a reduced rate of conversion of single-stranded AAV vector genomes to double-stranded genomes, a limitation that was preventable by pretreating with androgens.23 Improved rAAV2-mediated expression in female Pahenu2 mice has been observed following ovariectomy and testosterone treatment.22
Recombinant AAV2/5 have also been successfully employed to treat murine PKU, but extremely high doses of virus have been required. Administration of 3 × 1013 or 1 × 1014 rAAV2/5 vg per mouse via portal vein injection (MOI ≈ 6 × 105 or 2 × 106 vg/hepatocyte) yielded complete correction of serum phenylalanine levels by 2–4 weeks after injection in male Pahenu2 mice.12 This effect was stable up to 40 weeks after injection. In female mice, serum phenylalanine levels corrected completely only in mice that received 1 × 1014 vg per mouse. The effect was also only temporary as blood phenylalanine levels in treated female Pahenu2 mice began to rise again by 10 weeks after gene transfer and returned to pretreatment levels by 40 weeks. Essentially identical results with an analogous rAAV2/5 vector have been reported in an abstract by other investigators.13 No information regarding transduction frequency or liver PAH activity following rAAV2/5 administration to Pahenu2 mice has been reported.
We have previously employed hepatocyte-mediated therapeutic liver repopulation to explore the physiologic thresholds that influence the success of liver-directed gene therapy for PKU.24 Repopulation of PAH-deficient Pahenu2 liver with more than 10% wild-type PAH-expressing hepatocytes yielded complete correction of serum Phe levels, but only partial correction was achieved in animals with 5–10% repopulation frequency. The amount of PAH activity in the whole liver of a Pahenu2 mouse repopulated with 5% wild-type hepatocytes is capable of producing 31 500 nmol tyrosine/day, an amount that should have been more than sufficient to metabolize completely the entire daily dietary Phe intake (300 nmol/day) of the animal; yet, in the actual experiment serum Phe levels remained slightly elevated (316–685 µm) in mice with 4.8–9.3% repopulation. This result suggests that, at < 10% repopulation, Phe clearance was limited by some factor other than total PAH-activity and that this factor likely correlated with the absolute number of PAH-expressing hepatocytes in the liver. Possible factors could include limited blood flow to PAH positive hepatocytes, cell membrane Phe transport, or cellular BH4 supply. In isolated rat hepatocytes, flux control analysis has suggested that cellular PAH activity and transmembrane Phe transport make equal contributions (i.e., they have nearly equal control coefficients of approximately 0.5) to total Phe flux under conditions of basal enzyme activity.25 In fact, Phe flux was controlled predominantly by the rate of Phe transport into the hepatocyte once PAH activity was maximally activated by glucagon administration. Multiple animal trials with rAAV2 vectors have demonstrated transduction frequency equaling approximately 5% of hepatocytes. We propose that this level of transduction has been insufficient to correct Phe clearance completely in Pahenu2 mice regardless of the amount of PAH activity expressed per cell following vector administration. Transduction of at least 10% of hepatocytes is required to correct hyperphenylalaninemia in murine PKU completely.
The pseudotyping of rAAV2-based vectors with capsid proteins from alternative AAV serotypes has improved the tropism of rAAV vectors for the liver, and has led to enhanced therapeutic gene expression.14,26–28 Recombinant AAV2 vector genomes cross-packaged as rAAV2/1, rAAV2/5, rAAV2/7, or rAAV2/8 vectors all transduced the liver more efficiently than the original rAAV2 vector.14,27 Recombinant AAV2/8 vectors in particular have demonstrated increased frequency of hepatocyte transduction in mouse and dog liver (close to 100% when doses > 1012 vg per mouse are administered), 20 greater levels of stable gene expression, and therefore significantly improved potential efficacy over rAAV2 for liver-targeted gene therapy in hemophilia A and other genetic diseases.15 Whether rAAV2/8 vectors will demonstrate improved tropism in the human liver is yet unknown.
The LSPmPAH rAAV2/8 vector that we constructed has demonstrated vastly improved efficacy over previously employed rAAV vectors in the treatment of murine PKU. Liver PAH activity measured in the liver homogenate was restored to > 10% wild-type levels and presumably transduced more than 10% of hepatocytes. Phenylalanine clearance was restored and hyperphenylalaninemia was completely corrected in five mice that received 5 × 1011 vg per mouse (MOI ≈ 104 vg per hepatocyte) via portal vein injection. This effect was stable at least up to 17 weeks postinjection. Similar therapeutic results in Pahenu2 mice by other investigators using higher doses of a different rAAV2/8 vector are reported elsewhere in this issue.29 These investigators and others20 have reported that injection of rAAV2/8 vector via the tail vein is just as effective as portal vein injection for liver transduction but with the consequence of transducing multiple other tissues including heart and skeletal muscle. In our hands, portal vein administration of LSPmPAH rAAV2/8 more readily corrected serum Phe levels than tail vein injection at least with these relatively low vector doses, but the number of mice treated by tail vein injection was small (n = 2). We chose to further investigate portal vein injection only because of this apparent superior efficacy and the ability to restrict transduction primarily to the liver. Evaluation of multiple tissues other than liver for the presence of PAH activity and of vector genomes has demonstrated that transduction was successfully confined to the liver in our experiment. In our hands, rAAV2/8 has been the first gene transfer vector system to safely produce complete and stable correction of hyperphenylalaninemia in the Pahenu2 mouse model.
In contrast to previous work with rAAV2 and rAAV2/5 vectors, treatment with LSPmPAH rAAV2/8 was equally effective in four male and a single female mouse. Whereas previously employed rAAV vectors have often utilized strong promoters with expression potential in a broad range of tissues and therefore the potential for unwanted adverse effects, the LSP promoter employed here has yielded impressive PAH expression specifically in the liver. No short-term adverse effects of this therapy were noted, although detailed investigation of liver dysfunction using serum chemistries and markers of inflammation has not yet been completed. Likewise, treated animals have not yet been evaluated for humoral or cellular immune responses against either the rAAV2/8 vector or PAH protein. The Pahenu2 mouse model retains PAH protein, albeit catalytically inactive protein, in the liver; therefore, we propose that these animals are likely to be immune tolerant to PAH protein expressed from recombinant vectors.
Evaluation of longer-term expression stability is in progress. Although most of the vector genomes are expected to remain episomal,20 the potential for rare random integration of the rAAV2 genome into host chromosomes does exist. In mice, rAAV2 infection has led to small genomic deletions,30 and multiple chromosomal translocations have been observed in cultured mammalian cells following rAAV2 infection.31 The development of hepatic tumors 1 year after rAAV2 administration to mice has been reported by a single investigator.32 The long-term incidence of adverse effects following rAAV2/8 administration is yet to be evaluated. In comparison to rAAV2 or rAAV2/5 vectors previously used to treat Pahenu2 mice, lower doses of LSPmPAH rAAV2/8 were needed to produce a therapeutic effect; we propose that the administration of lower doses of vector will substantially reduce the risk of long-term adverse events.
Contemporary dietary therapy for inborn errors of intermediate metabolism must be rigorously applied throughout life to insure a satisfactory clinical outcome; nonadherence to the nutritional prescription and consequently adverse disease-associated complications are commonplace. The quest for a truly permanent, effective cure continues. Although questions concerning long-term stability of expression, the incidence of both acute and late adverse effects, and tropism for human liver remain, to date rAAV2/8 is the most promising vector system for liver-directed gene therapy of PKU and allied inborn errors of metabolism.
Acknowledgements
This work was supported by Doernbecher Children’s Hospital Foundation, Portland, OR (COH). Dr Koeberl was supported by the Muscular Dystrophy Association and Genzyme Corporation. The authors would like to thank Drs Markus Grompe and David Koeller for critical reviews of the manuscript. Dr Harding would like to thank the many families affected by PKU, who provided constant encouragement and inspiration for this work.
Abbreviations
- AAV
adeno-associated virus
- PKU
phenylketonuria
- Phe
phenylalanine
- PAH
phenylalanine hydroxylase
References
- 1.Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 1667–1724. [Google Scholar]
- 2.Udenfriend S, Cooper JR. The enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1952;194:503–511. [PubMed] [Google Scholar]
- 3.Azen CG, Koch R, Friedman EG, Berlow S, Coldwell J, Krause W, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am J Dis Child. 1991;145:35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- 4.Koch R, Hanley W, Levy H, Matalon K, Matalon R, Rouse B, et al. The Maternal Phenylketonuria International Study: 1984–2002. Pediatrics. 2003;112:1523–1529. [PubMed] [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference Statement. Phenylketonuria: screening and management, October 16–18, 2000. Pediatrics. 2001;108:972–982. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- 6.Ding Z, Harding CO, Thöny B. State-of-the-art 2003 on PKU gene therapy. Mol Genet Metab. 2004;81:3–8. doi: 10.1016/j.ymgme.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald JD, Bode VC, Dove WF, Shedlovsky A. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci USA. 1990;87:1965–1967. doi: 10.1073/pnas.87.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T-J, Kay MA, Darlington GJ, Woo SLC. Reconstitution of enzymatic activity in hepatocytes of phenylalanine hydroxylase-deficient mice. Somat Cell Mol Genet. 1992;18:89–96. doi: 10.1007/BF01233451. [DOI] [PubMed] [Google Scholar]
- 9.Fang B, Eisensmith RC, Li XHC, Finegold MJ, Shedlovsky A, Dove W, et al. Gene therapy for phenylketonuria: phenotypic correction in a genetically deficient mouse model by adenovirus-mediated hepatic gene therapy. Gene Therapy. 1994;1:247–254. [PubMed] [Google Scholar]
- 10.Laipis PJ, Reyes L, Embury JE, Alexander JJ, Hurt CB, Wein DA, et al. Long term reduction of serum phenylalanine levels in a mouse model of PKU by rAAV-mediated gene therapy. Mol Ther. 2001;3:S293. [Google Scholar]
- 11.Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Therapy. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- 13.Laipis PJ, Charron CE, Embury JE, Perera OP, Porvasnik SL, Fields CR, et al. Correction of maternal phenylketonuria syndrome in the Pahenu2 missense mutant mouse by r-AAV mediated gene therapy. Mol Ther. 2004;9:S334. [Google Scholar]
- 14.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 18.Harding CO, Wild K, Chang D, Messing A, Wolff JA. Metabolic engineering as therapy for inborn errors of metabolism – development of mice with phenylalanine hydroxylase expression in muscle. Gene Therapy. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCaman MW, Robins E. Fluorimetric method for the determination of phenylalanine in serum. J Lab Clin Med. 1962;59:885–890. [Google Scholar]
- 20.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto M, Fitzpatrick T. Competitive inhibition of mammalian tyrosinase by phenylalanine and its relationship to hair pigmentation in phenylketonuria. Nature. 1957;179:199–200. doi: 10.1038/179199b0. [DOI] [PubMed] [Google Scholar]
- 22.Laipis PJ, Charron CE, Ross K, Reyes L, Alexander JJ, Song S, et al. Long-term correction of phenylketonuria in an animal model by recombinant AAV-based gene therapy. J Inher Metab Dis. 2002;25:615–616. [Google Scholar]
- 23.Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- 24.Hamman K, Clark H, Montini E, Al-Dhalimy M, Grompe M, Finegold M, et al. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter M, Knowles RG, Pogson CI. Transport of the aromatic amino acids into isolated rat liver cells. Properties of uptake by two distinct systems. Biochem J. 1986;233:499–506. doi: 10.1042/bj2330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Z, Georgier P, Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Therapy. 2005 doi: 10.1038/sj.gt.3302684. [E-pub ahead of print: 17 November 2005; doi:10.1038/sj.gt.3302684]. [DOI] [PubMed] [Google Scholar]
- 30.Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- 31.Miller DG, Rutledge EA, Russell DW. Chromosomal effects of adeno-associated virus vector integration. Nat Genet. 2002;30:147–148. doi: 10.1038/ng824. [DOI] [PubMed] [Google Scholar]
- 32.Donsante A, Vogler C, Muzyczka N, Crawford JM, Barker J, Flotte T, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Therapy. 2001;8:1343–1346. doi: 10.1038/sj.gt.3301541. [DOI] [PubMed] [Google Scholar]
- 33.Ledley FD, Hahn T, Woo SL. Selection for phenylalanine hydroxylase activity in cells transformed with recombinant retroviruses. Somat Cell Mol Genet. 1987;13:145–154. doi: 10.1007/BF01534694. [DOI] [PubMed] [Google Scholar]