Abstract
Objective
To identify gaps in therapy with urate-lowering drugs for the treatment of gout as well as factors associated with resuming therapy.
Methods
We identified persons from two integrated delivery systems 18 years or older with a diagnosis of gout who initiated use of a urate-lowering drug from January 1, 2000 through June 30, 2006 and who had a gap in therapy. A gap was defined as a period of over 60 days after the completion of one prescription in which no refill for a urate-lowering drug was obtained. Survival curves were used to assess return to therapy of urate-lowering drugs. Cox proportional hazards analysis estimated the association between covariates and return to therapy.
Results
There were 4,166 new users of urate-lowering drugs (97% received allopurinol) of whom 2,929 (70%) had a gap in therapy. Among those with a gap, in 75% it occurred in the first year of therapy. Fifty percent of patients with a gap returned to therapy within 8 months, and by 4 years it was 75%. Age 45 to 74 (<45 referent) and greater duration of urate-lowering drug use prior to the gap was associated with resuming treatment within one year. In contrast, receipt of NSAIDs or glucocorticoids in the year prior to the gap was associated with a reduced likelihood of resuming therapy.
Conclusions
The majority of gout patients with gaps in urate-lowering drug use returned to treatment. More investigation is needed to better understand why patients may go for months without refilling prescriptions given the clinical consequences of nonadherence.
Keywords: persistence, adherence, compliance, gout, urate lowering drugs
Introduction
Gout is a common inflammatory condition involving joints and soft tissues and affects up to 5 million Americans.(1) Gout patients with recurrent disease flares and tophaceous deposits can be successfully treated with urate-lowering drugs. These agents have been shown to reduce the frequency of acute gouty attacks, decrease tophus area and prevent urate nephropathy and uric acid nephrolithiasis.(2, 3) Chronic use of urate-lowering drugs is necessary for lowering and maintaining serum uric acid levels to a target threshold of <6.0 mg/dL as this is associated with fewer gout flares and depletion of urate crystal stores in synovial tissues.(4-7) However, studies examining adherence with urate-lowering drugs in the treatment of gout have demonstrated that compliance with chronic medications is a substantial problem.(8-11) While the previous articles have led to important contributions regarding the problem of nonadherence, little is known regarding the dynamic patterns of medication use such as patients stopping and then resuming therapy.
While in some chronic diseases, such as osteoporosis, short-term gaps in therapy may have little clinical effect, intermittent urate-lowering drug use may trigger or prolong gouty attacks. Thus it is important to assess gaps in therapy and evaluate factors predicting return to treatment, paying particular attention to potentially modifiable determinates of reinitiation. The present study was conducted to characterize patterns of urate-lowering drug use, including gaps in treatment and return to treatment. Additionally, predictors of return to urate-lowering drug use were examined. We hypothesized that gaps in therapy as well as resuming use of urate-lowering drugs were common.
Methods
Setting
Two health care delivery systems involved in the Health Maintenance Organization (HMO) Research Network Center for Education and Research on Therapeutics (CERTs) participated in this study.(12) The two systems are located in the Northeast and Rocky Mountain region of the United States and had a combined population of approximately 650,000 in 2006. We identified the study cohort using the Virtual Data Warehouse (VDW), a previously-developed mechanism used to produce comparable data across participating health plans.(13) The VDW uses standardized file definitions, uniform data dictionaries for each content area, and common formats for each data element. These common file structures enable analysts at each site to write programs to extract and/or analyze data at all participating sites. For this study, the analytic dataset included computerized information on utilization of health care services including membership, pharmacy dispensing data, and selected hospital and ambulatory diagnoses and procedures for all enrollees of the health plans from 1/1/1999 to 6/30/07. The institutional review boards at each participating organization approved this study.
Study population and design
We identified members from the dataset who had an International Disease Classification code for a gout diagnosis, were 18 years or older at the time of the first urate-lowering drug dispensing and had continuous enrollment in the health plan with drug coverage during the period 12 months prior to and 12 months following the first urate-lowering drug dispensing. Our analysis focused on new users of therapy including those who had at least one gap in therapy. New use was defined as no dispensing of a urate-lowering drug in the prior 6 months.
Treatment gaps and return to therapy measures
Gaps in therapy were identified. These were defined as a period of greater than 60 days after the completion of one prescription (i.e., allowing a “grace period”) in which no refill for a urate-lowering drug was obtained. After a gap in therapy, patients could either have discontinued therapy or reinitiated sometime in the future. The number of gaps was identified in the study population. A return to medication use was defined as filling of any urate-lowering drug after a gap in therapy. This allowed patients to switch treatments and still be considered adherent.
Covariates
Patient characteristics were assessed as potential correlates of a gap in therapy as well as return to urate-lowering drug use. These included demographic factors (age and gender), health care utilization (hospitalization, number of provider visits for any diagnosis as well as visits associated with a gout diagnosis), comorbid conditions, medications used to treat symptomatic gout, and medications that can trigger gout. Age and sex were ascertained from the demographic data.
To compare those with a gap in therapy to those without, we assessed health care utilization and medication use during the time period 12 months prior to the first urate-lowering drug dispensing. We ascertained the presence of comorbidities from the ICD-9 diagnosis codes associated with ambulatory and inpatient care in the 12 months prior to and 12 months after the first urate-lowering drug dispensing. We then focused on those with a gap in therapy and compared those who resumed use of urate-lowering drugs to those who did not. For those analyses, we compared health care utilization and medication use during the time period 12 months prior to the first gap in urate-lowering drug use. We ascertained the presence of comorbidities from the ICD-9 diagnosis codes associated with ambulatory and inpatient care in the 12 months prior to and 12 months after the first gap in therapy.
Comorbidities of interest included coronary heart disease, diabetes mellitus, dyslipidemia, hypertension, nephrolithiasis, peripheral arterial disease, renal insufficiency and renal failure. A Charlson comorbidity score was ascertained based on a range of diagnoses in the 12 months prior to the first gap. Medication usage was identified by determining dispensings using national drug codes for the following drug classes: diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and glucocorticoids. In addition, the number of drugs used per person was identified using dispensing records based on the number of unique national drug codes numbers in the 60 days prior to the gap. At the two health plans the vast majority of patients received prescriptions with a days supply of either 30 or 60 days.
Analyses
The characteristics of the study populations were examined using descriptive statistics. Urate-lowering drug initiators with and without gaps in therapy were compared using t-tests for continuous variables and chi square tests for discrete variables. Similarly, among those with a gap in therapy, those who resumed treatment with a urate-lowering drug were compared to those who did not using t-tests and chi square tests as above. We calculated the number of gaps during the first year of therapy (all patients were required to have 12 months of enrollment following urate-lowering drug initiation) and over the study period. The Kaplan-Meier method was used to estimate the cumulative probability of return to treatment with urate-lowering drugs after a gap in therapy during the study period, censoring patients when they disenrolled in the health plan or at study end. Cox proportional hazards models were used to estimate the hazard of return to treatment within 1 year of having an interruption of therapy as well after 1 year controlling for covariates identified in the 12 months prior to the gap. We conducted an additional sensitivity analysis in which the grace period (the time between completing a prescription and filling a subsequent prescription) was defined as 90 days rather 60 days. All analyses were run using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
There were 4,166 new users of urate-lowering drugs, 97% of whom were initiated on allopurinol. Over 70 percent (n=2,929) had a gap in therapy, meaning they had at least one period lasting greater than 60 days during which no days were covered by a prescription for any urate-lowering drug. Those with a gap in therapy were more likely to be younger (mean age 62 versus 64, p <.0001) at the time of urate-lowering drug initiation, have fewer comorbid conditions based on the Charlson score (p<.0001), no hospitalizations (p=.0014), and fewer encounters for gout in the year prior to urate-lowering drug initiation (mean encounters 1.59 versus 1.86, p<.0001).
The characteristics of those with a gap in therapy are given in Table 1. The majority of patients were male (76%), and had a mean age of 63 (±15) at the time of the gap. On average, they had 1.97 (±2.76) visits with a provider for gout in the 12 months prior to the gap in therapy and used 3.46 (±3.47) different medications. In 75% of patients, the gap occurred in the first year of therapy. Over the study period 54% had one gap, 25% had two gaps and 22% had three or more gaps. Use of medications typically prescribed for gout flares in the 12 months prior to the gap in urate-lowering drug use, including nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine and glucocorticoids, occurred in 57%, 37% and 27% of the patients, respectively. The median duration of urate-lowering drug use in the 12 months prior to the gap was 120 days (IQR 170).
Table 1.
Characteristics of the study population with at least a 60 day gap in therapy after urate-lowering drug initiation.
| Population (n=2929 ) | |
|---|---|
| Mean age (in years ± SD) | 63 (15) |
| Gender, (N, % male) | 2213 (76) |
| Number of provider visits for gout prior to gap (mean, ± SD)* | 1.97 (2.76) |
| Number of physician visits for any diagnosis prior to gap (mean, ± SD)* | 6.81 (6.29) |
| Hospitalization (N, %)* | 584 (20) |
| Number of drugs dispensed prior to the gap (mean, ± SD)** | 3.46 (3.47) |
| Urate-lowering drug initiated (N, %) | |
| Allopurinol | 2832 (97) |
| Probenecid | 96 (3) |
| Sulfinpyrazone | 1 (0) |
| Number of days on a urate-lowering drug prior to gap (median, IQR)* | 120 (170) |
| Charlson comorbidity score (mean, ± SD)* | 1.16 (1.77) |
| Associated comorbidities (N, %)*** | |
| Hypertension | 1960 (67) |
| Dyslipidemia | 1405 (48) |
| Coronary heart disease | 706 (24) |
| Diabetes mellitus | 681 (23) |
| Renal insufficiency | 553 (19) |
| Renal failure | 503 (17) |
| Peripheral arterial disease | 186 (6) |
| Nephrolithiasis | 124 (4) |
| Use of medications associated with gout or difficulty treating gout (N, %)* | |
| Thiazide diuretics | 835 (29) |
| All diuretics | 1345 (46) |
| Use of acute gout medications prior to the gap* | |
| NSAIDs | 1659 (57) |
| Colchicine | 1095 (37) |
| Glucocorticoid | 779 (27) |
calculated based on the 12 months prior to the gap in therapy
calculated based on the number of unique national drug codes (NDC) for pharmacy dispensings in the 60 days prior to the gap
identified based on diagnostic codes in the 12 months prior and 12 months following the gap
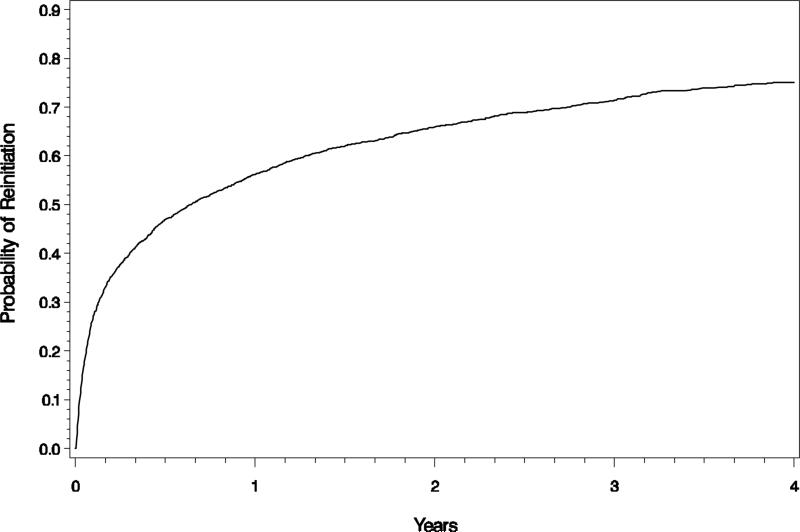
In Figure 1, we present the Kaplan-Meier estimates of the cumulative probability of return to urate-lowering drug therapy. An estimated 50% of patients returned to treatment within 8 months of the gap and by 4 years 75% had resumed urate-lowering drug use. Table 2 presents the results of the Cox proportional hazards model examining the association between patient characteristics and the rate at which medication use was resumed in the first year after the gap. After controlling for health plan, variables associated with return to treatment included age, duration of urate-lowering drug therapy prior to the gap and receipt of NSAIDS and glucocorticoids. Specifically, being aged 45 to 54, 55 to 64 and 65 to 75 was associated with resuming urate-lowering drug use as compared to those <45 years of age (Hazard Ratio [HR] 1.37, 95% CI, 1.14-1.65; HR 1.26, 95% CI, 1.05-1.53; and HR 1.38, 95% CI, 1.15-1.66 respectively). Greater duration of urate-lowering drug use prior to the interruption increased the probability of return to treatment (HR 1.45, 95% CI, 1.26-1.68; HR 2.15, 95% CI, 1.86-2.48; HR 1.92, 95% CI, 1.69-2.19 for the second, third and fourth quartile of use respectively as compared to the lowest quartile of use). Receipt of NSAIDs (HR 0.84, 95% CI, 0.75-0.93) and glucocorticoids (HR 0.83, 95% CI, 0.74-0.93) in the year prior to the gap was associated with a reduced likelihood of resuming therapy. Factors including number of comorbidities, number of encounters associated with a gout diagnosis or any diagnosis, number of medications, and hospitalization were not associated with a return to treatment in the first year.
Figure 1.
The Kaplan-Meir estimate of the cumulative probability of returning to treatment with urate-lowering drugs in gout patients who have had a gap in therapy.
Table 2.
Cox proportional hazards analysis of baseline variables associated with a return to urate-lowering drug use within the first year after an interruption in therapy after controlling for health plan.
| Variable Associated with Return to Therapy | |
|---|---|
| Hazard Ratio (95% CI) | |
| Demographics | |
| Gender (Male is reference) | 0.92 (0.82-1.04) |
| Age in years (<45 is the reference) | |
| 45-54 | 1.37 (1.14-1.65) |
| 55-64 | 1.26 (1.05-1.53) |
| 65-74 | 1.38 (1.15-1.66) |
| 75 and older | 1.19 (0.98-1.45) |
| Gout care | |
| Duration of urate-lowering drug use prior to the gap (lowest quartile of use is the reference) | |
| second quartile of use | 1.45 (1.26-1.68) |
| third quartile of use | 2.15 (1.86-2.48) |
| fourth quartile of use | 1.92 (1.69-2.19) |
| Use of acute gout medications | |
| NSAIDs | 0.84 (0.75-0.93) |
| Glucocorticoids | 0.83 (0.74-0.93 |
When the model was rerun assessing factors associated with return to therapy after 1 year (Table 3), the occurrence of a hospitalization (HR 0.65, 95% CI, 0.48-0.89) in the year prior to the gap was associated with a reduced likelihood. The number of gout encounters prior to the gap was inconsistently related to a return to therapy. Variables including number of comorbidities, number of medications, duration of urate-lowering drug use prior to the gap, and receipt of NSAIDs and glucocorticoids were not significantly associated. Finally, we tested the robustness of our observed associations between covariates and return to treatment by defining a gap in urate-lowering drug use of at least 90 days instead of 60 days. The strengths of the associations were essentially the same for return to therapy within 1 year except that use of glucocorticoids was no longer significant. For the model assessing return to therapy after 1 year, age was no longer significant.
Table 3.
Cox proportional hazards analysis of baseline variables associated with a return to urate-lowering drug use 1 year after an interruption in drug use after controlling for health plan.
| Variable Associated with Return to Therapy | |
|---|---|
| Hazard Ratio (95% CI) | |
| Demographics | |
| Gender (Male is reference) | 0.81 (0.62-1.06) |
| Age in years (<45 is the reference) | |
| 45-54 | 0.84 (0.60-1.17) |
| 55-64 | 0.85 (0.61-1.19) |
| 65-74 | 0.81 (0.59-1.12) |
| 75 and older | 0.73 (0.51-1.04) |
| Overall health status | |
| History of a hospitalization in the year prior to the gap | 0.65 (0.48-0.89) |
| Gout care (0 encounters is the reference) | |
| 1 encounter for gout in the year prior to the gap | 0.71 (0.54-0.94) |
| 2 encounters for gout in the year prior to the gap | 0.78 (0.58-1.07) |
| 3 or more encounters for gout in the year prior to the gap | 0.90 (0.68-1.19) |
Discussion
In our population of health plan enrollees with a diagnosis of gout who were new users of urate-lowering drugs, gaps in therapy greater than 60 days occurred in the majority of patients and most commonly occurred in the first year of treatment. Those with a gap were younger and healthier, and had fewer encounters for gout prior to urate-lowering drug initiation. Among patients with a gap in therapy, most returned to urate-lowering drug use suggesting adherence to urate-lowering drugs is dynamic, similar to what has been documented for other medication classes.(14-16) While several previous studies have reported that patient adherence with therapeutic regimens for gout is poor, to our knowledge this is first report suggesting that many patients return to urate-lowering drug use after periods of apparent nonuse. In addition, the factors associated with a return to therapy differed among those who reinitated within 1 year as compared to those who reinitiated after 1 year.
Previous studies on gout have examined adherence to urate-lowering drugs over a relatively short period of time (usually 1 year) using measures like the medication possession ratio or proportion of days covered.(9, 10, 17) These measures average medication use over a specified period of time, usually a year, and using a specified cutoff point, patients are characterized as adherent or not. However, use of a dichotomous outcome, leaves unclear the exact medication taking behavior on the part of patients. For example, a medication possession ratio of 50% could mean either that patients filled a monthly prescription every 2 months 6 times or that they only filled prescriptions for the first 6 months of the year.
Interruptions in medication drug use may occur for a variety of reasons. Patients may stop using newly initiated medications due to side effects, perceived drug ineffectiveness and financial concerns. However, over time patients may be motivated to return to urate-lowering drug use due to repeated gouty attacks and/or counseling by their provider. Longer duration of therapy prior to the gap was associated with return to therapy within one year, as has been seen in studies looking at a return to therapy for osteoporosis and hypertension.(14, 16) Interestingly there were very few covariates associated with a return to therapy after 1 year suggesting the factors that influence return to therapy after a prolonged interruption in drug treatment are complex and likely not easily identified using administrative data.
Of concern is that receipt of NSAIDs and glucocorticoids in the 12 months prior to the gap was associated with a reduced likelihood of return to therapy at 1 year. A previous study demonstrated that use of NSAIDs in gout patients who were new users of urate-lowering drugs was shown to be associated with nonadherence in the first year of urate-lowering drug therapy.(17) These findings taken together suggest that patients with more active gout—the very group who should be receiving urate-lowering drug therapy, are undertreated. Further examination of this issue is needed.
Our study has several strengths. It is the first study we know of to examine the dynamics of urate-lowering drug use in patients with gout. In addition, our population was limited to those with pharmacy benefits where the financial burden of multiple medications is reduced. Given that the urate-lowering drugs in this study were all available as generics, this also minimized the impact that financial factors had on drug use. There are some limitations. First, our population consisted of health plan enrollees. Therefore our results may not be generalizable to those with other types of insurance coverage or who are uninsured. Secondly, we used administrative claims data and thus are unable to assess reasons for stopping or starting urate-lowering drugs. Therefore some of the lapses in treatment may be physician-directed or otherwise appropriate. However, given the relative safety of urate-lowering drugs and the low frequency of adverse events, this is not a likely explanation for many of the gaps in therapy we observed. In addition, we can only capture pharmacy dispensings rather than actual patient use. Of note, while we classify patients as new users, given that patients may return to therapy after many years, it is likely some of our new users are actually returning users. Finally, we were unable to assess the relationship between acute gout attacks and return to therapy since there is no validated approach to identify gout flares in administrative data. Encounters associated with a gout diagnosis may reflect routine care rather than treatment of a flare. In addition, dispensing of medications used to treat acute gout can be used for non-gout related conditions and for prophylaxis against acute gout flares.
In summary, our results yield insight into the dynamics of urate-lowering drug use for gout. Gaps in therapy are common, usually occurring in the first year of therapy. However, the majority of patients with interruptions in urate-lowering drug use return to therapy. In patients who are not responding to therapy, providers should probe whether interruptions in urate-lowering drug use are playing a role. More investigation into the social, psychological and clinical factors associated with return to therapy is needed, as these factors should be targeted in order to improve medication adherence. Lastly, given that NSAIDs and glucocorticoids were associated with a reduced likelihood to return to therapy within one year, patients with active gout should be counseled regarding the short-term side effects (e.g., risk of flare with urate-lowering drug initiation) and chronic benefits of urate-lowering drug use (e.g., reduction in gout flares).
Acknowledgments
Funding: This work was supported by Grant Number K23AR053856 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kramer HJ, Choi HK, Atkinson K, et al. The association between gout and nephrolithiasis in men: The Health Professionals’ Follow-Up Study. Kidney Int. 2003;64(3):1022–6. doi: 10.1046/j.1523-1755.2003.t01-2-00171.x. [DOI] [PubMed] [Google Scholar]
- 2.Rott KT, Agudelo CA. Gout. Jama. 2003;289(21):2857–60. doi: 10.1001/jama.289.21.2857. [DOI] [PubMed] [Google Scholar]
- 3.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–5. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 4.Keith MP, Gilliland WR. Updates in the Management of Gout. The American Journal of Medicine. 2007;120(3):221–4. doi: 10.1016/j.amjmed.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Mikuls TR, MacLean CH, Olivieri J, et al. Quality of care indicators for gout management. Arthritis Rheum. 2004;50(3):937–43. doi: 10.1002/art.20102. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Ruiz F, Liote F. Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Rheum. 2007;57(7):1324–8. doi: 10.1002/art.23007. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedel AA, Nelson M, Joseph-Ridge N, et al. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004;31(8):1575–81. [PubMed] [Google Scholar]
- 9.Sarawate CA, Brewer KK, Yang W, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81(7):925–34. doi: 10.4065/81.7.925. [DOI] [PubMed] [Google Scholar]
- 10.Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008;67(5):609–13. doi: 10.1136/ard.2007.076182. [DOI] [PubMed] [Google Scholar]
- 11.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KA, Davis RL, Gunter MJ, et al. The HMO Research Network. In: Brian LS, editor. Pharmacoepidemiology. Fourth Edition 2007. pp. 261–9. [Google Scholar]
- 13.Wagner EH, Greene SM, Hart G, et al. Building a Research Consortium of Large Health Systems: The Cancer Research Network. J Natl Cancer Inst Monogr. 2005;2005(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 14.Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120(3):251–6. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167(8):847–52. doi: 10.1001/archinte.167.8.847. [DOI] [PubMed] [Google Scholar]
- 16.van Wijk BLG, Avorn J, Solomon DH, et al. Rates and determinants of reinitiating antihypertensive therapy after prolonged stoppage: a population-based study. J Hypertens. 2007;25(3):689–97. doi: 10.1097/HJH.0b013e3280148a58. [DOI] [PubMed] [Google Scholar]
- 17.Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46. doi: 10.1186/ar2659. [DOI] [PMC free article] [PubMed] [Google Scholar]