Abstract
The skull is distinguished from other parts of the skeleton by its composite construction. The sutures between bony elements provide for interstitial growth of the cranium, but at the same time they alter the transmission of stress and strain through the skull. Strain gages were bonded to the frontal and parietal bones of miniature pigs and across the interfrontal, interparietal and coronal sutures. Strains were recorded 1) during natural mastication in conjunction with electromyographic activity from the jaw muscles and 2) during stimulation of various cranial muscles in anesthetized animals. Vault sutures exhibited vastly higher strains than did the adjoining bones. Further, bone strain primarily reflected torsion of the braincase set up by asymmetrical muscle contraction; the tensile axis alternated between +45° and −45° depending on which diagonal masseter/temporalis pair was most active. However, suture strains were not related to overall torsion but instead were responses to local muscle actions. Only the coronal suture showed significant strain (tension) during jaw opening; this was caused by the contraction of neck muscles. All sutures showed strain during jaw closing, but polarity depended on the pattern of muscle usage. For example, masseter contraction tensed the coronal suture and the anterior part of the interfrontal suture, whereas the temporalis caused compression in these locations. Peak tensile strains were larger than peak compressive strains. Histology suggested that the skull is bent at the sutures, with the ectocranial surface tensed and the endocranial surface predominantly compressed. Collectively, these results indicate that skulls with patent sutures should be analyzed as complexes of independent parts rather than solid structures.
Keywords: skull, cranial sutures, biomechanics, bone strain, pig
For purposes of modeling, the mammalian cranium is often considered a one-piece, albeit complex structure (Badoux, 1970; Demes, 1982; Alexandridis et al., 1985). Sutures are presumed to be rigid, or at least to transmit loads efficiently from one bone to another. This necessary assumption is justified by the fact that most sutures in many taxa are only temporary structures. When fusion occurs, the cranium is literally a one-piece element. Nevertheless, there is accumulating evidence that patent sutures are relatively flexible and constitute zones where loads are damped and energy is absorbed (Behrents et al., 1978; Jaslow, 1990; Persson, 1995).
In contrast to a large body of clinical, surgical, histological, and organ culture studies on sutures (for a review, see Persson, 1995), actual measurement of mechanical conditions has rarely been attempted. In vitro loading of sutures has generally indicated that sutures are less stiff and less strong than surrounding bones (but see Hubbard et al., 1971; Jaslow, 1990; Sutton, 1993; Jaslow and Biewener, 1995). In intact but anesthetized animals, stimulation of jaw muscles resulted in sutural strains that were much higher than strains in neighboring bones (Behrents et al., 1978; Herring and Mucci, 1991), as would be expected from the difference in stiffness. In vivo studies of normally functioning animals are rare, but work on the skull roof of the lizard Varanus exanthematicus (Smith and Hylander, 1985) and the zygomatic arch of the pig, Sus scrofa, showed the same discrepancy between suture strain and bone strain (Herring and Mucci, 1991; Herring et al., 1996).
Debate has surrounded the issue of whether braincase loading is predominantly tensile or compressive. Loading due to growth, e.g. expansion of the brain, is usually assumed to be tensile at the sutural margins and thus to promote osteogenesis (Smith and McKeown, 1974). Unfortunately, these low, chronic loads are difficult to measure, and there is no experimental confirmation of their existence.
Loading due to function, e.g. mastication, is usually assumed to be compressive, because bite force imposes a compressive load on the teeth (e.g., Badoux, 1966). However, a few authors have pointed out that masticatory muscle contraction should tense some sutural areas (Rak, 1978; Kimbel and Rak, 1985; Katsaros et al., 1994). Greaves (1985) has proposed that unilateral bite forces and contralateral joint reaction loads torque the braincase during chewing, thus giving rise to alternate tension and compression, depending on chewing side. These dynamic loads can be assessed experimentally using strain gages. Little evidence on the braincase is available, and results on adjacent areas are mixed. The supraorbital region of several anthropoid primates exhibits principal tensile strains directed roughly laterally, but with some suggestion of slight twisting (albeit usually opposite to the direction predicted by Greaves) during mastication (Hylander et al., 1991a,b; Hylander and Ravosa, 1992; Ross and Hylander, 1996). The anthropoid postorbital septum has proved to be a very complex and variable system that is not amenable to simple mechanical analogies, including the notion of facial torsion, although torsion may be a factor (Oyen and Tsay, 1991; Oyen et al., 1996: Ross and Hylander, 1996). Stronger evidence for torsion in the frontal bone was shown by a simulation of bite loads in pig skulls, but strain magnitudes were inconsistent (Fisher et al., 1976). Only two studies to date have examined braincase sutures, and these investigations were not on awake animals. Behrents and coworkers (1978) found tensile strain at the sagittal suture after stimulating the temporalis in rhesus macaques. Jaslow and Biewener (1995) loaded goat skulls under impact, which resulted in substantial compressive strains on all braincase bones and sutures, as well as hinge-like bending in the interfrontal suture.
In view of these conflicting results, the absence of studies on braincase behavior during mastication, and the probable influence of sutural strain on cranial growth, we undertook this investigation of the braincase. Our primary goals were 1) to delineate the pattern of strain during mastication, in particular to assess whether torsion occurs; and 2) to compare the relative contributions of bony elements and sutures to the overall strain pattern. The pig, Sus scrofa, was used in order to build on a database that already includes similar information about strain patterns in the zygomatic arch and suture (Herring and Mucci, 1991; Herring et al., 1996).
Methods and Materials
A total of 17 juvenile female Hanford miniature swine (Charles River Labs, Wilmington, MA), ranging in age from 75 to 161 days and in weight from 10.4 to 31.8 kg, were studied (Table 1). All procedures were approved by the University of Washington Animal Care Committee. Animals had undergone at least one session of fine-wire electromyography (EMG) and video recording of mastication (for technique, see Huang et al., 1994). In addition to accustoming the animals to the experimental equipment, these sessions served to verify that they were subjectively normal in function. A few animals had had minor surgery to the jaws (e.g., to implant radio-opaque markers) as part of other investigations, but none of these procedures affected the braincase, nor did they modify function.
TABLE 1. Subjects and strain gage placements1.
| Pig no. | Weight (kg) |
Age (days) |
Bone (Rosette gage) | Suture (Single element gage) | |||
|---|---|---|---|---|---|---|---|
| Frontal | Parietal | Coronal | Interfrontal | Interparietal | |||
| 147 | 22.6 | 115 | R | ||||
| 154 | 20.0 | 96 | R, L | ||||
| 155 | 18.1 | 98 | R, L | P2 | |||
| 156 | 17.2 | 102 | R, L | A2 | |||
| 157 | 21.8 | 131 | L | L | |||
| 158 | 15.0 | 133 | L | L | |||
| 159 | 15.0 | 100 | R, L | P | |||
| 160 | 16.8 | 102 | R, L | P | |||
| 161 | 17.2 | 114 | R, L | ||||
| 162 | 27.7 | 125 | L | P, MP, M | P, A | ||
| 163 | 15.4 | 102 | L | MP | A | ||
| 164 | 16.8 | 99 | L | L | P | A | |
| 165 | 31.8 | 136 | L | MP, M | P2 | ||
| 166 | 14.1 | 84 | P | A | |||
| 167 | 31.8 | 161 | R | P, MP, M | P, A | ||
| 183 | 10.4 | 75 | R | P, A | |||
| 184 | 12.7 | 75 | L | P, A | |||
| N = 17 | 9 (8) | 6 (4) | 11 (8) | 13 (8) | 11 (7) | ||
| Avg. | 19.1 ± 6.2 | 109 ± 23 | |||||
Midline gages were placed in the posterior (P), middle-posterior (MP), middle (M) and/or anterior (A) sections of their respective sutures. Bone and coronal suture gages were placed on right (R) and/or left (L). Totals are given for total gage sites (number of subjects).
The interparietal sutures of pigs 155, 156 and 165 gave very low strains (less than 10 με) under all conditions. Examination of the dried skulls revealed synostosis in 155 and 165. Although the suture appeared patent in 156 (a sibling of 155), we assume that some internal fusion had occurred and was the cause of the low strains. Data from these gages were not used in the calculations.
Pigs were anesthetized by mask with a mixture of halothane and nitrous oxide. The braincase was exposed by a sagittal and two transverse (typically over the orbits and near the nuchal crest) incisions. The periosteum was similarly incised. Gage sites were prepared by sanding, cauterizing, degreasing, neutralizing and drying the bony surface. Foil strain gages, soldered in a three-wire configuration and coated with insulation (M-coat A followed by M-coat D, Measurements Group, Raleigh, NC), were affixed to the sites using cyanoacrylate glue (Measurements Group). Stacked rosette gages (SA-06-030WR-120, Measurements Group) were used for the frontal and parietal bones, and single-element (EP-08-125BT-120, Measurements Group) gages were placed over sutures. As in our previous work (e.g., Herring and Mucci, 1991) the sutural gages were placed at right angles to the suture with tabs bonded to the adjacent bones; the gage elements were isolated from sutural soft tissues by narrow strips of Teflon tape. Lead wires from the gages were secured using 4-0 silk stitches, and the incisions were closed. Wherever possible, the periosteal incisions were closed separately. Three to eight channels of strain (1–7 gages) were recorded from each pig (Table 1). Some animals had additional transducers placed on the zygomatic arch, jaw joint or temporal fossa, but these will be reported elsewhere.
Following strain gage implantation, fine-wire EMG electrodes (0.05 mm nickel-chromium) were placed percutaneously into 4–6 masticatory muscles, usually including bilateral masseter (equivalent to the human superficial masseter) plus zygomaticomandibularis (ZM, equivalent to the human deep masseter) and/or temporalis muscles (Fig. 1). The strain gage leads were connected to Model 2120A (Measurements Group) conditioner/amplifiers, and the EMG wires were input to high-impedance probes (Model 7HIP5G, Grass Instruments, Quincy, MA) and amplified (Model 7P3C, Grass). After recovery from the anesthetic (typically less than 30 min) and administration of analgesic (buprenorphine hydrochloride, Reckitt and Colman, Richmond, VA, 0.005 mg/kg IM), animals were allowed to feed unrestrained. In addition to pelleted pig chow, hard corn and candy were usually offered. Amplified signals were viewed on oscilloscopes and recorded on magnetic tape (Hewlett Packard, Palo Alto, CA) and/or sampled at 1 kHz and stored to computer (EGAA software, RC Electronics, Santa Barbara, CA). The animal and the oscilloscope screens were simultaneously videotaped (Panasonic, Secaucus, NJ) to provide a record of behavior.
Fig. 1.
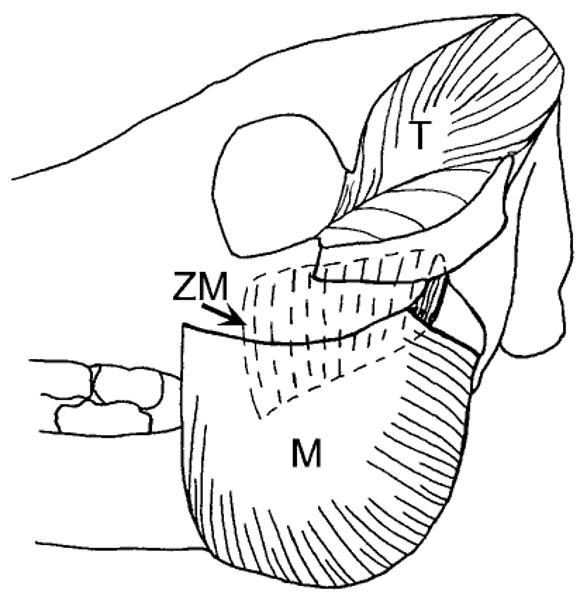
Lateral view of a pig skull showing masseter (M), temporalis (T) and zygomaticomandibularis (ZM) muscles. The ZM is equivalent to the deep masseter of human anatomy. The three muscles are well differentiated posteriorly but blend anteriorly.
Following 10–30 min of mastication, pigs were re-anesthetized and placed in a prone position with the mandible on the table and the teeth in occlusion. EMG electrodes were removed, and stimulating needle electrodes were placed in the masseter/zygomaticomandibularis and (in a subsample of animals) temporalis muscles. One electrode was placed near the entry of the motor nerve into the muscle, and the second as far away as possible. This arrangement was intended to maximize contraction by stimulating muscle fibers directly and by activating the entire motor nerve. Muscles, singly and in pairs, were tetanized at 0.5/sec by 600 msec trains of 5 msec pulses delivered at 60 pps (Model S48 and SIU, Grass). Voltage was adjusted to produce maximal muscle contraction without spread to adjacent muscles; typical values were 30 V for unilateral and 50 V for bilateral stimulations. Contraction of the correct muscle and lack of spread were assessed by palpation and observation of the resulting movement. Although the anatomical relationship of the masseter and the ZM prevents their separate stimulation, we believe that effects observed were mainly due to the masseter, which comprises 88% of their combined mass (Herring, 1985). Because the first experiments involving the coronal suture indicated that strains occurred during jaw opening as well as jaw closing, some later experiments included passive opening of the jaw (three animals) and stimulation of the medial pterygoid (two animals), digastric (one animal) and neck extensor (two animals) muscles using methods analogous to those for the masseter and temporalis. For the neck extensors, electrodes were inserted to be parallel with the occipital plane, with one needle within 1 cm of the plane and the other 1.5 cm more caudal. These electrodes traversed the splenius capitis and semispinalis capitis muscles, which are very large in pigs. Bone and suture strains caused by muscle stimulation were photographed from the oscilloscope screen and/or digitized at 1 kHz and recorded to computer using EGAA. Animals were sacrificed by an intracardiac injection of pentobarbital and gages were inspected to verify proper placement and fixation. Samples were taken from two of the subjects (pigs 183 and 184) for histological analysis. Blocks of bone were taken from the interparietal suture (anterior and posterior locations at the strain gage sites), the interfrontal suture (middle-posterior location) and right coronal suture. The undecalcified blocks were fixed in ethanol, embedded in polymethyl methacrylate, and sectioned perpendicular to the suture at 10 μm using a Polycut E microtome (Leica Instruments, Nussloch, Germany). Sections were stained with the Villanueva MIBS method (Villanueva and Lundin, 1989).
According to convention, tensile strains were expressed as positive values and compressive strains as negative values. Strain measurements from each element of the rosette gages were used to calculate magnitude and orientation of tensile (maximum principal) and compressive (minimum principal) strains on the bones (ROSETTE-Plus, Measurements Group). The sutural gages reported only the orthogonal component of strain (tension or compression). Thus shearing movements between the bones would register as slight tension. Because gages sites were limited to the outer cranial surface, axial loading could not be distinguished from bending.
Results
General
Instrumented pigs appeared to function normally, using the same alternation of chewing side that we have reported previously (Herring and Scapino, 1973; Herring, 1976). All locations registered strain associated with the closing/power stroke phase of mastication. Only the coronal suture consistently showed strain during the opening phase. In anesthetized animals, masseter and temporalis stimulations produced strains at all locations. Medial pterygoid and digastric stimulations did not result in any braincase strain, nor did passive jaw opening. Stimulation of the neck extensors caused strain in one location only, the coronal suture (see below).
There was no apparent relationship between strain and body size/age. For example, pigs 183 and 184, at 75 days old the youngest in the study, and pig 165, the oldest at 136 days, gave unremarkable strain values for both bones and sutures (data for bones in Table 2; data for sutures not shown). However, the sample was limited in age range, and an ontogenetic effect cannot be ruled out. Two (possibly three; see footnote to Table 1) animals were found postmortem to have partially synostosed interparietal sutures. These sutures produced only negligible strain and were not used in the analysis, but it is interesting to note that one of the animals involved was only 98 days old.
TABLE 2. Masticatory strain in the parietal and frontal bones1.
| Ipsilateral chewing | Contralateral chewing | |||||||
|---|---|---|---|---|---|---|---|---|
| Cycles analyzed | Tensile strain | Compressive strain | Orientation of tension | Cycles analyzed | Tensile strain | Compressive strain | Orientation of tension | |
| Early strain | ||||||||
| 147, R frontal | 12 | 81 ± 23 | −77 ± 20 | 35° ± 3 | 12 | 96 ± 63 | −62 ± 39 | −63° ± 5 |
| 154, L frontal | 8 | 15 ± 13 | −27 ± 8 | 59° ± 20 | 5 | 19 ± 8 | −30 ± 3 | −52° ± 5 |
| 157, L frontal | 4 | 25 ± 15 | −27 ± 16 | 44° ± 3 | 3 | 23 ± 10 | −20 ± 3 | −47° ± 5 |
| 158, L frontal | 3 | 22 ± 4 | −14 ± 6 | 52° ± 7 | 10 | 37 ± 16 | −19 ± 6 | −54° ± 3 |
| 164, L frontal | 16 | 25 ± 6 | −50 ± 11 | 55° ± 21 | 9 | 23 ± 6 | −7 ± 3 | −32° ± 8 |
| 165, L frontal | 10 | 54 ± 18 | −69 ± 29 | 77° ± 4 | 3 | 36 ± 17 | −24 ± 12 | −30° ± 2 |
| Frontal average | n = 6 | 37 ± 25 | −44 ± 25 | 54° ± 14 | n = 6 | 39 ± 29 | −27 ± 19 | −46° ± 13 |
| 155, R parietal | 7 | 60 ± 35 | −22 ± 31 | −71° ± 11 | ||||
| 155, L parietal | 13 | 38 ± 11 | −38 ± 23 | −71° ± 14 | ||||
| 156, L parietal | 11 | 41 ± 11 | −31 ± 7 | −41° ± 5 | ||||
| 183, R parietal | 12 | 15 ± 4 | −6 ± 3 | 37° ± 8 | 8 | 31 ± 9 | −15 ± 5 | −58° ± 8 |
| 184, L parietal | 13 | 18 ± 5 | −16 ± 6 | 43° ± 7 | 11 | 17 ± 6 | −11 ± 3 | −39° ± 6 |
| Parietal average | n = 2 | 16 ± 2 | −11 ± 7 | 40° ± 4 | n = 5 | 37 ± 16 | −23 ± 11 | −56° ± 16 |
| Late strain | ||||||||
| 147, R frontal | 12 | 124 ± 37 | −74 ± 28 | −59° ± 3 | 12 | 88 ± 26 | −80 ± 27 | 42° ± 5 |
| 154, R frontal | 9 | 22 ± 5 | −3 ± 1 | −19° ± 6 | ||||
| 154, L frontal | 11 | 18 ± 7 | −36 ± 14 | −55° ± 3 | 16 | 21 ± 8 | −27 ± 8 | 49° ± 7 |
| 157, L frontal | 14 | 45 ± 16 | −37 ± 12 | −48° ± 2 | 12 | 47 ± 13 | −58 ± 12 | 43° ± 2 |
| 158, L frontal | 12 | 52 ± 15 | −31 ± 7 | −52° ± 2 | 13 | 37 ± 7 | −37 ± 8 | 45° ± 2 |
| 162, L frontal | 10 | 16 ± 6 | −12 ± 4 | −41° ± 4 | 10 | 18 ± 6 | −25 ± 7 | 40° ± 7 |
| 164, L frontal | 16 | 26 ± 5 | −8 ± 3 | −31° ± 5 | 20 | 30 ± 7 | −66 ± 16 | 57° ± 2 |
| 165, L frontal | 8 | 44 ± 11 | −24 ± 7 | −41° ± 8 | 15 | 41 ± 17 | −75 ± 25 | 31° ± 3 |
| Frontal average | n = 8 | 43 ± 35 | −28 ± 23 | −43° ± 13 | n = 7 | 40 ± 23 | −53 ± 23 | 44° ± 8 |
| 155, R parietal | 18 | 89 ± 21 | −19 ± 10 | −77° ± 7 | ||||
| 155, L parietal | 10 | 33 ± 10 | −29 ± 16 | −63° ± 9 | ||||
| 156, R parietal | 12 | 42 ± 12 | −18 ± 10 | −31° ± 7 | 8 | 39 ± 15 | −20 ± 13 | 34° ± 19 |
| 156, L parietal | 9 | 46 ± 12 | −41 ± 5 | −41° ± 5 | 9 | 22 ± 11 | −48 ± 22 | 78° ± 11 |
| 183, R parietal | 7 | 33 ± 8 | −57 ± 5 | −57° ± 5 | 9 | 27 ± 7 | −15 ± 8 | 35° ± 6 |
| 184, L parietal | 13 | 15 ± 8 | −41 ± 4 | −41° ± 4 | 14 | 24 ± 6 | −21 ± 5 | 43° ± 5 |
| Parietal average | n = 6 | 43 ± 25 | −23 ± 8 | −52° ± 17 | n = 4 | 28 ± 8 | −26 ± 15 | 48° ± 21 |
Tensile and compressive principal strains, με ± standard deviation. For ease of comparison, orientation is given as if all gages were on the left (as in Figure 2). The sagittal axis is 0°; positive angles are posterolateral to anteromedial (as in element C in Figure 2) and negative angles are posteromedial to anterolateral (as in element A in Figure 2). Most gages showed a change in the orientation of strain in the middle of each masticatory cycle. Thus, two peak strains are given, early and late.
Frontal and parietal bones
Figure 2 illustrates a typical recording from a rosette gage during mastication. Little or no strain was seen along the sagittal axis, whereas the 45° elements were reciprocally tensed or compressed, indicating torsion to alternate sides, often within the same masticatory cycle. Comparison with muscle activity (Fig. 2) suggested that the tensile axis ran anteriorly to the left (axis A of the gage in Fig. 2) when the left masseter and right ZM were predominant, and anteriorly to the right (axis C, Fig. 2) when the right masseter and left ZM were predominant. In pig (as in human) mastication, the masseters contribute to contralateral movement and the temporalis/ZM muscles to ipsilateral movement (Herring, 1992). Thus, for a chewing cycle on the right, the left masseter/right ZM and temporalis are active early in the cycle to position the mandible to the right, and the right masseter/left ZM and temporalis are predominant late in the cycle, moving the mandible leftward during the power stroke. This reciprocal activity is mirrored in the alternating strain pattern seen in Figure 2, particularly when the pig is chewing on the right.
Fig. 2.
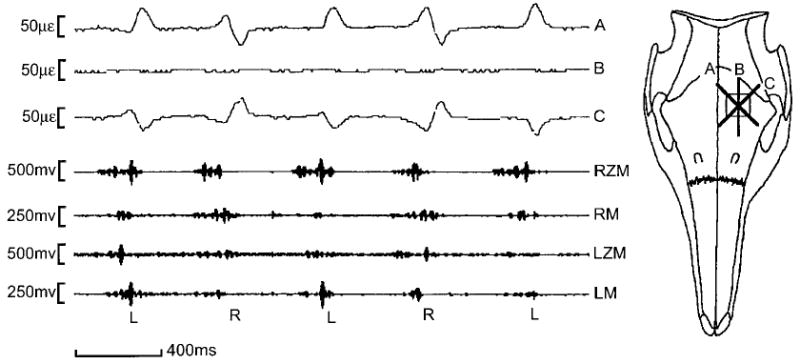
Left frontal bone strain during mastication, pig 158. The B element of the rosette is parallel to the sagittal axis and records no strain, whereas the 45° A and C elements are reciprocally tensed (positive signal) or compressed (negative signal). The pig alternates between left (L) and right (R) side chewing. Chewing on the left features early activity in the right masseter (RM) and left zygomaticomandibularis (LZM) to move the mandible to the left, and late activity in the left masseter (LM) and right zygomaticomandibularis (RZM) to complete the power stroke. For chewing on the right, LM and RZM are early, RM and LZM are late. Strain in the left frontal bone is best predicted by masseteric activity—predominance of the LM is associated with tension along the A element and compression in the C element, predominance of the RM causes the opposite pattern. Note that strain is slightly delayed relative to muscle contraction; this is because the EMG signal of the pig masseter precedes the development of mechanical force by approximately 50 msec (Anapol and Herring, unpublished data).
Quantitative results from mastication are displayed in Table 2. Calculation of principal strains confirmed an alternating orientation of approximately +45° or −45°, with tensile axis always directed toward the origin of the dominant masseter. Standard deviations were never higher than 21°. At any given time, all four cranial bones had essentially identical strain patterns. However, strain magnitudes were very low, usually less than 50 microstrain (με) and exceeding 100 με in only one animal. Tensile (maximum principal) and compressive (minimum principal) strains were comparable, but Table 2 reveals a tendency for tension to exceed compression when the ipsilateral masseter was dominant (early strain in contralateral chewing, late strain in ipsilateral chewing) and for compression to exceed tension when the contralateral masseter was dominant (early strain in ipsilateral chews and late strain in contralateral chews).
Muscle stimulations gave results very similar to mastication (Table 3, Fig. 3). For any given muscle stimulation, frontal and parietal bones showed the same pattern. Strain magnitudes were typically less than 50 με. Unilateral muscle contractions caused principal strains roughly oriented at 45° to the sagittal axis; the tensile strain was always directed toward the origin of the contracting muscle. Particularly interesting is the fact that opposite masseter and temporalis pairs (e.g., ipsilateral masseter and contralateral temporalis), which contract together during mastication, resulted in the same 45° orientation. The tendency for increased compression during contralateral contraction of the masseter could again be seen (P < 0.05 for the frontal bone, t-test), although no such effect could be demonstrated for the temporalis. Unilateral masseter contractions caused higher strains on the frontal bone than did unilateral temporalis contractions (3 of 4 comparisons significant at P < 0.05 or better, t-tests), but the parietal bone was similarly strained by both muscles. Bilateral muscle contraction did not increase strain magnitude, but did change its orientation so that the tensile axis approximated the sagittal axis.
TABLE 3. Strain in frontal and parietal bones resulting from muscle stimulation1.
| Frontal bone | Parietal bone | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Tension (με) |
Compression (με) |
Orientation of tension | n | Tension (με) |
Compression (με) |
Orientation of tension | |
| Masseter | ||||||||
| Ipsilateral | 7 | 44 ± 24 | −23 ± 12 | −41° ± 9 | 4 | 31 ± 9 | −12 ± 7 | −48° ± 22 |
| Contralateral | 7 | 45 ± 19 | −52 ± 28 | 39° ± 14 | 4 | 18 ± 9 | −29 ± 13 | 24° ± 12 |
| Bilateral | 6 | 40 ± 27 | −17 ± 14 | −2° ± 29 | 6 | 24 ± 9 | −20 ± 12 | 0° ± 21 |
| Temporalis | ||||||||
| Ipsilateral | 7 | 19 ± 14 | −28 ± 7 | 45° ± 17 | 4 | 27 ± 6 | −12 ± 9 | 25° ± 73 |
| Contralateral | 6 | 20 ± 12 | −18 ± 10 | −32° ± 18 | 4 | 31 ± 22 | −27 ± 6 | −39° ± 14 |
| Bilateral | 5 | 24 ± 17 | −11 ± 8 | −17° ± 19 | 6 | 39 ± 19 | −24 ± 6 | 2° ± 65 |
Maximum and minimum principal strains ± standard deviation. As in Table 2, 0° represents the sagittal axis and orientations are given as if all gages were on the left. A positive orientation means the tensile (maximum principal) axis is oriented toward the anterior left. n-values are the number of gages.
Fig. 3.
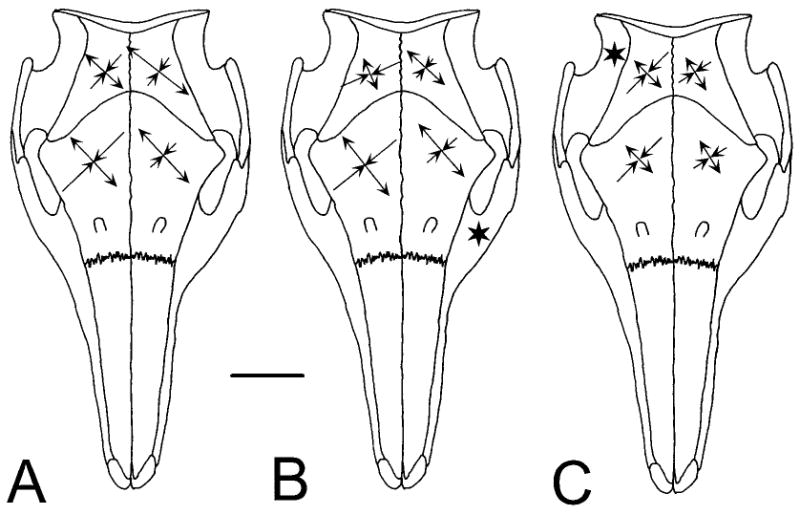
Comparison of principal strains in the parietal and frontal bones under three conditions (data from Tables 2, 3). Tensile strain is indicated by divergent and compressive strain by convergent arrowheads. The horizontal scale bar indicates 40 με. A: Late in a left side masticatory cycle. B: Stimulation of the left masseter (star). C: Stimulation of the right temporalis (star). The pattern of strain is similar in all cases, but the magnitudes suggest that A is a combination of B and C.
Thus, the complex strain patterns of neurocranial bones during mastication can essentially be accounted for by muscle contraction, particularly the “diagonal couple” of a masseter and its opposite temporalis.
Interfrontal and interparietal sutures
Locations of the midline sutural gages and average strains are illustrated in Figure 4; examples of masticatory strain are shown in Figure 5. Data on peak masticatory and stimulated strains are presented in Table 4. Typical sutural strains were about an order of magnitude larger than bone strains. During chewing the highest strains were seen anteriorly in the interfrontal suture (middle location, averaging over 700 με) and posteriorly in the interparietal suture (averaging over 400 με). Except for the posterior interfrontal site, peak strains were always tensile. However, the course of strain development was often complex, exhibiting compressive intervals and asymmetry for left- vs. right-side chewing (Fig. 5).
Fig. 4.
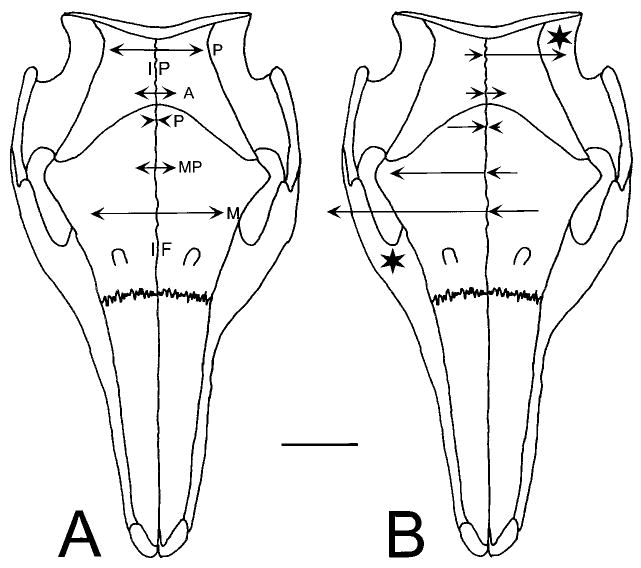
Strain in the midline sutures. Three locations were recorded for the interfrontal suture (IF): posterior (P), middle-posterior (MP), and middle (M). Two locations were recorded for the interparietal suture (IP): posterior (P) and anterior (A). The uniaxial gages used reported only strain at right angles to the suture, either as tension (arrow points away from the suture) or compression (arrow points toward the suture). Data from Table 4. A: Mastication (average peak values). B: Unilateral stimulation of the masseter (left side of figure) and temporalis (right side of figure). The stars indicate the contracting muscles. Masticatory strains in the interfrontal suture resemble unilateral masseter contraction, whereas those in the interparietal suture resemble unilateral temporalis contraction. The scale bar represents 400 με (an order of magnitude larger than the bar in Figure 3).
Fig. 5.
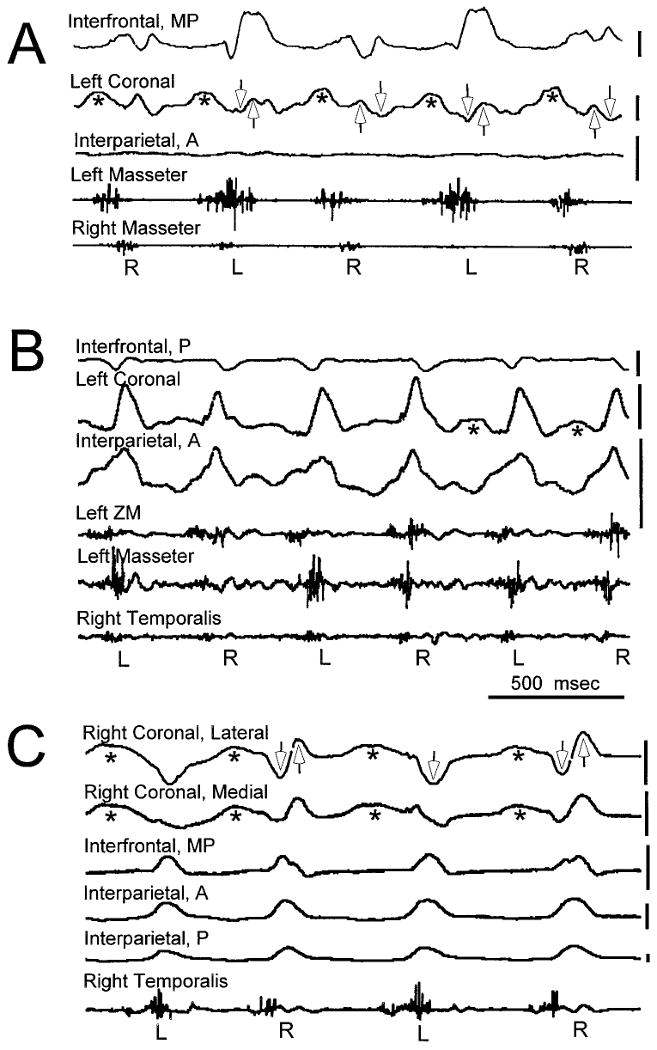
Examples of sutural strain and muscle activity during mastication. Tension is upward and compression is downward. The vertical scale next to each strain channel indicates 250 με. Abbreviations for locations within sutures follow Figure 4. The side of chewing (L or R) is indicated beneath each cycle. The early part of the closing cycle is typically dominated by the ipsilateral temporalis and contralateral masseter, whereas the late part features the ipsilateral masseter and contralateral temporalis. Opening strain is seen only in the coronal suture (asterisks). The coronal suture usually shows biphasic strain associated with muscle activity, compressive (downwardly directed open arrows) when the ipsilateral temporalis is predominant and tensile (upwardly directed open arrows) when the ipsilateral masseter is predominant. A: Pig 163. The interparietal location shows very little strain. The interfrontal suture is unusual for a midline suture in that strain is different depending on chewing side. The large tensile peaks are associated with activity in the left masseter. The coronal suture shows the typical pattern of strain. B: Pig 164. This animal had the simplest coronal suture strain pattern. During opening strain was often lacking and during closing it was exclusively tensile. C: Pig 167. Strain gages placed medially and laterally on the right coronal suture gave essentially identical patterns; their output was averaged. The coronal suture shows the typical strain pattern except that during contralateral (left) cycles there is only compressive strain.
TABLE 4. Midline sutural strains during mastication (peak strains) and muscle stimulations1.
| Masseter stimulation | Temporalis stimulation | ||||
|---|---|---|---|---|---|
| Mastication | Unilateral | Bilateral | Unilateral | Bilateral | |
| Interfrontal | |||||
| Posterior | −80 ± 192 (6) | −197 ± 152 (5) | −110 ± 146 (5) | −58 ± 118 (2) | −6 ± 67 (2) |
| Mid-post | 182 ± 26 (4) | 488 ± 459 (4) | 1,290 ± 932 (4) | −143 ± 43 (3) | −170 ± 59 (3) |
| Middle | 729 ± 221 (2) | 859 ± 62 (3) | 2,226 ± 277 (3) | −266 ±119 (3) | −327 ± 95 (3) |
| Interparietal | |||||
| Posterior | 594 ± 314 (4) | −100 ± 71 (2) | −221 ± 517 (4) | 422 ± 314 (2) | 575 ± 274 (4) |
| 438 ± 8 (3)2 | −150 (1)2 | −463 ± 224 (3)2 | 200 (1)2 | 449 ±129 (3)2 | |
| Anterior | 221 ± 198 (7) | −90 ± 115 (5) | −73 ± 290 (7) | 101 ± 190 (4) | −23 ± 321 (4) |
| 150 ± 68 (6)2 | −127 ± 94 (4)2 | −156 ± 208 (6)2 | 14 ± 91 (3)2 | −182 ± 60 (3)2 | |
Mean με ± standard deviation (number of gages). There were no significant differences between right and left chewing cycles or between right and left side stimulations; thus the sides were averaged.
Pig 167 had very high and sometimes paradoxical peak interparietal strains, inflating both the average and standard deviation. These values are calculated without pig 167.
For the interfrontal suture, masticatory strains were mimicked by masseter stimulation (Table 4). In particular, the posterior location showed relatively low, predominantly compressive strains whereas the middle-posterior and middle locations were progressively tensed. The only significant difference was the very high tensile strain observed at the middle location during bilateral masseteric stimulation (2,226 με) compared to unilateral stimulation and mastication (700–850 με, P < 0.01, t-test). Temporalis stimulations gave a strikingly different result, with both unilateral and bilateral stimulations causing compression that increased progressively from the posterior to the middle location.
The interparietal suture gave opposite results from the interfrontal in that it was unilateral temporalis stimulations that most closely mimicked mastication, with diminishing tension from posterior to anterior. Bilateral temporalis stimulation was similar, except that the anterior location became predominantly compressive. In contrast, masseteric stimulation compressed the interparietal suture, especially the posterior location.
Taken in combination, these results suggest that the masseter muscles tend to split the anterior part of the braincase while compressing its posterior part. The temporalis muscles have the opposite tendency. Masticatory strain clearly reflects a predominant influence of the temporalis muscles on the interparietal suture and of the masseter muscles on the interfrontal suture. Because sutural strain is so sensitive to the pattern of muscle contraction, variation in the details of muscle activity (e.g., greater use of the masseter muscle during left-side than right-side chewing, see pig 163, Fig. 5) can account for the complex or asymmetrical patterns sometimes observed.
Coronal suture
Data for the coronal suture are displayed in Table 5 and illustrated in Figures 5 and 6. Although strain gages were placed in various locations (medial and lateral) along the suture, no consistent differences among locations were observed. This was even true in one experiment where medial and lateral gages were simultaneously placed over the same suture (pig 167—the readings from the two gages were averaged for Table 5). Thus all coronal suture locations were considered together.
Fig. 6.
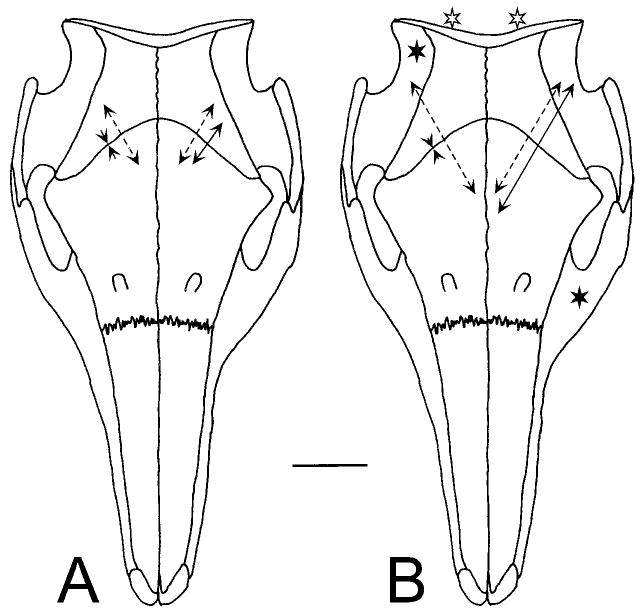
Strain in the coronal suture. Conventions as in Figure 4, data from Table 5. The scale bar represents 400 με. A: Mastication. Solid lines: late in a left side masticatory cycle when the left masseter and right temporalis are the most active muscles. Dotted lines: during the opening portion of the same cycle. Values are relative only, because a baseline could not be established during mastication. B: Muscle stimulations. The dotted lines show the result of bilateral stimulation of the neck extensor muscles (open stars). The solid lines on the left side of the figure show strain arising from the right temporalis (solid star). On the right side of the figure, the solid lines indicate strain from stimulation of the left masseter (solid star).
TABLE 5. Coronal suture strain during mastication (peak strains) and muscle stimulation1.
| Closing2 | |||
|---|---|---|---|
| Mastication | Tension2 | Compression2 | Opening2 |
| Ipsilateral | 278 ± 150 (7) | −160 ± 63 (8) | 365 ± 208 (10) |
| Contralateral | 269 ± 134 (8) | −151 ± 73 (8) | 373 ± 216 (10) |
| Stimulation | Ipsilateral | Contralateral | Bilateral |
| Masseter | 816 ± 435 (11) | 280 ± 319 (10) | 1,156 ± 557 (10) |
| 665 ± 337 (3)3 | 182 ± 279 (3)3 | 820 ± 389 (3)3 | |
| Temporalis | −111 ± 167 (3) | −22 ±98 (3) | −273 ± 256 (3) |
| Neck extensors | — | — | 699 ± 295 (4) |
με ± standard deviation (number of gages).
Because the coronal suture was strained during all phases of mastication, it was impossible to establish a definite baseline. The figures presented here should be considered as relative, not absolute, values. The most common pattern during ipsilateral mastication was early compression followed by late tension. For contralateral mastication, early tension was followed by late compression. However, some animals showed only compression (157, 160) or tension (158). All but one (164) exhibited tension during jaw opening. Values in the table are averages from all gages showing nonzero strain.
Subsample of three animals (157, 158, 167) that were also used for temporalis stimulations.
Because both opening and closing gave rise to strain in the coronal suture, a true baseline for mastication was impossible to define. The values for mastication are thus relative rather than absolute, although the peak-to-peak differences between tension and compression are accurate. Nevertheless, it is clear that the magnitudes of coronal strain were similar to those of the midline sutures and much larger than bone strain values. During jaw opening, almost all coronal sutures exhibited an ill-defined but usually large peak in tensile strain (pig 164 was the exception; see Fig. 5). Strain during closing was more complex and showed both inter- and intra-individual variability. The most frequent pattern was a reversal in strain polarity accompanying the early/late change in muscle activity (pigs 163 and 167 in Fig. 5; compare with pig 164, which did not show clear reversals). In particular, when the pigs were chewing on the side of the coronal strain gage, early strain (corresponding to ipsilateral temporalis/contralateral masseter activity) was compressive and late strain (corresponding to ipsilateral masseter and contralateral temporalis activity) was tensile. For contralateral cycles, the pattern was reversed. Magnitudes of strain were approximately the same regardless of the side of chewing (Table 5).
Muscle stimulations again illuminated the masticatory pattern. As in the midline sutures, the masseter and temporalis caused coronal strains of opposite polarity. Masseteric stimulation caused strong tension, particularly ipsilateral and bilateral muscle contractions. The sample size for temporalis stimulation was unfortunately small (n = 3), but showed clearly that this muscle produced compression, at least during ipsilateral and bilateral contractions. As might be expected, ipsilateral muscles produced larger strains than contralateral muscles, significantly so for the masseter (P < 0.01, t-test). Bilateral contractions tended to cause larger strains than ipsilateral contractions, but these trends were not statistically significant.
In contrast to the other braincase locations, the coronal suture also showed strain that clearly could not have resulted from masseter/temporalis contraction, i.e. that observed during the jaw opening phase of mastication. Jaw opening itself was not the cause, because neither passive opening nor digastric stimulation elicited recordable strain from the coronal suture (or any other location). However, stimulation of the neck extensor musculature, which is very large in pigs, resulted in substantial tensile strain at the coronal suture (Fig. 7). These strains were unaffected by jaw position and none were seen elsewhere in the braincase.
Fig. 7.
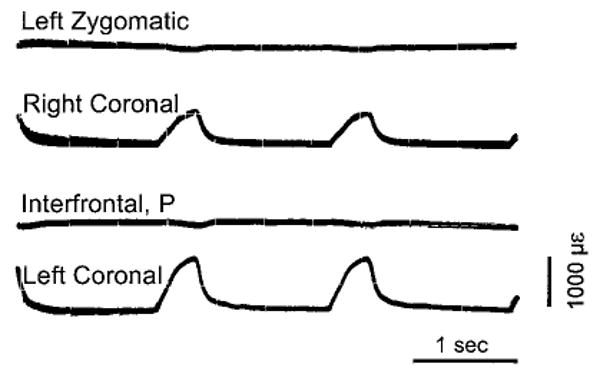
Sutural strain resulting from bilateral stimulation of neck extensor muscles. Pig 160, photograph of oscilloscope screen. In addition to three braincase sutures, strain from the left zygomatic suture is shown. Contraction of the neck muscles caused substantial tensile strain in both right and left coronal sutures, but no measurable deflection for the interfrontal or zygomatic.
Suture histology
The complexity of calvarial construction is not apparent from a surface view of the flat bones and slightly irregular suture lines but can be seen in the histological sections. The two animals studied (pigs 183 and 184) were very similar in their morphology. As illustrated in Figure 8, the skull roofing bones were 4–5 mm thick, with a gradual decrease from posterior to anterior. They were composed of a very dense trabecular network without large marrow cavities or cortices. At the endocranial surface trabeculae were generally more robust and the bone was more mature, as indicated by the occasional presence of osteons.
Fig. 8.
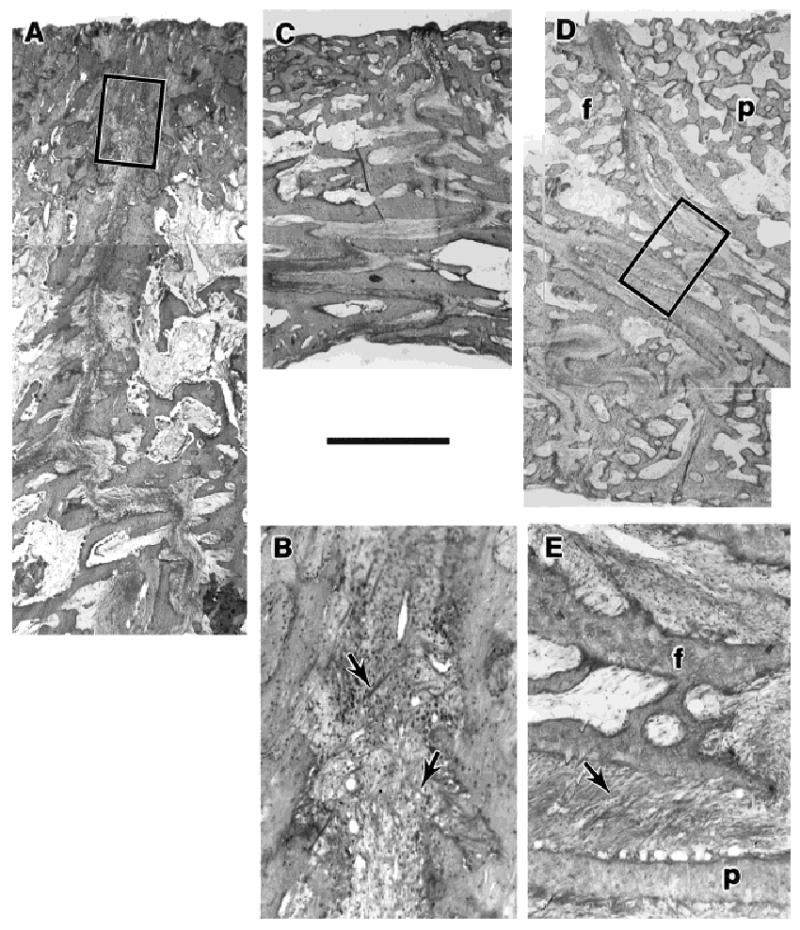
Histological sections through sutures. The calvarial bones are densely trabecular, with the youngest bone on the dorsal, ectocranial side (toward the top of the page). A: Interparietal suture, pig 184, just beneath the posterior strain gage (montage). The suture is relatively butt-ended but becomes irregular toward the endocranial side. B: Enlargement of the boxed area of A. The sutural ligament shows some large fibers in a cruciate configuration (arrows). C: Posterior interfrontal suture, pig 183. The endocranial portion of the suture is interdigitated and shows some ligamentary fibers in an oblique, compression-resisting conformation. D: Right coronal suture, pig 184 (montage). The endocranial portion is very strongly interdigitated. E: Enlargement of the boxed area of D, showing the oblique arrangement of many ligamentary fibers (arrow). f, frontal bone; p, parietal bone. Undecalcified sections, MIBS stain, size marker 1 mm for A, C and D.
All three sutures were relatively butt-ended near the ectocranial surface and became rougher endocranially (Fig. 8). The roughness of the endocranial portion developed into interdigitations at the posterior part of the interfrontal suture and even more prominently at the coronal suture. At the ectocranial butt intersections, much of the sutural ligament lacked a predominant orientation, but a number of very large fibers ran between the bones dorsoventrally in cruciate fashion. This arrangement would not resist compression. It could, however, resist tension, although not optimally, and it could effectively resist bending or shearing forces tending to separate the bones dorsoventrally. At the interdigitated endocranial portions, fibers were often seen running obliquely, connecting the apical regions of overlapping bony spicules. This arrangement is analogous to the periodontal ligament and is well suited to resist compression.
Discussion
Magnitude and polarity of strain
There was a striking, order-of-magnitude difference in the degree of strain recorded from the braincase bones as opposed to the sutures. This was in large part due to exceptionally low strains in the bones; sutural strains were not unusual (see below). Not only were braincase bone strains lower than sutural strains, they were also much less than zygomatic arch bone strains measured from pigs of comparable age under identical conditions (Herring et al., 1996). Although they challenged our technical abilities, the low frontal and parietal bone strains are in accord with previous studies, including the anthropoid supraorbital region (Hylander et al., 1991; Ross and Hylander, 1996) and the rat parietal bone (Rawlinson et al., 1995). Nevertheless, low braincase bone strains do not imply that the braincase is not loaded, because if that were the case, the sutures would also show low strains. Rather, the most likely explanation is that the bones themselves, thick and solidly constructed, are unusually stiff and unlikely to deform greatly under load. It remains to be seen whether maturation of the cortex and invasion of air sinuses alter the amount of strain observed.
In contrast to the bones, braincase sutural strain magnitudes were comparable to those measured previously from the zygomatic suture (Herring and Mucci, 1991). One interesting difference pertains to the polarity of strain. Compression and tension typically alternated rapidly in each braincase suture (Fig. 5); muscle stimulation clearly showed that polarity of strain depended on which muscle was active. The zygomatic suture was more uniform in that its vertical segment was primarily under compression and its horizontal segment was tensed; reversals could be caused by asymmetrical muscle activation, but during mastication these reversals were rare and small compared to the dynamic behavior of the braincase. In that study, the ranges for average stimulated strain (in με) were −750 to + 300 for the vertical segment and −450 to +1500 for the horizontal part. These ranges are similar for those observed in the present study: −220 to +600 for the interparietal, − 300 to +2200 for the interfrontal, and − 300 to +1200 for the coronal (Tables 4, 5). An additional interesting point is that at every sutural location except the posterior interfrontal, the largest and most frequent strains were tensile. In our earlier investigation of the zygomatic suture (Herring and Mucci, 1991), we noted that internal architecture reflected the predominant strain polarity. Specifically, the tensed portion had smooth bone edges directly united by the fibers of the sutural ligament, whereas the compressed portion had long bony interdigitations with obliquely oriented ligamentary fibers. By analogy, the histological structure of the braincase sutures strongly suggests bending, with tension ectocranially and compression endocranially. This interpretation of sutural architecture is consistent with the preponderance of tensile strain, because the strain gages were located exclusively on the ectocranial surface. The increasing prominence of the interdigitated portion in the posterior part of the interfrontal suture and especially in the coronal suture suggests that compression is relatively more important at these locations.
Role of individual muscles: Suture strain
Braincase strain observed in this study was clearly a consequence of muscle contraction, because we were able to duplicate all masticatory strains by stimulating the appropriate muscles. Each muscle tested caused a unique braincase strain pattern.
A general question is whether the muscle-induced strains are direct loads from muscular forces on the bones, or indirect, such as reaction loading at the teeth or at the jaw joint. This point is particularly important for evaluating Greaves' (1985) model of braincase torsion, which is predicated on indirect loading. Both direct and reaction loads are present, but for several reasons we believe that the main effects are direct loads applied by the muscles. First, taking the teeth out of occlusion by opening the jaw (which also modified jaw joint position) had no important effect on braincase strain. Second, the medial pterygoid muscle, which is distant from the braincase but does create occlusal and joint reaction forces, did not strain the calvarial bones or sutures. Third, given their lines of action, both masseter and temporalis would be expected to compress the jaw joints, yet contraction of these muscles gave rise to very different strains in the braincase. Fourth, our previous work on the zygomatic arch (Herring and Mucci, 1991; Herring, 1996) indicated that strain was a local phenomenon with proximity of the applied force being important. The present findings also indicate a neighborhood effect in that the parietal bone and interparietal suture were most influenced by the temporalis muscle, whereas the frontal bone and interfrontal suture were more strongly affected by the masseter (Tables 3, 4). Because jaw joints, and even more so the teeth, are distant from recording sites, they should be less important sources of strain than the close-by masseter and temporalis muscles. For all these reasons, strain in the neurocranium appears to result directly from muscle loading. We proceed to consider individual muscle actions on the sutures.
Only one strain pattern was independent of the masseter and temporalis muscles, the tension in the coronal suture that accompanied jaw opening. Because chewing pigs characteristically raise their heads as the mandible is lowered (Herring, 1976), we tested the effect of neck extensor contraction and were able to reproduce the opening strain pattern (Fig. 7). These muscles pull ventrally (downward) and posteriorly (back-ward) on the supraoccipital bone, which joins the parietals at a transversely oriented suture. Assuming the muscle load on the supraoccipital is at least partly transferred across the suture to the parietals, the tensile strain on the coronal suture can be easily understood as a consequence of 1) the posterior pull of the muscles causing axial tension on the skull roof, and 2) the ventral pull of the muscles on the parietals, causing shear and bending at the coronal suture. The parietals themselves are presumably too stiff to be deformed by the nuchal muscles, a finding also reported by Mowbray and Lieberman (1997).
The masseter and temporalis muscles accounted for all strain patterns other than that during jaw opening. Because these two muscles had almost opposite effects on the bones and sutures of the calvaria, strain patterns during mastication were often complex and reflected the choreography of muscle contraction. Interpretation was simple, however, because pigs almost always use these muscles in opposite-side pairs. Mastication thus consists of rapidly alternating reversals of right masseter/left temporalis vs. left masseter/right temporalis. The strains from all braincase locations clearly reflect these reversals (Figs. 2–6).
Stimulations of masseter and temporalis enabled their effects on strain to be dissected. The skull roof can be imagined as four movable plates of bone (Fig. 9A). The area of least midline strain is the posterior interfrontal suture, near bregma (the intersection of the bones). This point can be regarded as relatively stable in terms of transverse movements.
Fig. 9.
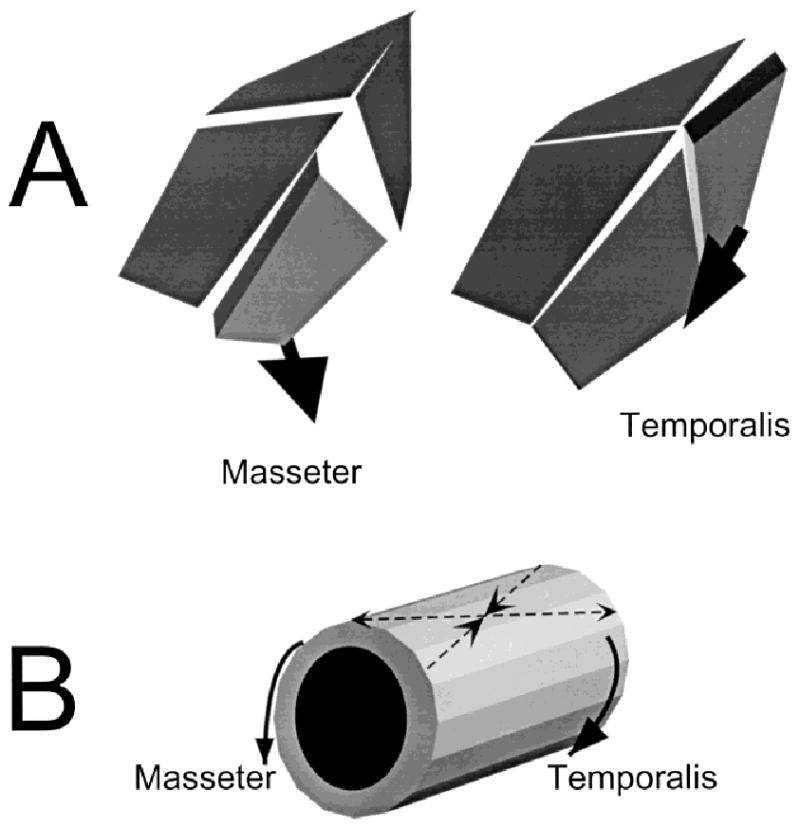
A: Suture strain as a result of muscle contraction. The masseter pulls ventrally and laterally. The resulting rotation and bending are read as tension in the coronal suture and the anterior part of the interfrontal suture. The temporalis pulls anteriorly and ventrally, causing compression in the coronal suture but tension in the interparietal. B: The muscles also torque the braincase, producing the 45° pattern reported in the frontal and parietal bones.
The masseter muscle caused the midline suture to open anteriorly (interfrontal) and to close posteriorly (interparietal), that is a rotation approximately around the stable point. The masseter also tensed the coronal suture, especially ipsilaterally. Because all points on the coronal suture behaved similarly, this is not a rotation. All these actions can be understood as direct results of muscle pull. Because the masseters do not attach directly to the braincase, their ventral and posterior pull on the zygomatic bone is transmitted via the lacrimal bone to the anterolateral corner of the frontal bone. Through this connection, the masseter pulls the anterolateral corner of the frontal ventrally, laterally and somewhat posteriorly. The lateral and posterior components would cause the rotation around the stable point by separating the interfrontal suture anteriorly. The ventral component would shear the frontal bone relative to the parietal, resulting in bending of the interfrontal suture, increasing the tension observed here, and bending of the coronal suture, accounting for its tension.
The temporalis muscle also caused the midline suture to rotate around the stable point, but in the reverse direction, with opening posteriorly (interparietal) and closing anteriorly (interfrontal). It compressed the coronal suture, especially ipsilaterally. The temporalis attaches directly to the side of the parietal bone and pulls the bone anteriorly, ventrally, and somewhat laterally. The muscle is especially thick posteriorly (Fig. 1), so the center of force application approximates the posterolateral corner of the parietal bone (Fig. 9A). These actions can account for the observed strains as follows: 1) the anterior and lateral components would cause the rotation around the stable point by opening the interparietal suture posteriorly; 2) the ventral component would bend the interparietal suture, adding to the tension observed; this component would presumably bend the coronal suture as well, but that effect is evidently swamped by 3) the larger anterior component, jamming the parietal into the frontal and thus compressing the coronal suture.
Twisting of the braincase: Bone strain
Although the sutural strains are easily explained by muscle action lines, bone strains are not. Bone strains were always ±45° to the sagittal axis, but neither the masseter nor the temporalis runs in this direction. Furthermore, the four calvarial bones showed very similar strain patterns even when muscle force was applied to only one of them, indicating a common deformation over the entire skull roof. This is very different for the unique pattern of sutural strain corresponding to each muscle.
The 45° orientation, its invariance and its universality in all bones point strongly toward torsion of the braincase as a whole, as predicted by Greaves (1985). However, Greaves envisioned that the forces that induced twisting were the molar bite force and the reaction force on the opposite jaw joint. We found, as did Hylander and colleagues (Hylander et al., 1991a,b; but not Ross and Hylander, 1996), that torsion during the power stroke was opposite to that which would be set up by a bite point. In fact, our observations on anesthetized animals indicated that the side of the bite point was irrelevant; because both sides of the jaws were in occlusion, the bite point was effectively in the midline. The side of muscle contraction (which would determine which jaw joint received most reaction load) was also not critical, because almost identical torsion occurred for left masseter as for right temporalis contractions (Fig. 3). Rather, the only important determinant of the direction of twisting was which muscle, with the masseter and temporalis on the same side giving opposite results. Clearly, the muscles themselves are causing the torsion. The masseter, attaching near the front of the braincase, and the temporalis, attaching near the back, set up opposite rotations (Fig. 9B). When either muscle contracts bilaterally, the opposite torques cancel and the braincase can be considered under cantilever bending, accounting for the axial tension observed (Table 3).
A final problem is to reconcile the twisting of the braincase with sutural strain. Muscle couples such as the left masseter and the right temporalis, which give identical bone strains, give radically different sutural strains (Tables 4, 5). Why is twisting not seen in the sutures and why are the individual muscle forces not seen in the bones?
A consideration of geometry suggests that the sutures are not strained by braincase twisting. Braincase torsion would not be expected to produce net deformation of the midline suture, because the ±45° compressive and tensile axes would set up equal and opposite axial and shearing loads. Thus, the interparietal and interfrontal sutures would be “blind” to torsion. This is not the case for the coronal suture, which is oriented at about 60° to the sagittal axis. When, for example, the braincase is twisted toward the ipsilateral masseter (creating a tensile strain at 15° to the suture), the axial compression should exceed axial tension, but the shearing component (which is recorded as tension) should be large. The reverse would be true for the contralateral coronal suture. To the extent that these conflicting loads cancel, then the coronal suture would also be blind to torsion.
With regard to why individual muscle forces are not seen in the bones, the answer is probably that they are, but the overall strain level is so low that they are difficult to detect. That these effects exist is indicated by 1) the larger strains on the frontal bone caused by masseter as contrasted to temporalis muscles, and a tendency for the reverse on the parietal bone (Table 3); and 2) the predominance of tension for ipsilateral masseter contractions and compression for contralateral contractions (Table 3). The variability of orientation during stimulations was, however, too high to allow any differences to be detected.
Overview: Nature of functional strain in the braincase
During mastication the pattern of muscle contraction is an alternating series of “diagonal couple” contractions of one masseter and the temporalis from the opposite side. Both members of this “diagonal couple” produce the same bone strain pattern of 45° tension directed at the respective origins of the muscles (Figs. 1, 2), accounting for the stability of this result during chewing (Table 1). In terms of sutural strain, however, the members of the “diagonal couple” are antagonistic rather than synergistic. Thus the strain environment of the sutures is constantly varying according to the details of muscle contraction. Nevertheless, sutural peak strains during mastication seem to be dominated by the closest muscle. Thus, peak masticatory strain in the interparietal suture reflects the temporalis effect, whereas the interfrontal suture reflects the masseter (Table 4). Masticatory strain in the intermediately positioned coronal suture is a blend of temporalis and masseter (Table 5). Importantly, peak sutural strains during mastication are all tensile (on the ectocranial surface), except for the stable point just anterior to bregma.
These findings on pigs may be relevant to previous studies on primates. The arrangement and relative size of the muscles of mastication are comparable in pigs and primates (Fig. 1). Primates and pigs (and indeed most mammals with a transverse chewing stroke) use similar muscle coordination to produce the power stroke of mastication (Weijs, 1994). Like anthropoid primates, pigs have a fused mandibular symphysis, and thus forces should be transmitted from the mandible in similar ways. Therefore, the mild torsion seen in the supraorbital regions of Macaca and Papio (Hylander et al., 1991a,b) may stem from the same coupling of a masseter and its opposite temporalis as in pigs. The primate interparietal suture, like that of pigs, is tensed by temporalis contraction (Behrents et al., 1978). However, there are points of difference as well. The early fusion of the interfrontal suture in primates implies that masseteric forces must be transmitted across the midline rather than dissipated at the suture. Finally, in place of a postorbital bar or septum, pigs have a ligament, which can convey only tensile forces from the zygomatic arch to the braincase. The presence of a supraorbital ridge in primates but not pigs may be related to these differences.
In conclusion, the pig braincase acts as a tubular structure with a roof composed of movable plates connected by flexible junctions. The contractions of jaw muscles attached to the sides of the tube twist the overall structure (Fig. 9B) and simultaneously bend and stretch the junctions (Fig. 9A). The junctional deformations are much larger than the plate strains and are resisted by ligaments generally arranged to counteract tension on the outside and compression on the inside. Although the junctions are easily strained because of their low stiffness, their angulation prevents them from being deformed by the twisting. For the much stiffer plates, twisting is the major strain regime, with the local effects of muscle contraction contributing only minor alterations.
Acknowledgments
These studies were supported by PHS grant DE08513 from the National Institute of Dental Research. We thank Patricia Emry for help with the histology, Jay Decker for photography, Kathy Rafferty, Scott Pedersen, and Betty Sindelar for help with the experiments and discussions, and Callum Ross and Christine Wall for the opportunity to present our work at the AAPA meeting.
Grant sponsor: National Institute of Dental Research; Grant number: DE08513.
Literature Cited
- Alexandridis C, Caputo AA, Thanos CE. Distribution of stresses in the human skull. J Oral Rehab. 1985;12:499–507. doi: 10.1111/j.1365-2842.1985.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Badoux DM. Framed structures in the mammalian skull. Acta Morphol Neerl-Scand. 1966;6:239–250. [PubMed] [Google Scholar]
- Badoux DM. Some notes on the biostatics of the skull cap in domesticated dog. Koninkl Nederl Akad Wetensch Proc Ser C. 1970;73:343–352. [PubMed] [Google Scholar]
- Behrents RG, Carlson DS, Abdelnour T. In vivo analysis of bone strain about the sagittal suture in Macaca mulatta during masticatory movements. J Dent Res. 1978;57:904–908. doi: 10.1177/00220345780570091401. [DOI] [PubMed] [Google Scholar]
- Demes B. The resistance of primate skulls against mechanical stresses. J Hum Evol. 1982;11:687–691. [Google Scholar]
- Fisher JL, Godfrey K, Stephens RI. Experimental strain analysis of infant, adolescent and adult miniature swine skulls subjected to simulated mastication forces. J Biomech. 1976;9:333–338. doi: 10.1016/0021-9290(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Greaves WS. The mammalian postorbital bar as a torsion-resisting helical strut. J Zool (A) 1985;207:125–136. [Google Scholar]
- Herring SW. The dynamics of mastication in pigs. Arch Oral Biol. 1976;21:473–480. doi: 10.1016/0003-9969(76)90105-9. [DOI] [PubMed] [Google Scholar]
- Herring SW. Morphological correlates of masticatory patterns in peccaries and pigs. J Mamm. 1985;66:603–617. [Google Scholar]
- Herring SW. Muscles of mastication: architecture and functional organization. In: Davidovitch Z, editor. The biology of tooth movement and craniofacial adaptation. Columbus: The Ohio State University; 1992. pp. 541–548. [Google Scholar]
- Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. J Morphol. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol. 1973;141:427–460. doi: 10.1002/jmor.1051410405. [DOI] [PubMed] [Google Scholar]
- Herring SW, Teng S, Huang X, Mucci RJ, Freeman J. Patterns of bone strain in the zygomatic arch. Anat Rec. 1996;246:446–457. doi: 10.1002/(SICI)1097-0185(199612)246:4<446::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang G, Herring SW. Age changes in mastication in the pig. J Comp Biochem Physiol. 1994;107A:647–654. doi: 10.1016/0300-9629(94)90364-6. [DOI] [PubMed] [Google Scholar]
- Hubbard RP, Melvin JW, Barodawala IT. Flexure of cranial sutures. J Biomech. 1971;4:491–496. doi: 10.1016/0021-9290(71)90039-x. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Picq PG, Johnson KR. Function of the supraorbital region of primates. Arch Oral Biol. 1991a;36:373–381. doi: 10.1016/0003-9969(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Picq PG, Johnson KR. Masticatory-stress hypotheses and the supraorbital region of primates. Am J Phys Anthropol. 1991b;86:1–36. doi: 10.1002/ajpa.1330860102. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Ravosa MJ. An analysis of the supraorbital region of primates: a morphometric and experimental approach. In: Smith P, Tchernov E, editors. Structure, function and evolution of teeth. London: Freund; 1992. pp. 223–255. [Google Scholar]
- Jaslow CR. Mechanical properties of cranial sutures. J Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- Jaslow CR, Biewener AA. Strain patterns in the horncores, cranial bones and sutures of goats (Capra hircus) during impact loading. J Zool. 1995;235:193–210. [Google Scholar]
- Katsaros C, Kiliaridis S, Berg R. Functional influence on sutural growth. A morphometric study in the anterior facial skeleton of the growing rat. Eur J Orthod. 1994;16:353–360. doi: 10.1093/ejo/16.5.353. [DOI] [PubMed] [Google Scholar]
- Kimbel WH, Rak Y. Functional morphology of the asterionic region in extant hominoids and fossil hominids. Am J Phys Anthropol. 1985;66:31–54. doi: 10.1002/ajpa.1330660104. [DOI] [PubMed] [Google Scholar]
- Mowbray KM, Lieberman DE. The effects of nuchal muscle activity on occipital morphology: experimental evidence from swine. Am J Phys Anthropol Suppl. 1997;24:175. [Google Scholar]
- Oyen O, Melugin MB, Indresano AT. Strain gauge analysis of the frontozygomatic region of the zygomatic complex. J Oral Maxillofac Surg. 1996;54:1092–1095. doi: 10.1016/s0278-2391(96)90167-6. [DOI] [PubMed] [Google Scholar]
- Oyen OJ, Tsay TP. A biomechanical analysis of craniofacial form and bite force. Am J Orthod. 1991;99:298–309. doi: 10.1016/0889-5406(91)70012-L. [DOI] [PubMed] [Google Scholar]
- Persson M. The role of sutures in normal and abnormal craniofacial growth. Acta Odont Scand. 1995;53:152–161. doi: 10.3109/00016359509005965. [DOI] [PubMed] [Google Scholar]
- Rak Y. The functional significance of the squamosal suture in Australopithecus boisei. Am J Phys Anthropol. 1978;49:71–78. doi: 10.1002/ajpa.1330490111. [DOI] [PubMed] [Google Scholar]
- Rawlinson SCF, Mosley JR, Suswillo RFL, Pitsillides AA, Lanyon LE. Calvarial and limb bone cells in organ and monolayer culture do not show the same early responses to dynamic mechanical strain. J Bone Min Res. 1995;10:1225–1232. doi: 10.1002/jbmr.5650100813. [DOI] [PubMed] [Google Scholar]
- Ross CF, Hylander WL. In vivo and in vitro bone strain in the owl monkey circumorbital region and the function of the postorbital septum. Am J Phys Anthrop. 1996;101:183–215. doi: 10.1002/(SICI)1096-8644(199610)101:2<183::AID-AJPA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Smith HG, McKeown M. Experimental alteration of the coronal sutural area: a histological and quantitative microscopic assessment. J Anat. 1974;118:543–559. [PMC free article] [PubMed] [Google Scholar]
- Smith KK, Hylander WL. Strain gauge measurement of mesokinetic movement in the lizard Varanus exanthematicus. J Exp Biol. 1985;114:53–70. doi: 10.1242/jeb.114.1.53. [DOI] [PubMed] [Google Scholar]
- Sutton G. Master's thesis, Civil Engineering. University of Washington; Seattle: 1993. The use of a non-contact video method to compare the compressive properties of bone and ligament in the zygomatic arch of pigs. [Google Scholar]
- Villanueva AR, Lundin KD. A versatile new mineralized bone stain for simultaneous assessment of tetracycline and osteoid seams. Stain Technol. 1989;64:129–137. doi: 10.3109/10520298909106985. [DOI] [PubMed] [Google Scholar]
- Weijs WA. Evolutionary approach of masticatory motor patterns in mammals. In: Bels VL, Chardon M, Vandewalle P, editors. Biomechanics of feedng in vertebrates. Berlin: Springer-Verlag; 1994. pp. 281–320. [Google Scholar]


