Abstract
The incidence of overweight and obesity is increasing among children with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) or mitochondrial trifunctional (TFP) deficiency. Traditional treatment includes fasting avoidance and consumption of a low-fat, high-carbohydrate diet. A diet higher in protein and lower in carbohydrate may help to lower total energy intake while maintaining good metabolic control. To determine the short-term safety and eYcacy of a high protein diet, subjects were admitted to the General Clinical Research Center and fed an ad-libitum high-protein diet and a high-carbohydrate diet for 6 days each using a randomized, crossover design. Nine subjects with LCHAD or TFP deficiency, age 7–14 were enrolled. Body composition was determined by DEXA. Total energy intake was evaluated daily. Resting energy expenditure and substrate utilization were determined by indirect calorimetry. Post-prandial metabolic responses of plasma glucose, insulin, leptin, ghrelin, acylcarnitines, and triglyceride were determined in response to a liquid meal. Subjects had a higher fat mass, lower lean mass and higher plasma leptin levels compared to reference values. While on the high protein diet energy consumption was an average of 50 kcals/day lower (p=0.02) and resting energy expenditure was an average of 170 kcals/day higher (p=0.05) compared to the high carbohydrate diet. Short-term higher protein diets were safe, well tolerated, and resulted in lowered energy intake and increased energy expenditure than the standard high-carbohydrate diet. Long-term studies are needed to determine whether higher protein diets will reduce the risk of overweight and obesity in children with LCHAD or TFP deficiency.
Keywords: Dietary protein, Fatty acid oxidation disorders, Long-chain 3-hydroxyacylCoA dehydrogenase deficiency, Trifunctional protein deficiency, Energy intake, Energy expenditure
Introduction
deficiency of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), either isolated or as a part of generalized trifunctional protein (TFP) deficiency, typically manifests with acute episodes of fasting or illness-induced hypoketotic hypoglycemia [1]. Cardiomyopathy, liver damage, and rhabdomyolysis are frequent clinical complications associated with acute exacerbation and may be lethal. Acute and chronic complications of LCHAD and TFP deficiency can be tempered by dietary therapy, including fasting avoidance and consumption of an energy-sufficient diet low in long-chain fatty acids (LCFA) but supplemented with medium-chain triglycerides (MCT) [2-4].
These rare disorders can be caused by mutations in the genes for either the α-subunit (OMIM # 600890) or β-subunit (OMIM #143450) of the mitochondrial trifunctional protein, but a missense mutation (c.1528G>C) in the α-subunit is the most prevalent. The biochemical hallmarks of this disorder are accumulation of long-chain 3-hydroxyacylcarnitines and free fatty acids in plasma and dicarboxylic acids in urine. Dietary therapy minimizes LCFA oxidation as evidenced by lower plasma long-chain 3-hydroxyacylcarnitine proWles and reduces the occurrence of acute exacerbations.
In our prospective cohort, we have noted excess weight gain with time and increased risk of overweight and obesity in this group of patients. Excess body weight poses a particular therapeutic dilemma to children with LCHAD or TFP deficiency. Once overweight, a reduction in body fat stores cannot be accomplished through traditional weight loss strategies such as energy restriction or increased exercise without potentially jeopardizing the metabolic control and health of the subject. High-protein diets have been shown to enhance satiety, reduce food intake, and lead to weight loss when fed ad-libitum [5,6]. Therefore, in this study we investigated the short-term effects of an alternate dietary treatment, a long chain fat restricted diet that was higher in protein and lower in carbohydrate content than typically consumed, on energy balance, metabolic control and levels of leptin and ghrelin in nine subjects with LCHAD or TFP deficiency.
Methods
Subjects
Nine subjects, seven years of age or older, with a conWrmed diagnosis of LCHAD or TFP deficiency were recruited from a previous cohort for this study [7,8]. Six cases had previously published mutation analyses [9-12]. The subjects' ages ranged from 7 to 14 years. Data collected during the high protein diet from one subject was excluded due to objection to the diet and oral intake less than 50% of her estimated needs. The diets were otherwise well tolerated by all subjects with no adverse events. The Institutional Review Board at OHSU approved the study protocol, each subject's legal guardian gave written informed consent and each subject gave written assent.
Study protocol
Subjects were admitted to the OHSU General Clinical Research Center (GCRC) inpatient facility for 2 weeks. Body composition was measured by DEXA at the beginning of the study (Discovery QDR Series Hologic, Bedford, MA). They were then fed a high-carbohydrate and a high-protein diet for 6 days in a randomized, crossover study design. There was no washout period between the diets.
Diet composition
The composition of the experimental diets is given in Table 1. Diets were served in three meals plus a snack each day with a 3-day cycle menu. The daily menu provided 1800, 2200 or 2400 kcals per day depending on the age and body weight of the subject. This provided approximately 110–120% of estimated energy needs for the subjects. All meals were prepared in the Bionutrition Research Kitchen at the GCRC. During each controlled diet phase, participants were asked to eat only the food provided to them by the study and nothing else. Total food intake (weight of food dispensed minus weight of uneaten food returned) was recorded and energy and nutrient intakes were calculated using Pronutra® software by the GCRC bionutrition staff (Viocare Technologies, Inc., Princeton, NJ). Body weight was measured each morning prior to breakfast.
Table 1.
Macronutrient composition of experimental diets
| Diet | Protein | CHO | LCFA | MCT | CHO : Protein ratio (gm : gm) |
|---|---|---|---|---|---|
| (% total energy) | |||||
| High-CHO | 11 | 67 | 10 | 12 | 6.1 |
| High-Protein | 30 | 48 | 10 | 12 | 1.4 |
CHO, carbohydrate; LCFA, long-chain fatty acids; MCT, medium-chain triglyceride.
Energy expenditure
Subjects were awakened, allowed to use the restroom and resting energy expenditure was then measured while the subject rested in bed. Oxygen consumption and CO2 production were measured and resting energy expenditure (REE) and respiratory quotient (RQ) were calculated by indirect calorimetry on the last two days of each diet phase (days 6 and 7, Sensormedics Corp. model 29n, Yorba Linda, CA). The results of the two measurements were averaged to provide a better estimate of true resting energy expenditure. A 24-h urine collection was analyzed for markers of protein metabolism (urinary urea nitrogen and creatinine concentration). Substrate oxidation was calculated using resting VO2 consumption (L/min), VCO2 production (L/min) and 24-h urea excretion (g/min) by the equations of Jequier, et al. [13].
Metabolic response to a meal
The metabolic response to a single liquid test meal was measured on the last day (day 7) of each diet phase. The macronutrient content of the liquid meal was the same as the assigned research diet (either high protein or high carbohydrate) and provided 500 kcals. An intravenous catheter was placed in a peripheral arm vein to facilitate multiple blood draws. Blood samples were drawn immediately before consuming the liquid meal (fasting) and again 1, 2 and 4 h post-prandially. Blood samples were analyzed for plasma glucose, insulin, triglyceride, leptin, total ghrelin, and hydroxyacylcarnitine concentrations. Blood concentrations of measured parameters were plotted over time to create a post-prandial response curve. Total area under the curve (AUC) was calculated for each blood analyte using the trapezoidal method.
Blood measurements
Plasma glucose and triglyceride concentrations were measured in the OHSU clinical laboratory using an autoanalyzer. Plasma insulin was measured by ELISA using a kit purchased from Diagnostic Products Corporation (Los Angeles, CA). Plasma leptin and total ghrelin concentrations were measured by radioimmunoassay using kits purchased from Diagnostic Systems Laboratories (Webster, TX) and Linco Research, Inc. (St. Louis, MO), respectively. Total ghrelin concentrations were measured in six of nine subjects whose blood was properly processed with phenylmethylsulfonyl Xuoride (PMSF) and then acidified with hydrochloric acid (HCL). Plasma hydroxyacylcarnitine concentrations were measured by tandem mass spectroscopy at the Biochemical Genetics Laboratory, Mayo Clinic (Rochester, MN) [14]. The sum of the long-chain hydroxyacylcarnitine concentrations for each blood sample was calculated and used in the data analysis. Acylcarnitine species included in the calculated total were C14:0-OH, C14:1-OH, C16:0-OH, C16:1-OH, C18:0-OH, C18:1-OH and C18:2-OH.
Data analysis
Differences in outcome variables after consuming the high protein and high carbohydrate diets were analyzed with a mixed model in which the fixed effects included diet (high protein or high carbohydrate) and the order in which the diets were provided. AUC for blood parameters was considered a good representation of the effect of diet and was used as the dependent variable in the model. Differences were considered statistically significant when p ⩽ 0.05 and analysis was performed with the PROC MIXED procedure of SAS (SAS Institute, Inc., Cary, NC). Fasting blood levels of insulin, leptin and ghrelin were compared to a group of normal children with a similar age, sex and BMI distribution as our study subjects by two-sample t-test.
Results
Three males and six females completed the 2-week study. Subject characteristics are given in Table 2. Three of the subjects were classified as at risk for obesity with a BMI ⩾ 95 percentile. Two subjects were classified as underweight with a BMI < 5th percentile. Four subjects were within the normal range (5–85 percentile) of BMI for children. Regardless of BMI percentile, all subjects had higher percent fat mass and lower percent lean mass than predicted based on published reference data [15].
Table 2.
Subject characteristics
| Age (years) | Sex | Mutations | Body composition |
Leptin(mg/L) |
Ghrelin (pg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) |
BMI %tile |
Lean mass (kg) |
Subjects' lean mass index (kg/m2) |
Predicted lean mass index (kg/m2)(16) |
Fat mass (kg) |
Subjects' fat mass index (kg/m2) |
Predicted fat mass index (kg/m2) (16) |
High CHO |
High protein |
Control subjects |
High CHO |
High protein |
Control subjects |
|||
| 7.5 | F | c.1528G>C /c.274_278del | 12.5 | 3 | 6.62 | 9.2 | 12.1 | 1.20 | 2.8 | 1.9 | 1.2 | NA | 1.11 | 1850 | 1975 | |
| 8.4 | F | c.1528G>C /c.274-278del | 21.6 | 96 | 10.41 | 11.6 | 14.5 | 8.40 | 9.1 | 9.3 | 70.3 | 50.9 | 5.97 | 1484 | 1577 | 1669 |
| 8.7 | F | c.901G>A β-subunit/ ? | 13.3 | 3 | 7.90 | 10.8 | 12.1 | 0.75 | 2.0 | 1.9 | 4.6 | 2.7 | 2.8 | 2133 | 1916 | 1516 |
| 10.3 | M | c.1528G>C/c.1528G>C | 15.3 | 25 | 13.18 | 11.4 | 13.1 | 2.73 | 3.3 | 1.6 | 7.3 | 8.0 | 1.69 | 1234 | ||
| 12.6 | M | c.1528G>C/? | 19.5 | 70 | 14.33 | 14.0 | 17 | 3.79 | 4.8 | 3.1 | 8.3 | 6.0 | 3.4 | 913 | ||
| 12.7 | F | c.1528G>C/c.1528G>C | 24.7 | 95 | 18.08 | 13.6 | 16 | 14.22 | 10.6 | 7.6 | 46.0 | 49.0 | 1.14 | 1038 | ||
| 13.6 | M | c.1528G>C/c.1678C>T | 24.5 | 95 | 22.98 | 16.3 | 17.7 | 10.22 | 7.8 | 5.2 | 15.8 | 17.3 | 3.68 | 673 | 1622 | 1285 |
| 14.1 | F | c.1528G>C/c.479-482T AGC>AATA |
18.9 | 50 | 16.85 | 13.8 | 17.9 | 3.01 | 4.2 | 2.6 | NA | 4.5 | 19.03 | 1423 | 632 | 889 |
| 14.4 | F | c.901G>A β-subunit/ ? | 17.7 | 25 | 17.02 | 13.3 | 17.4 | 2.77 | 3.8 | 2 | 8.3 | 7.9 | 7.83 | 888 | 956 | 2016 |
| Mean±SD: | 18.7±4.4 | 51±39 | 14.15±5.24 | 12.7±2.1 | 15.3±2.4 | 5.2±4.6 | 5.4 +3.0 | 3.9±2.8 | 20.2±24.6 | 18.3±20.0 | 5.18±5.6 | 1408±554 | 1341±528 | 1393±428 | ||
BMI, body mass index; BMI % tile, percentile BMI for age on US growth charts. Lean mass and fat mass measured by DEXA. Subjects' lean mass index was calculated as kg lean mass/ht in m2. Subjects' fat mass index was calculated from kg fat mass/ht in m2. These indices are compared to values of children of similar age and BMI (15). Leptin=fasting plasma leptin (mg/L) and ghrelin=fasting ghrelin (pg/ml) compared to fasting concentrations in unaffected control children of similar age, sex and BMI.
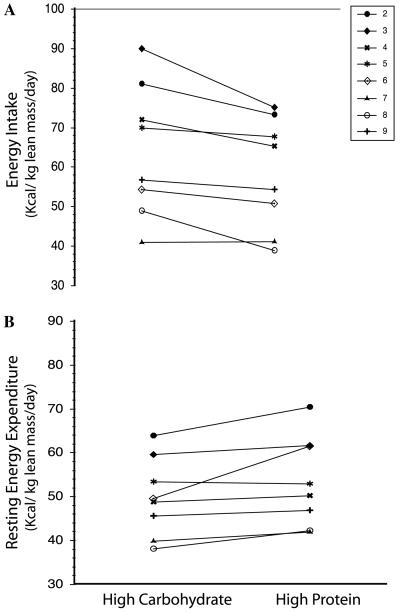
Body weight remained relatively stable over the course of the study with a mean change in body weight of +0.6 kg and a range of −0.2 to +1.9 kg. The goal of the study was to test the effects of differential dietary macronutrient content without the confounding effects of a change in body weight. Macronutrient content of the two diets is given in Table 1. During the high protein diet phase, energy intake was an average of 6 kcal/kg lean mass/day lower (p=0.02), and resting energy expenditure (REE) was an average of 4 kcals/kg lean mass/day higher (p=0.05) compared to the high carbohydrate diet phase (Fig. 1). One subject (subject 6) had a proportionately larger change in REE during the high protein diet (Table 3). The difference in mean REE among all subjects remained significant whether subject 6 was included or excluded from the analysis. Resting RQ was lower during the high-protein diet phase most likely due to the lower RQ of protein oxidation (Table 3). The estimated substrate oxidation data confirm this hypothesis: CHO oxidation was lower and protein oxidation was higher during the high protein diet phase compared to the high carbohydrate diet phase but fat oxidation was not different (Table 4).
Fig. 1.
Effect of diet on energy intake and resting energy expenditure in subjects with LCHAD or TFP deficiency. (A) Change in daily energy intake (kcals/day) from the high carbohydrate diet phase (High CHO) and the high protein diet phase (High Protein). Energy intake was significantly lower during the high protein diet phase (High CHO 1726 ± 349, High Protein 1676 ± 271, p = 0.021). (B) Change in resting energy expenditure from the high carbohydrate diet phase (High CHO) and the high protein diet phase (High Protein). Resting energy expenditure was significantly higher during the high protein diet phase (High CHO 1398 ± 305 High Protein 1569 ± 329, p=0.05).
Table 3.
Resting energy expenditure (REE), respiratory quotient (RQ)
| Age | REE (kcal/day)# |
RQ |
||
|---|---|---|---|---|
| High CHO | High protein | High CHO | High protein | |
| 7.5 | 926 | 0.91 | ||
| 8.4 | 1346 | 1483 | 0.965 | 0.945 |
| 8.7 | 984 | 1018 | 0.97 | 0.925 |
| 10.3 | 1338 | 1381 | 1.02 | 0.95 |
| 12.6 | 1598 | 1583 | 0.885 | 0.895 |
| 12.7 | 1730 | 2148 | 0.895 | 0.865 |
| 13.6 | 1813 | 1905 | 0.89 | 0.885 |
| 14.1 | 1321 | 1468 | 0.97 | 0.89 |
| 14.4 | 1528 | 1570 | 0.95 | 0.875 |
| Mean±SD | 1398±305 | 1569±339* | 0.94±0.05 | 0.90±0.03* |
REE, resting energy expenditure; kcal, kilocalorie. Respiratory Quotient (RQ) was calculated from VO2 and VCO2 values measured by indirect calorimetry on the last two days of the high carbohydrate and the high protein diet. The two measures were averaged and the average gas exchange (VO2 (L/min) and VCO2(L/min)) plus urinary urea nitrogen (UUN) were used to calculate estimated substrate oxidation by the formulas of Jeuquier et al.
=statistically-significant difference (p ⩽ 0.05).
kcal *4.184 = kilojoules (kJ, SI units).
Table 4.
Substrate oxidation
| High carbohydrate | High protein | |
|---|---|---|
| Substrate oxidation (% of REE) | ||
| CHO | 81±16 | 58±10* |
| Protein | 8±3 | 22±4* |
| Fat | 14±11 | 17±10 |
Results are the mean±SD.
=statistically-significant difference (p < 0.05).
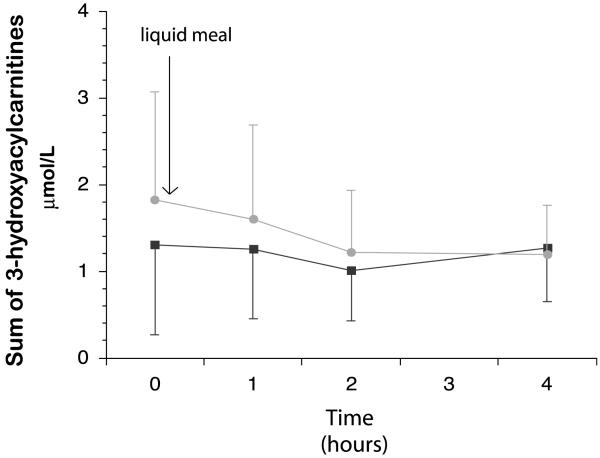
There was a non-significant trend (p=0.07) for the sum of fasting long-chain hydroxyacylcarnitine concentrations to be higher during the high protein diet phase (Fig. 2). There were no significant differences in the sum of the long-chain acylcarnitine AUC following the high carbohydrate test meal (4.28±2.8 μmol/L/4 h) and the high protein test meal (5.18±3.2 μmol/L/4 h; p=0.09). Fasting and AUC plasma insulin levels did not differ between the high carbohydrate and high protein diet periods (Table 3). Fasting insulin levels were not different from control subjects. Even though there was a trend (p=0.054) for plasma glucose AUC to be lower during the high protein diet, no episode of hypoglycemia was noted during the entire 2-week study. Fasting and AUC plasma triglyceride concentrations were low on both diets, but significantly lower during the high protein diet than the high carbohydrate diet (Table 5).
Fig. 2.
Effect of a High CHO or a High Protein liquid test meal on sum of long-chain hydroxyacylcarnitine concentrations in subjects with LCHAD or TFP deficiency. Long-chain hydroxyacylcarnitine concentrations immediately before (time 0) and 1, 2 and 4 h after the test meal. There were no significant differences in the sum of the long-chain acylcarnitine AUC following the high carbohydrate test meal (4.28 ± 2.8 μmol/L/4 h) and the high protein test meal (5.18 ± 3.2 μmol/L/4 h; p = 0.09).
Table 5.
Plasma insulin, glucose, and triglyceride concentrations
| Fasting |
AUC |
|||
|---|---|---|---|---|
| High carbohydrate |
High protein |
High carbohydrate |
High protein |
|
| Insulin (μU/ml) | 8±4 | 9±5 | 177±150 | 120±62 |
| Glucose (mg/dl)a | 94±4 | 90±5 | 451±59 | 436±69 |
| Triglycerides (mg/dl)b | 71±22 | 48±17* | 312±132 | 207±87 |
Results are the mean±SD.
Glucose (mg/dl)/18 = mmol/L (SI units).
Triglycerides (mg/dl) * 0.011 = mmol/L (SI units).
=statistically-significant difference (p ≤ 0.05).
Plasma levels of leptin did not differ between the diet phases (Table 2). However, compared to control values, fasting plasma leptin concentrations were higher in the study participants. Ghrelin data presented here represent samples collected from six subjects to which an appropriate protease inhibitor and acid were added. Fasting and post-prandial ghrelin AUC was similar between the two diets (mean±SD AUC ghrelin: high carbohydrate diet=5124±2178 pg/ml; high protein diet=5017 ±1320 pg/ml). Fasting ghrelin concentrations were not significantly different from controls. Maximum ghrelin suppression following the research meal (difference in fasting and 1 h ghrelin concentrations) was approximately 14% in both diets (p=0.5).
Discussion
As diagnosis and dietary treatment for inherited disorders of long-chain fatty acid oxidation improves, more children are living into adolescence and beyond. An emerging problem among these patients is a high incidence of pediatric obesity (defined as a BMI ⩾ 95% for age). Data from National Health and Nutrition Examination Survey (NHANES) III indicate that 16% of the general pediatric population is now considered obese [16,17]. In the current study we found 30% of subjects were obese. We have also observed increasing BMI percentiles with time in most of our LCHAD or TFP deficient subjects. Current dietary therapy restricts fat and calls for avoidance of prolonged fasting. Because higher protein diets may have favorable effects on body weight, including a reduced induction of fatty acid synthesis, compared to a high carbohydrate diet, we compared the effects of these diets on hormonal mediators of body weight regulation and metabolic control (Table 5).
Results of body composition analysis indicate that subjects with LCHAD and TFP deficiency have a higher fat mass and lower lean mass in comparison to published data on unaffected children [15,18]. This result could be predicted as dietary fat has two possible fates: either oxidation for energy production or storage as a structural moiety or as tissue triglyceride. If the ability to oxidize long-chain fatty acids, the primary component of dietary fat, is dramatically reduced, then the remaining amount of dietary fat must be stored. The etiology of the characteristic lower lean mass is unknown but may be related to lower activity levels compared to unaffected children.
The feeding study was designed so that total fat, long-chain fat and medium chain triglyceride (MCT) content did not differ between the two diets, but the carbohydrate to protein ratio was very different. The high protein diet resulted in a lower total energy intake and higher resting energy expenditure compared to the high carbohydrate diet. Differences in energy balance and metabolic control between the two diets can therefore be attributed to the altered carbohydrate to protein ratio and not to altered fat intake. Even though energy intake was lower and resting energy expenditure was higher during the high protein diet, due to the short period of time the subjects were followed we did not observe a change in body weight. Looking at the growth charts of these subjects before study entry, we noted a rapid increase in BMI percentile compared to the increase in height (data not shown). Over a longer period of time, the influence of a high protein diet on energy balance may reduce the rate at which subjects gain weight relative to height, rather than lead to absolute weight loss.
Weight loss studies in adults have reported no change or a lower resting energy expenditure with higher protein diets compared with a high carbohydrate diet [6,19]. An increased thermic effect of food (TEF) following consumption of a high protein diet has been reported but increased resting energy expenditure is not consistently observed [20]. It is possible that higher resting energy expenditure, despite a lower energy intake, is unique to children with long-chain fatty acid oxidation disorders. Others have postulated that patients with fatty acid oxidation disorders have decreased tricarboxcylic acid (TCA) cycle intermediates and limited oxaloacetate concentrations because of their defect in fatty acid oxidation [21]. Amino acids from protein can be anaplerotic and replenish the cycle to enhance energy production. Higher resting energy expenditure in the face of lower energy intake supports this hypothesis.
Concerns regarding lowering the carbohydrate content of diets of children with LCHAD or TFP deficiency are that the incidence of hypoglycemia will increase, that metabolic control will deteriorate, or both. In this short-term study we did not observe hypoglycemic symptoms during the high protein diet in any of the subjects. We also evaluated insulin concentrations and found they were similar to published data in children suggesting that these children with LCHAD or TFP deficiency had normal insulin sensitivity despite increased adiposity. The sum of long-chain hydroxyacylcarnitine concentrations, a measure of partial fatty acid oxidation metabolites in subjects with LCHAD or TFP deficiency and an indicator of metabolic control, did show a non-significant trend toward increased levels in the fasting state but postprandial AUC levels were not different. A rise in hydroxyacylcarnitine concentrations with a high protein diet could be detrimental to long-term outcomes even if it prevents obesity [8]. Further long-term study is needed to determine the metabolic safety of a high-protein diet in individuals with fatty acid oxidation disorders.
A role in the regulation of fatty acid oxidation has recently been reported for numerous hormones, including leptin and ghrelin, that are involved in the control of food intake and body weight. Plasma total ghrelin concentrations and post-prandial suppression of ghrelin in our subjects was similar to control values and did not differ between the high protein and high carbohydrate diets in our study. Total plasma ghrelin does not appear to be altered in children with TFP/LCHAD deficiency. Leptin, an adipocytokine synthesized and secreted by adipose tissue, is believed to signal energy sufficiency to the brain and promote energy expenditure in the periphery via alterations in the fatty acid oxidation system [22,23]. Leptin levels increase with increasing adiposity. The subjects studied here had higher leptin levels than predicted even for their increased adiposity. Possible explanations for increased leptin concentration include abnormalities in adipocyte fatty acid metabolism may result in increased leptin secretion, TFP/LCHAD deficiency may lead to leptin resistance, or that any leptin-mediated central inhibitory signal is overcome by the need to eat frequent meals to avoid hypoglycemia. Future studies comparing children with LCHAD and TFP deficiency and controls are needed to further explain this observation.
In conclusion, subjects with LCHAD and TFP deficiency appear to have abnormal body composition and elevated circulating leptin concentrations compared to reference data. Short-term consumption of a high protein diet was associated with decreased energy intake and increased resting energy expenditure without exacerbating their metabolic control or causing hypoglycemia. Future studies should test the long-term effects of high protein diets on metabolic outcomes, weight gain, body composition and levels of hormones involved in body weight regulation in this patient population. Such studies would be not only important for clinical management but could provide new insights into the role of fatty acid oxidation in insulin signaling and weight regulation in humans.
Acknowledgments
We thank Dr. Arnold Strauss (Vanderbilt University, Nashville, TN) for providing the mutation analysis results of subjects not available in the medical record and Dr. Dawn Peters for assistance with the statistical analysis. This research was supported by PHS Grants RR000334, F32-DK065400 and K23-RR-021979.
References
- 1.den Boer ME, Wanders RJ, Morris AA, L IJ, Heymans HS, Wijburg FA. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics. 2002;109:99–104. doi: 10.1542/peds.109.1.99. [DOI] [PubMed] [Google Scholar]
- 2.Gillingham M, Van Calcar SC, Ney DM, Wolff J, Harding CO. Dietary Management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD). A case report and survey. J. Inher. Metab. Dis. 1999;22:123–131. doi: 10.1023/a:1005437616934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J. Inherit. Metab. Dis. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 4.Tyni T, Palotie A, Viinikka L, Valanne L, Salo MK, von Dobeln U, Jackson S, Wanders R, Venizelos N, Pihko H. Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G15 28C mutation: clinical presentation of thirteen patients [see comments] J. Pediatr. 1997;130:67–76. doi: 10.1016/s0022-3476(97)70312-3. [DOI] [PubMed] [Google Scholar]
- 5.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int. J. Obes. Relat. Metab. Disord. 1999;23:528–536. doi: 10.1038/sj.ijo.0800867. [DOI] [PubMed] [Google Scholar]
- 6.Weigle DS, Breen P, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005;82:1495–1502. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, Harding CO. Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 2003;79:114–123. doi: 10.1016/s1096-7192(03)00073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillingham MB, Weleber RG, Neuringer M, Connor WE, Mills M, van Calcar S, Ver Hoeve J, Wolff J, Harding CO. Effect of optimal dietary therapy upon visual function in children with long-chain 3-hydroxyacyl CoA dehydrogenase and trifunctional protein deficiency. Mol. Genet. Metab. 2005;86:124–133. doi: 10.1016/j.ymgme.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, Strauss AW. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N. Engl. J. Med. 1999;340:1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs JD, Jr., Sims HF, Powell CK, Bennett MJ, Hale DE, Treem WR, Strauss AW. Maternal acute fatty liver of pregnancy associated with fetal trifunctional protein deficiency: molecular characterization of a novel maternal mutant allele. Pediatr. Res. 1996;40:393–398. doi: 10.1203/00006450-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Spiekerkoetter U, Sun B, Khuchua Z, Bennett MJ, Strauss AW. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- 12.Treem WR, Rinaldo P, Hale DE, Stanley CA, Millington DS, Hyams JS, Jackson S, Turnbull DM. Acute fatty liver of pregnancy and long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Hepatology. 1994;19:339–345. [PubMed] [Google Scholar]
- 13.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu. Rev. Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 14.Matern D, Strauss AW, Hillman SL, Mayatepek E, Millington DS, Trefz FK. Diagnosis of mitochondrial trifunctional protein deficiency in a blood spot from the newborn screening card by tandem mass spectrometry and DNA analysis. Pediatr. Res. 1999;46:45–49. doi: 10.1203/00006450-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 16.Troiano RP, Flegal KM. Overweight prevalence among youth in the United States: why so many different numbers? Int. J .Obes. Relat. Metab. Disord. 1999;23(Suppl 2):S22–S27. doi: 10.1038/sj.ijo.0800855. [DOI] [PubMed] [Google Scholar]
- 17.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 18.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy X-ray absorptiometry in white children and young adults. Arch. Dis. Child. 2002;87:341–347. doi: 10.1136/adc.87.4.341. discussion 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 20.Luscombe ND, Clifton PM, Noakes M, Farnsworth E, Wittert G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int. J. Obes. Relat. Metab. Disord. 2003;27:582–590. doi: 10.1038/sj.ijo.0802270. [DOI] [PubMed] [Google Scholar]
- 21.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J. Clin. Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 23.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]