Abstract
Background
Osteosarcoma, the most common primary bone tumor, occurs most frequently in adolescents, but a second incidence peak among individuals over age 60 exists. Most osteosarcoma epidemiology studies have been embedded in large analyses of all bone tumors, or focused on cases occurring in adolescence. Detailed descriptions of osteosarcoma incidence and survival specifically, with direct comparisons among subjects of all ages and ethnicities, are not available.
Methods
Frequency, incidence and survival rates for 3,482 patients with osteosarcoma from the National Cancer Institute’s population-based Surveillance, Epidemiology, and End Results (SEER) program between 1973 and 2004 are presented by age (0–24, 25–59, and 60–85+ years), race, sex, pathology subtype, stage, and anatomic site.
Results
There were large differences in incidence and survival rates by age. Osteosarcoma incidence in the youngest cases was greatest in the Other race designation, while it was greatest in Blacks and Whites in the middle age and elderly patients, respectively. There was a high percentage of osteosarcoma with Paget’s disease and osteosarcoma as a second or greater cancer among the elderly. Tumor site differences among age groups were noted. Survival rates varied by anatomic site and disease stage, and have not significantly improved from 1984 to 2004.
Conclusions
This comprehensive, population-based description of osteosarcoma, identified important differences in incidence, survival, pathologic subtype, and anatomic site among age groups, and quantified the impact of osteosarcoma in Paget’s disease or as a second cancer on incidence and mortality rates. These findings may have implications in understanding osteosarcoma biology and epidemiology.
Keywords: osteosarcoma, bone cancer, epidemiology, SEER, incidence, survival
Introduction
Osteosarcoma is the most commonly diagnosed primary malignancy of bone, particularly among children and adolescents.1–5 Nonetheless, it is rare, representing less than 1% of all cancers diagnosed in the United States, and there is a paucity of population-based comprehensive data on its occurrence and outcome. In young patients, it most often arises in the metaphyses of long bones, such as the distal femur, proximal tibia, and proximal humerus.6, 7 Although the majority of osteosarcoma cases occur in adolescence, there is a second incidence peak in the elderly (seventh and eighth decades).8, 9 Osteosarcomas in elderly patients are often considered secondary neoplasms attributed to sarcomatous transformation of Paget’s disease of bone or some other benign bone lesion.10–13 In elderly patients, tumors occur more commonly in axial locations and in areas that have been previously irradiated or have underlying bone abnormalities.14 At all ages, males are affected more frequently than females.4, 5, 8, 15, 16 Most previous studies describing incidence and survival rates focused only on children and young adults,16–28 or combined patients of all ages,4, 5, 10, 29–31 and many were brief sections in reports on all bone cancers combined 4, 5, 9, 29–32 or childhood cancer.16–20, 22, 23, 25–28, 33
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program of United States population-based data from 1973 to 2004 offers a unique opportunity to perform detailed analyses of incidence and survival of rare neoplasms. The SEER program now covers approximately 26% of the U.S. population, and consists of 17 geographically-defined central cancer registries; the population covered by these registries is comparable to the general U.S. population.34 In this study, we compare osteosarcoma incidence and survival in three age groups (0–24, 25–59, and 60–85+) by race, sex, calendar year, disease stage, pathology subtype, and anatomic site.
Methods
Data were extracted from the 2006 SEER Public Access database of the National Cancer Institute, covering years 1973–2004. SEER limited-use data files and SEER*Stat software (version 6.3.6)35 were used to calculate frequency (i.e., number of cases), incidence rates, and survival statistics. All rates are per 1,000,000, and the age-adjusted rates were calculated with the 2000 U.S. standard population (19 age groups - Census P25-1130).
All malignant bone tumors and osteosarcoma cases were classified according to the International Classification of Childhood Cancers (ICCC)36 and/or the International Classification of Disease for Oncology (ICD-O-3),37 and all osteosarcoma histology subtypes and anatomic site codes were classified as described by ICD-O-3.37 No additional detailed site descriptions, i.e. the specific bones, or epiphyseal, metaphyseal, and diaphyseal location distinctions, were available.
Frequency and incidence statistics were calculated for the entire time period (1973–2004), and survival rates were calculated from 1973 to 2003 (allowing for at least one year of follow-up for all cases) based on: age group (0–24, 25–59, and 60–85+ years), race [using SEER designations of White, Black, and Other (including American Indian, Alaskan Native, and Asian/Pacific Islanders)], sex (male or female), osteosarcoma subtype (all osteosarcoma cases, without Paget’s disease; first neoplasm, without Paget’s disease; second or greater neoplasm, without Paget’s disease and sequence number ≥ 2; and, with Paget’s disease), pathology (ICD-O-3 codes 9180-9186, 9192-9194), primary anatomic site (ICD-O-3 codes C40.0-C41.9), stage (localized, regional, or distant), and SEER registry. More specific race designations [White, Black, American Indian/Alaska Native, Asian/Pacific Islander, and Hispanic (an origin recode, not mutually exclusive from the other race designations)] were available after 1992 in the SEER 13 registry38, and were used for a more detailed investigation of racial differences. All incidence rates were calculated using SEER 939 data (except those for the new race designations using SEER 13), and all frequency and survival statistics were determined with SEER 1740. Rates based on less than 10 cases are considered unstable and should be interpreted with caution, indicated by italics in the tables. Time trends were evaluated by dividing the study period into 5-or 10-year intervals and using the mean value over this period, and by estimating the annual percent change (APC; including 95% confidence intervals [95% CI]) in incidence rates. The percent relative survival was estimated at yearly intervals for a total of 5 years.
Results
Incidence rates by age, subtype, and race
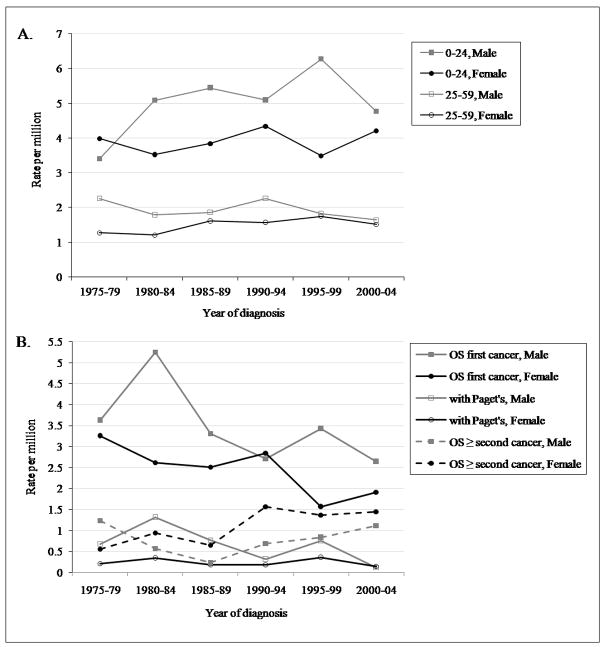
The SEER 17 database consists of 3,482 cases of osteosarcoma reported between 1973 and 2004. Osteosarcoma was the most common specified malignant bone tumor, followed by chondrosarcoma and Ewing’s sarcoma, respectively. Osteosarcoma as a first cancer represented 88% (n = 3071) of all reported osteosarcoma cases, osteosarcoma as a second or greater cancer 10% (n = 344), and osteosarcoma with Paget’s disease 2% (n = 67). A primary peak in osteosarcoma incidence occurred in children and adolescents ages 0–24, a plateau of incidence was observed for ages 25–59, and there was a secondary peak of osteosarcoma incidence in the elderly (60–85+) (Figure 1). There were few cases of osteosarcoma as a second or greater cancer and osteosarcoma with Paget’s disease among the younger age groups, but among the elderly these subtypes made up a significant proportion of all cases (Figure 1 and Table 1). All osteosarcoma rates are calculated after excluding those occurring with Paget’s disease, and these results are shown separately for the elderly age group.
Figure 1.
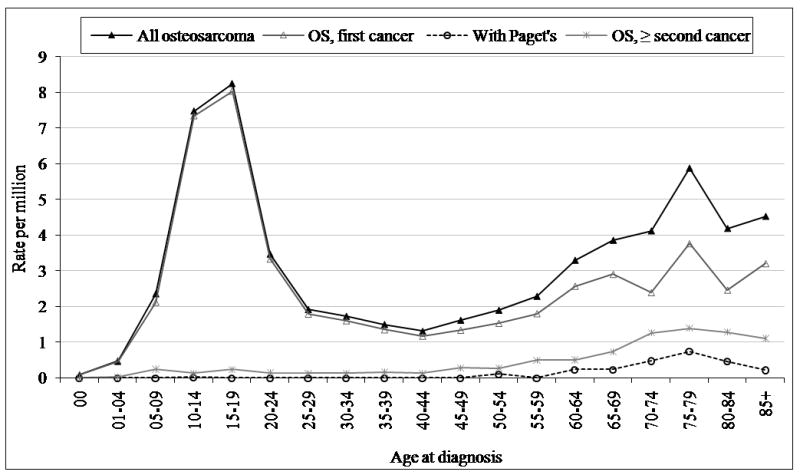
Osteosarcoma incidence by disease sequence, SEER 9 1973–2004.
Table 1.
Age-adjusted incidence rates per million of osteosarcoma by sex, race, and subtype, SEER 9 1973–2004.
| Age | All races |
White |
Black |
Other* |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both |
Male |
Female |
Both |
Male |
Female |
Both |
Male |
Female |
Both |
Male |
Female |
||||||||||||||
| Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | Rate | n | ||
| 0–24 | Osteosarcoma † | 4.4 | 1,253 | 5.0 | 717 | 3.9 | 536 | 4.2 | 923 | 4.6 | 520 | 3.8 | 403 | 5.0 | 181 | 5.9 | 107 | 4.1 | 74 | 5.4 | 140 | 6.2 | 82 | 4.5 | 58 |
| 25–59 | Osteosarcoma† | 1.7 | 607 | 1.9 | 339 | 1.5 | 268 | 1.7 | 483 | 1.9 | 283 | 1.4 | 200 | 2.3 | 83 | 2.2 | 36 | 2.5 | 47 | 1.2 | 37 | 1.3 | 18 | 1.1 | 19 |
| 60+ | Osteosarcoma | 4.2 | 471 | 4.9 | 226 | 3.8 | 245 | 4.4 | 423 | 5.2 | 205 | 3.9 | 218 | 3.1 | 27 | 3.1 | 11 | 3.2 | 16 | 2.6 | 20 | 3.1 | 10 | 2.3 | 10 |
| OS, first cancer | 2.8 | 321 | 3.5 | 163 | 2.4 | 158 | 2.9 | 284 | 3.7 | 147 | 2.4 | 137 | 2.4 | 21 | 1.8 | 7 | 2.8 | 14 | 1.9 | 15 | 2.7 | 9 | 1.3 | 6 | |
| OS, ≥ second cancer | 1.0 | 108 | 0.8 | 36 | 1.1 | 72 | 1.0 | 98 | 0.8 | 31 | 1.2 | 67 | 0.7 | 6 | 1.3 | 4 | 0.4 | 2 | 0.5 | 4 | 0.3 | 1 | 0.7 | 3 | |
| OS with Paget’s | 0.4 | 42 | 0.6 | 27 | 0.2 | 15 | 0.4 | 41 | 0.7 | 27 | 0.2 | 14 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.1 | 1 | 0.0 | 0 | 0.3 | 1 | |
| All | Osteosarcoma | 3.1 | 2,336 | 3.5 | 1,285 | 2.7 | 1,051 | 3.0 | 1,833 | 3.4 | 1,011 | 2.6 | 822 | 3.4 | 292 | 3.7 | 154 | 3.2 | 138 | 2.9 | 197 | 3.3 | 110 | 2.5 | 87 |
| OS, first cancer | 2.7 | 2,065 | 3.1 | 1,165 | 2.3 | 900 | 2.6 | 1,605 | 3.0 | 906 | 2.3 | 699 | 3.1 | 266 | 3.2 | 141 | 2.9 | 125 | 2.6 | 180 | 3.2 | 108 | 2.0 | 72 | |
| OS, ≥ second cancer | 0.3 | 223 | 0.3 | 90 | 0.3 | 133 | 0.3 | 182 | 0.3 | 75 | 0.3 | 107 | 0.4 | 25 | 0.5 | 13 | 0.3 | 12 | 0.3 | 16 | 0.1 | 2 | 0.4 | 14 | |
| OS with Paget’s | 0.1 | 47 | 0.1 | 30 | 0.0 | 17 | 0.1 | 45 | 0.1 | 30 | 0.0 | 15 | 0.0 | 1 | 0.0 | 0 | 0.0 | 1 | 0.0 | 1 | 0.0 | 0 | 0.0 | 1 | |
Other includes American Indian, Alaskan Native, and Asian/Pacific Islander;
osteosarcoma without Paget’s disease; OS, osteosarcoma; rates in italics are based on less than 10 cases.
Osteosarcoma incidence rates were highest in Blacks, as were osteosarcomas occurring as a second or greater cancer. Osteosarcomas with Paget’s disease rates were highest in Whites in the oldest age group, where there were sufficient evaluable cases (Table 1). The average male-to-female ratio of osteosarcoma was 1.22:1. The characteristics of subjects (age, sex, race, and survival) with osteosarcoma throughout the 9 SEER registries did not vary significantly by registry (Supplemental Table 1).
Since osteosarcoma incidence is clearly age-dependent, more specific analyses were conducted within each age group (0–24, 25–59, and 60–85+ years). These age groups were chosen to focus on the two incidence peaks in children and adolescents and the elderly, and the incidence plateau persons aged 25 to 59 years.
Ages 0–24 years
Osteosarcoma represented the majority of specified malignant bone tumors in all children and adolescents (n = 1855; 55%), followed by Ewing’s sarcoma (n = 1198; 36%). The age-adjusted incidence per million of osteosarcoma in this age group was 4.4. There was one case of osteosarcoma with Paget’s disease and 70 cases in which osteosarcoma occurred after one or more prior malignancies. Among the 9 SEER registries, osteosarcoma in this age group represented approximately 53% of all reported osteosarcoma cases. Osteosarcoma occurred more in males than females of each race category (overall male:female ratio of 1.34:1; Table 1). However, the incidence was slightly higher in females in each age stratum less than 15 years of age (0–4, 5–9, and 10–14 years). Incidence peaked earlier in females (age 12) than males (age 16) (Supplemental Figure 1), with some disparity by race. Osteosarcoma was more common in Blacks than Whites; however, the greatest incidence occurred in the Other race category (Table 1).
The age-adjusted incidence rates of osteosarcoma overall increased from 1975 until 1995–1999, and then decreased until 2000–04 (APC = 0.73*, 95% CI: 0.12, 1.34, significantly different from zero P < 0.05), although these changes were observed primarily in males (APC = 1.01*, 95% CI: 0.01, 2.02; female APC = 0.30, 95% CI: −0.47, 1.07) (Figure 2). Over the entire study period, 1973–2004, there was some variability in osteosarcoma incidence. Small increases were noted in Whites (3.5 to 4.3) and Blacks (4.4 to 4.8). The Other race category incidence decreased from 5.1 to 4.5. Osteosarcoma incidence increased in males of all race designations over this period and, overall, remained relatively unchanged in females of all races designations (data not shown).
Figure 2.
Age-adjusted incidence of osteosarcoma by age group over 5-year intervals, SEER 9 1975–2004. A. ages 0–24 and 25–59; B. ages 60+.
In 1992, the SEER race classification system was expanded to include Whites, Blacks, American Indian/Alaska Natives, and Asian/Pacific Islanders. From 1992 to 2004, rates tended to be lower in non-Hispanic Whites and indigenous Americans, and highest in Asian/Pacific Islanders (Table 2). Rates were higher in males of each race designation (Table 2).
Table 2.
Osteosarcoma incidence by race and sex, SEER 13 1992–2004.
| Both | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| Age | SEER race designations | Rate | n | Rate | n | Rate | n |
| 0–24 | non-Hispanic White | 4.4 | 394 | 5.1 | 231 | 3.7 | 163 |
| Black | 5.1 | 116 | 6.2 | 70 | 4.0 | 46 | |
| American Indian/Alaska Native* | 3.0 | 7 | 4.1 | 5 | 1.8 | 2 | |
| Asian/Pacific Islander | 5.3 | 97 | 6.2 | 58 | 4.4 | 39 | |
| Hispanic† | 4.9 | 205 | 5.2 | 115 | 4.4 | 90 | |
| 25–59 | non-Hispanic White | 1.8 | 269 | 1.9 | 139 | 1.8 | 130 |
| Black | 3.0 | 79 | 2.6 | 32 | 3.3 | 47 | |
| American Indian/Alaska Native* | 1.9 | 4 | 0.9 | 1 | 2.7 | 3 | |
| Asian/Pacific Islander | 1.5 | 40 | 1.6 | 21 | 1.3 | 19 | |
| Hispanic† | 2.2 | 94 | 2.5 | 54 | 1.9 | 40 | |
| 60+ | non-Hispanic White | 3.7 | 197 | 4.1 | 89 | 3.6 | 108 |
| Black | 4.6 | 27 | 5.4 | 13 | 4.1 | 14 | |
| American Indian/Alaska Native* | 2.9 | 1 | 6.4 | 1 | 0.0 | 0 | |
| Asian/Pacific Islander | 1.9 | 13 | 2.3 | 6 | 1.7 | 7 | |
| Hispanic† | 3.0 | 18 | 3.1 | 8 | 2.8 | 10 | |
| All | non-Hispanic White | 3.0 | 860 | 3.4 | 459 | 2.8 | 401 |
| Black | 4.0 | 222 | 4.4 | 115 | 3.7 | 107 | |
| American Indian/Alaska Native* | 2.4 | 12 | 3.0 | 7 | 2.0 | 5 | |
| Asian/Pacific Islander | 2.9 | 150 | 3.3 | 85 | 2.5 | 65 | |
| Hispanic† | 3.3 | 317 | 3.6 | 177 | 3.0 | 140 | |
restricted to only CHSDA county registries;
excluding the Alaska Native registry; rates in italics are based on less than 10 cases.
The overwhelming majority of early-onset osteosarcomas occurred in the lower long bones (74.5%), whereas 11% occurred in the upper long bones (Table 3). All other sites represented less than 5% of cases. Anatomic site differed little among the osteosarcoma pathology subtypes (data not shown). Osteosarcoma pathology was classified as “NOS” (not otherwise specified) for 75% of cases; otherwise, chondroblastic (11.9%) was the most common, followed by parosteal (4.1%), fibroblastic (4.1%), and telangiectatic (3.1%); the other pathology subtypes made up less than 1% of cases. Osteosarcoma after a previous malignancy occurred most frequently in the lower long bones (39%) and the face or skull (24%). The anatomic site distributions and pathology subtype did not differ significantly among sex, race, or over time (data not shown), although fibroblastic, parosteal, and periosteal osteosarcomas occurred slightly more often in females than males. “NOS” reporting has decreased steadily from 1973 to 2004, most likely reflecting the osteosarcoma ICD-O coding system changes made in 1976, 1990, and 2000.
Table 3.
5 year relative survival (RS) by anatomic site* (AS) and age group, SEER 17 1973–2004.
| Age group | ||||||
|---|---|---|---|---|---|---|
| 0–24 | 25–59 | 60+ | ||||
| Anatomic site | RS | % of AS (n) | RS | % of AS (n) | RS | % of AS (n) |
| Face or skull | 64.9% | 3.2% (60) | 61.0% | 10.0% (97) | 32.5% | 5.3% (31) |
| Mandible | 64.5% | 1.9% (35) | 71.7% | 7.2% (70) | 36.0% | 5.8% (34) |
| Chest region | 51.9% | 1.8% (33) | 45.8% | 3.7% (36) | 19.7% | 4.1% (24) |
| Vertebral column | 58.9% | 1.2% (22) | 28.8% | 3.8% (37) | 5.7% | 4.9% (29) |
| Upper long bones | 56.6% | 11.2% (208) | 53.9% | 9.8% (95) | 7.3% | 7.5% (44) |
| Upper short bones | 75.0% | 0.3% (5) | 100.0% | 0.9% (9) | 78.0% | 0.7% (4) |
| Pelvic region | 25.4% | 3.6% (67) | 30.8% | 10.9% (106) | 4.4% | 18.8% (111) |
| Lower long bones | 64.1% | 74.5% (1381) | 67.1% | 43.4% (421) | 29.8% | 26.7% (158) |
| Lower short bones | 86.8% | 1.1% (20) | 53.4% | 1.3% (13) | 27.6%† | 1.4% (8) |
only the most common bone sites are included;
only the 48mo survival rate was calculated.
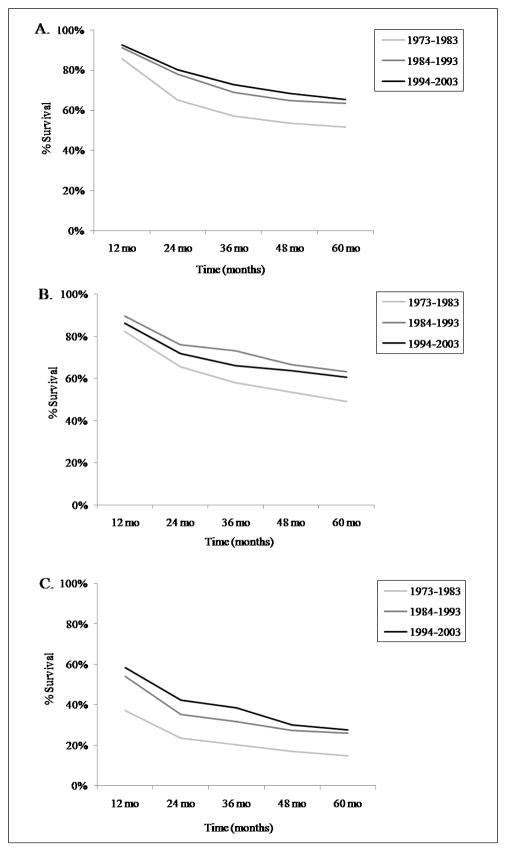
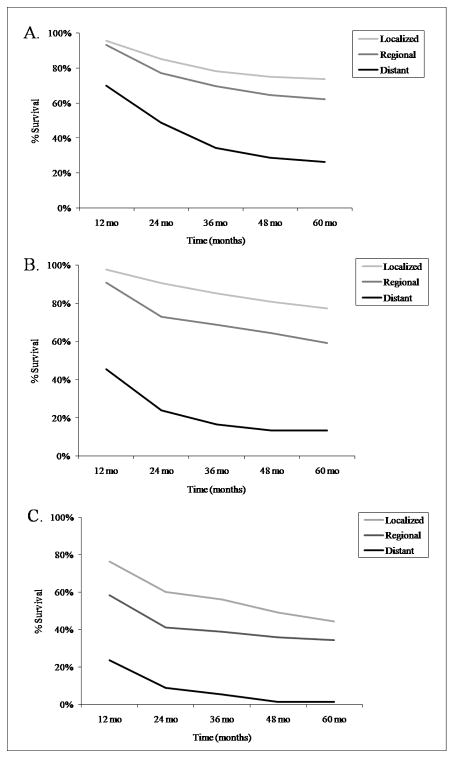
The relative 5-year survival rate of young-onset osteosarcoma was 61.6%. Survival increased significantly between 1973–83 and 1984–93, but there was little change subsequently (Figure 3A). The 5-year osteosarcoma survival rates among the 9 registries varied within ±15% of the mean (Supplemental Table 1). Survival was slightly higher for females (65.8%) than males (58.4%), and for the Other race category (66.0%) compared with Whites (60.8%) and Blacks (61.0%). Based on cases from 1992–2004, the 5-year survival rate was highest in Asian/Pacific Islanders (72.2%), and lower for those of Hispanic origin (58.1%), White Hispanics (57.8%) and American Indian/Alaska Natives (56.0%). Survival varied by anatomic site, with the upper and lower short bones having the highest survival rates, and the pelvic region the poorest (Table 3). The 5-year survival rates by osteosarcoma pathology (including those with >10 reported cases), in frequency order, were: chondroblastic 66.7%, fibroblastic 65.5%, telangiectatic 65.3%, central 61.2% (only 3-yr survival rate available), and small cell 41.6% (survival rates could not be confidently calculated for periosteal or parosteal since they were only added to ICD-O-3 in 2001). We also evaluated survival based on osteosarcoma stage (localized n = 612, regional n = 735, or distant n = 286). Subjects with distant disease had much poorer 5-year survival rates (Figure 4A).
Figure 3.
Five year survival rates of osteosarcoma by age group over 10-year intervals, SEER 17 1973–2003. A. ages 0–24; B. ages 25–59; C. ages 60+.
Figure 4.
Five year survival rates of osteosarcoma by age group and stage, SEER 17 1973–2003. A. ages 0–24; B. ages 25–59; C. ages 60+.
Ages 25–59 years
Individuals ages 25–59 had the lowest incidence rates of osteosarcoma, 1.7 per million (n = 974). There were 4 cases of osteosarcoma with Paget’s disease and 115 cases in which osteosarcoma occurred after one or more prior malignancies. Since this is a broad age group we also looked at rates for 25–39 and 40–59 years; both of these age groups had incidence rates of 1.7 per million. Osteosarcoma in the 25 to 59 age group comprised approximately 28% of all reported cases (data not shown). Overall, osteosarcoma in this age group occurred more in males than females (1.2:1). However, osteosarcoma occurring after one or more prior malignancies was more common in females (0.7:1). Osteosarcoma incidence was highest in Blacks (Table 1). From 1973 to 2004, the age-adjusted incidence rates of osteosarcoma showed minimal variability (APC = 0.27, 95% CI: −0.54, 1.09; Figure 2), with an overall decrease in males (APC = −0.51, 95% CI: −1.60, 0.59) and increase in females (APC = 1.36*, 95% CI: 0.07, 2.67, significantly different from zero P < 0.05). Over the entire study period, the most notable change was an incidence increase in Black females, from 0.97 to 2.9 (data not shown). During 1992 to 2004, incidence rates were highest in Blacks, and lowest in Asian/Pacific Islanders (Table 2).
The most common anatomic site of osteosarcoma in this age group was the lower long bones (43%; Table 3). The anatomic site distributions did not vary significantly by sex or over time (data not shown). There were only small differences among the races; osteosarcoma of the mandible was twice as common in Blacks as it was in Whites or the Other race category. Anatomic site was consistent amongst pathology subtypes. Osteosarcoma after a previous malignancy also occurred most commonly in the lower long bones (25%), but had a broader distribution than for osteosarcomas occurring as the first cancer, including pelvic region (17%), face or skull (14%), upper long bones (10%), and the mandible (10%). Osteosarcoma pathology was classified as NOS for 67% of cases. Otherwise, chondroblastic (10.5%) was the most common osteosarcoma pathology, followed by fibroblastic (9.4%), parosteal (8.7%), telangiectatic (2.2%), and the other pathology subtypes made up less than 1% of all cases. Osteosarcoma pathology did not vary significantly by sex or race (data not shown); the most marked sex difference was a greater occurrence of parosteal osteosarcoma in females.
The relative 5-year survival rate of osteosarcoma in this age group (25–59 years) was 58.7%. Osteosarcoma survival was consistent among the 9 SEER registries (Supplemental Table 1). As described in the youngest age group, there was a large improvement in survival between 1973–83 and 1984–93 (Figure 3B). 5-year survival was higher for females (64%) than males (54.6%), highest in the Other race category (66.9%) and lowest in Blacks (47.6%; Whites survival rate: 59.9%). The greater survival in females was most notable in Blacks (female rate of 56% and male rate of 36.8%). Based on cases from 1992–2004, the 5-year survival rate was 69.6% in Asian/Pacific Islanders, and 63.6% for those of Hispanic origin. Survival varied by anatomic site, with the upper short bones having the highest percent survival, and the vertebral column the lowest (Table 3). The relative 5-year survival rates for osteosarcoma pathology (including those with >10 cases), in frequency order, were: chondroblastic 54%, fibroblastic 73%, and telangiectatic 59%. As expected, osteosarcoma stage significantly affected survival (localized n = 319, regional n = 341, distant n = 118; Figure 4B). Distant disease had much lower 5-year survival rates than localized or regional disease.
Ages 60–85+ years
The incidence of osteosarcoma in this elderly age group was 4.2 per million (n = 653). There were 62 cases osteosarcoma with Paget’s disease and 159 cases of osteosarcoma as a second or greater cancer. Osteosarcoma in this age group represented approximately 19% of all reported osteosarcoma cases (data not shown). Incidence fluctuated among single ages in this elderly group. The highest peak in osteosarcoma incidence occurred in males age 79, and in females ages 77, and the highest peak in osteosarcoma with Paget’s disease was in males age 75. Osteosarcoma was more common in Whites than Blacks or the Other race category (Table 1). In this age group, osteosarcoma occurred less commonly in males than females (0.89:1), particularly when osteosarcoma was the second or greater malignancy (0.66:1) (based on 17 SEER registries). Osteosarcoma with Paget’s disease occurred more in males (1.58:1). However, the overall age-adjusted incidence rate of osteosarcoma was greater in males, and the male-to- female incidences for each osteosarcoma subtype varied somewhat by race (Table 1; based on 9 SEER registries).
Overall, from 1973 to 2004, the age-adjusted incidence rates of all osteosarcoma cases decreased in both males and females (overall APC = −1.39*, 95% CI: −2.28, −0.49, significantly different from zero P < 0.05; Figure 2). Osteosarcoma with Paget’s disease decreased from 1975–2004, particularly in males, and osteosarcomas after a previous malignancy increased (Figure 2). In Blacks and the Other race category, there was an overall incidence increase after some fluctuation (Blacks: 2.6 to 4.2; Other: 2.1 to 3.3) in both males and females. In Whites, the incidence decreased in both males and females (overall, 4.6 to 3.4).
During 1992 to 2004, osteosarcoma incidence rates were highest in Blacks and lowest in Asian/Pacific Islanders (Table 2). Osteosarcoma incidence after a previous cancer was 1.3 for both non-Hispanic Whites and Blacks, followed by 0.8 in persons of Hispanic origin, 0.4 in Asian/Pacific Islanders, and absent in American Indian/Alaska Natives.
The anatomic site distributions of osteosarcoma in this elderly age group were variable. The majority (27%) of osteosarcomas occurred in the lower long bones, and the next most common site was the pelvic region (19%; Table 3). There were more unspecified bone sites (6%, compared to <1% in those age 0–24 and 1% in those age 25–59) and non-bone sites (18%, compared to <1% in 0–24 and 7% in 25–59) in this elderly group compared to the younger ages. In Blacks and the Other race category, the second most common anatomic site was the mandible and in Whites, it was the pelvic region. For osteosarcoma with Paget’s disease the anatomic sites were more limited, with no reported cases occurring in the short bones, mandible, or chest region (data not shown). The most common site of osteosarcomas after a previous malignancy was the pelvic region (25%), followed by the lower long bones (14.5%; data not shown). There was more anatomic site disparity by sex in this group (data not shown). Females had a higher occurrence of osteosarcoma after a previous cancer in the chest region and upper long bones while in males it was more common in the vertebral column, pelvic region, and mandible. The anatomic site distribution did not differ much among most osteosarcoma pathology subtypes; except in telangiectatic osteosarcoma where the upper long bones were the most prominent site (although based on <10 cases).
Osteosarcoma pathology was classified as NOS for 87% of cases; otherwise, chondroblastic (6.3%) was the most common pathology, followed by fibroblastic (3.6%), telangiectatic (1.2%), and parosteal (1.4%), the other pathology subtypes made up less than 1% of all cases. Fibroblastic osteosarcoma was more common in females than males (n = 17, 6.7% of cases vs. n = 4, 1.4% of cases).
The relative 5-year survival rate of osteosarcoma in this age group was 24.2%. The survival rate dropped drastically from approximately 50% for patients in their 50s to 17% for those in their mid-late 60s, and was the lowest for those age 80–84 (10.8%). Again, a marked improvement in survival between 1973–83 and 1984–93 was noted with little increase after that (Figure 3C). The 5-year osteosarcoma survival rates varied little among SEER registry sites (Supplemental Table 1). Osteosarcoma survival was higher for females (27.0%) than males (19.9%) and for Blacks (27.1%) compared to the Other race category (25.7%) and Whites (23.6%). The 5-year survival rate of osteosarcoma with Paget’s disease was 18.9% (17.6% for males and 19.3% for females). Based on cases from 1992–2004, the 5-year survival rate for Asian/Pacific Islanders was the highest (60.8%), and was 26.1% for those of Hispanic origin. Survival varied by anatomic site, with the upper short bones having the greatest percent survival and the pelvic region the lowest (Table 3). The 5-year survival rates for osteosarcoma pathology (including those with >10 cases) were: chondroblastic 55.3%, and fibroblastic 42.6%. Osteosarcoma stage significantly influenced survival (localized n = 103, regional n = 131, distant n = 128; Figure 4C) with distant disease resulting in lower 5-year survival rates.
Discussion
This study presents detailed incidence and survival data by age, sex, race, and pathology. Prior SEER studies consisted of reports on either all types of bone cancer combined5 or were limited to those occurring in childhood.21, 22, 28 Our study confirms the commonly-observed bimodal age distribution of osteosarcoma incidence.5, 8, 29, 30, 32 Although a recent National Cancer Database report suggested there was no bimodal age distribution, that report was based on absolute case numbers, not population-based incidence rates.4 Our use of a large, population-based data set largely avoided the possibility of selection bias, which limits the interpretation of data from hospital-based studies.
The present study shows that females of each age stratum less than 15 years had slightly higher incidence rates than males, consistent with many earlier reports,10, 16–18, 22, 29 and in contrast to a single report suggesting that females only have higher rates in the 10–14 age stratum.21 We also observed that the first incidence peak occurs earlier in females than in males. This correlates with an earlier onset of puberty and the adolescent growth spurt. These findings suggest that bone growth and/or hormonal changes during puberty may contribute to osteosarcoma pathogenesis.18, 41
In reports on osteosarcoma that combine patients of all ages, it is likely (but unspecified) that osteosarcoma with Paget’s and osteosarcoma occurring after a previous malignancy are included in the incidence and survival rate estimates. Here, we show distinct trends for these subtypes in the elderly. The age-, gender-, site-, and race-specific patterns we observed for osteosarcoma with Paget’s and osteosarcoma occurring after a previous malignancy were similar to those described in the few other reports focused on these neoplasms.42–45 Several reports have indicated that the majority of osteosarcoma in elderly patients occur with Paget’s disease or consider it a secondary lesion,10–13 while other reports suggest that primary osteosarcoma (occurring as the first cancer) occurs more commonly in older patients.43, 46–49 The interpretation of osteosarcoma incidence after a previous malignancy is challenging, since osteosarcoma is a common treatment-related cancer. We were not able to link the osteosarcoma cases occurring as a second or greater cancer to a specific primary cancer type.
We were able to assess 653 elderly patients with osteosarcoma, to our knowledge the largest series yet reported, and found that osteosarcoma with Paget’s and osteosarcoma occurring after a previous malignancy represent only approximately 34% of cases, as compared with the 56% observed by Huvos.13 Two recent studies in Japan46 and Europe43 also found that the majority of elderly osteosarcoma patients had primary osteosarcoma, and suggest that misdiagnosis may cause an overestimation of osteosarcoma as a secondary lesion.46 From 1973 to 2004, the most significant overall change in osteosarcoma incidence in our study was the decline observed in elderly patients, possibly due to changes in reporting Paget’s and secondary osteosarcoma in this age group.
The epidemiologic patterns of osteosarcoma were different among the three age groups. The age groups chosen are based on the overall osteosarcoma incidence peaks and plateau. Osteosarcoma incidence varied uniquely by race and sex among the age groups. Many earlier studies that relied on data for Whites and Blacks only5, 15, 21, 22, 26, 30 suggested that incidence was greatest in the latter, and our data for all ages combined agrees with this conclusion. However, our comparison among the age groups with additional racial designations, as defined by SEER, found that the incidence is actually greatest in the Other race designation among patients < 25 years, specifically in Asian/Pacific Islanders, in agreement with a recent childhood cancer incidence study,33 while in elderly patients incidence was greatest among Whites. In all age groups, osteosarcoma incidence was higher in males, except in middle age and elderly Black patients where the incidence was slightly higher in females. There was a much higher incidence of distant disease in elderly patients compared with the younger age groups, perhaps in part due to delayed diagnosis or age-specific differences in tumor biology.
The anatomic site distributions were more diverse for the 25–59 and 60–85+ age groups compared with the 0–24 group; however, the lower long bones were always the most common site. Among the elderly, there were considerable differences in anatomic location between males and females for osteosarcoma occurring after a previous malignancy. Although we were not able to determine the previous cancer diagnoses for those subjects, it is theoretically possible that the anatomic site differences could be related to differences in the location of their primary cancer and the resultant radiation field; for example, therapeutic radiation for breast cancer could contribute to the observed osteosarcoma increase in the chest region of elderly females.
Differences in incidence and survival were also noted between pathology subtypes, although these analyses are somewhat limited because the majority of osteosarcomas are reported as histology Not Otherwise Specified. In the future, as NOS reporting continues to decrease, we may achieve more insight into the characteristics of pathologic subtypes. We were able to show that parosteal osteosarcoma was more common in females age 0–24 and 25–59, corroborating the National Cancer Database report (all ages combined).4 In addition, we demonstrated that chrondroblastic and parosteal osteosarcoma were more commonly reported in the adolescent and middle age groups, and parosteal and fibroblastic osteosarcoma were significantly more frequent in the middle age group.
Survival after osteosarcoma diagnosis improved significantly for all three age groups between 1973–83 and 1984–93. Our analyses also showed that survival varies by gender, age, anatomic site, pathology sub-type, and disease stage. Survival rates were highest in females and in the youngest age group, and lowest in elderly patients. The greatest indicators of survival in all age groups were anatomic site and disease stage. In all age groups, the highest survival rate was observed with osteosarcoma of the short bones and localized disease, and the poorest with osteosarcoma of the pelvic region and/or vertebral column and distant disease. Survival in patients with tumors in the upper long bones was particularly poor in elderly patients, but not in the other age groups. Osteosarcoma pathology also affected survival, although it is difficult to evaluate the accuracy of these measures, as many of the pathology subtypes were underrepresented (<10 cases) in each age group.
Prior to the 1980s, the osteosarcoma survival rate in the U.S. was approximately 20%.50, 51 Five-year osteosarcoma survival rates for children and adolescents in Europe also show marked improvement up to the 1980s and little improvement thereafter.24, 53 Clinical trials including chemotherapy, given both before and after definitive surgical resection, began in the early 1980s. These studies resulted in rapid improvement in 5-year survival, to approximately 70%. However, improvements in osteosarcoma survival during the last decade have been limited; clearly, new treatment strategies are needed (recent treatment developments reviewed in Ferrari and Palmerini52).
We have confirmed that osteosarcoma incidence is bimodal, more common in males, occurs most frequently in the lower long bones, and that survival rates have leveled off since the mid-1980s. We have also, for the first time, demonstrated the impact of osteosarcoma associated with Paget’s disease or occurring as a second malignancy on the incidence and survival of “pure” osteosarcoma. However, among the three age groups there are many unique epidemiologic features, illustrating that these age groups should be studied separately in order to more completely understand osteosarcoma epidemiology and underlying biology.
Supplementary Material
Acknowledgments
We would like to thank Dr. Susan Devesa and Ms. Carol Kosary, of the National Cancer Institute for helpful advice and discussions. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
References
- 1.Sweetnam R. Osteosarcoma. Br J Hosp Med. 1982;28(2):112, 16–21. [PubMed] [Google Scholar]
- 2.Mascarenhas L, Siegel S, Spector L, Arndt C, Femino D, Malogolowkin M. Malignant bone tumors: cancer in 15- to 29-year-olds in the United States. In: Bleyer A, OLM Barr R, Ries LAG, editors. Cancer Epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Cancer Institute; 2006. pp. 98–109. NIH Pub. No. 06-5767. [Google Scholar]
- 3.Dahlin DC, Unni KK. Bone Tumors: general aspects and data on 8,542 cases. 4. Springfield, IL: Thomas; 1986. [Google Scholar]
- 4.Damron TA, Ward WG, Stewart A. Osteosarcoma, Chrondrosarcoma, and Ewing’s Sarcoma. National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman HA, Czerniak B. Bone Cancers. Cancer supplement. 1995;75(1):203–10. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Kramárová E, Stiller CA. The international classification of childhood cancer. Int J Cancer. 1996;68:759–65. doi: 10.1002/(SICI)1097-0215(19961211)68:6<759::AID-IJC12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CDM, Unni KK. Pathology and genetics of soft tissue and bone. Lyon, France: IARC Press; 2002. World Health Organization classification of tumours. [Google Scholar]
- 8.Unni KK. Dahlin’s bone tumors: general aspects and data on 11,087 cases. 5. Philadelphia: Lippincott-Raven; 1996. pp. 143–83. [Google Scholar]
- 9.Dorfman HA, Czerniak B. Bone Tumors. St. Louis: Mosby; 1997. [Google Scholar]
- 10.Price CHG. Osteogenic sarcoma: an analysis of the age and sex incidence. Br J Cancer. 1955;9:558–74. doi: 10.1038/bjc.1955.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabanas AO, Dahin DC, Childs DS, Ivings JC. Postirradiation sarcoma of bone. Cancer. 1956;9:528–42. doi: 10.1002/1097-0142(195605/06)9:3<528::aid-cncr2820090316>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Murray RO. Tumor and tumorlike lesions of bone. In: Sutton DGR, editor. A textbook of radiology. 2. New York: Churchill Livingstone; 1975. pp. 107–37. [Google Scholar]
- 13.Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;57:1442–9. doi: 10.1002/1097-0142(19860401)57:7<1442::aid-cncr2820570734>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JB, Hoang BH. Osteosarcoma: Basic Science and Clinical Implications. Orthopedic Clinics of North America. 2006;37(1):1–7. doi: 10.1016/j.ocl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Gurney JG, Swensen AR, Bulterys M. Malignant bone tumors. In: Reis LAGSM, Gurney JG, et al., editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program, 1975–1995. Bethesda, MD: [Google Scholar]
- 16.Polednak AP. Human biology and epidemiology of childhood bone cancers: a review. Hum Biol. 1985;57:l–26. [PubMed] [Google Scholar]
- 17.Hanson MR, Mulvihill JJ. Epidemiology of cancer in the young. New York: Masson; 1982. [Google Scholar]
- 18.Glass AG, Fraumeni JF. Epidemiology of bone cancer in children. J Natl Cancer Inst. 1970;44:187–99. [PubMed] [Google Scholar]
- 19.Kramer S, Meadows AT, Jarret P, Evans AE. Incidence of childhood cancer: Experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 20.Young JL, Jr, Ries LG, Silverberg E, Horn JW, Miller RW. Cancer incidence, survival, and mortality for children younger than age 15 years. Cancer. 1986;58:598–602. doi: 10.1002/1097-0142(19860715)58:2+<598::aid-cncr2820581332>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Homa DM, Sowers MR, Schwartz AG. Incidence and survival rates of children and young adults with osteogenic sarcoma. Cancer. 1991;67:2219–24. doi: 10.1002/1097-0142(19910415)67:8<2219::aid-cncr2820670837>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Cancer. 1995;75(8):2186–95. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Gatta G, Capocaccia R, Coleman MP, Ries LAG, Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95(8):1767–72. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- 24.Foster L, Dall GF, Reid R, Wallace WH, Porter DE. Twentieth-century survival from osteosaroma in children: Trends from 1933 to 2004. J Bone Joint Surg. 2007;89-B(9):1234–38. doi: 10.1302/0301-620X.89B9.19255. [DOI] [PubMed] [Google Scholar]
- 25.Herzog CE. Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol. 2005;27(4):215–18. doi: 10.1097/01.mph.0000161762.53175.e4. [DOI] [PubMed] [Google Scholar]
- 26.Parkin DM, Stiller CA, Draper GJ, Bieber CA. The international incidence of childhood cancer. Int J Cancer. 1988;42:511–20. doi: 10.1002/ijc.2910420408. [DOI] [PubMed] [Google Scholar]
- 27.Ajiki W, Hanai A, Tsukuma H, Hiyama T, Fujimoto I. Survival rates of childhood cancer patients in Osaka, Japan, 1975–1984. Jpn J Cancer Res. 1995;86(1):13–20. doi: 10.1111/j.1349-7006.1995.tb02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novakovic B. U.S. childhood cancer survival, 1973–1987. Medical and Pediatric Oncology. 1994;23:480–86. doi: 10.1002/mpo.2950230606. [DOI] [PubMed] [Google Scholar]
- 29.Larsson SE, Lorentzon R. The incidence of malignant primary bone tumors in relation to age, sex, and site: a study of osteogenic sarcoma, chondrosarcoma, and Ewing’s sarcoma diagnosed in Sweden from 1958 to 1968. J Bone Joint Surg. 1974;56B:534–40. [PubMed] [Google Scholar]
- 30.Polednak AP. Primary bone cancer incidence in black and white residents of New York state. Cancer. 1985;55:2883–88. doi: 10.1002/1097-0142(19850615)55:12<2883::aid-cncr2820551231>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Blackwell JB, Threlfall TJ, McCaul KA. Primary malignant bone tumours in Western Australia, 1972–1996. Pathology. 2005;37(4):278–83. doi: 10.1080/00313020500168737. [DOI] [PubMed] [Google Scholar]
- 32.Ji J, Hemminki K. Familial risk for histology-specific bone cancers: An updated study in Sweden. Eur J Cancer. 2006;42:2343–49. doi: 10.1016/j.ejca.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2007;112(2):416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 34.Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. SEER Registries: Surveillance Research Program, NCI. 2007 Dec; http://seer.cancer.gov/registries/
- 35.Surveillance Research Program, National Cancer Institute SEER*Stat software ed. version 6.3.6. Sept, 2007. [Google Scholar]
- 36.Kramárová E, Stiller CA. The international classification of childhood cancer. Int J Cancer. 1996;68:759–65. doi: 10.1002/(SICI)1097-0215(19961211)68:6<759::AID-IJC12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. International classification of diseases for oncology: Morphology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 38.Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence - SEER 13 Regs Limited-Use, Nov 2006 Sub (1992–2004) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch., released April 2007, based on the November 2006 submission.
- 39.Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973–2004) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch released April 2007 based on November 2006 submission.
- 40.Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973–2004 varying) -Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch released April 2007, based on the November 2006 submission. Available from www.seer.cancer.gov.
- 41.Miller RW. Contrasting epidemiology of childhood osteosarcoma, Ewing’s sarcoma, and rhabdomyosarcoma. Nat Cancer Inst Monogr. 1981;56:9–15. [PubMed] [Google Scholar]
- 42.Hamre MR, Severson RK, Chuba P, Lucas DR, Thomas RL, Mott MP. Osteosarcoma as a second malignant neoplasm. Radiotherapy and Oncology. 2002;65:153–57. doi: 10.1016/s0167-8140(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 43.Grimer RJ, Cannon SR, Taminiau AM, Bielack S, Kempf-Bielack B, Windhager R, Dominkus M, Saeter G, Bauer H, Meller I, Szendroi M, Folleras G, San-Julian M, van der Eijken J. Osteosarcoma over the age of forty. Eur J Cancer. 2003;39:157–63. doi: 10.1016/s0959-8049(02)00478-1. [DOI] [PubMed] [Google Scholar]
- 44.Healey JH, Buss D. Radiation and pagetic osteogenic sarcomas. Clin Orthop Relat Res. 1991;270:128–34. [PubMed] [Google Scholar]
- 45.Hanson MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. Journal of bone and mineral research. 2006;21(S2):P58–P63. doi: 10.1359/jbmr.06s211. [DOI] [PubMed] [Google Scholar]
- 46.Okada K, Hasegawa T, Nishida J, Ogose A, Tajino T, Osanai T, Yanagisawa M, Hatori M. Osteosarcomas after the age of 50: a clinicopathologic study of 64 cases--an experience in northern Japan. Ann Surg Oncol. 2004;11(11):998–1004. doi: 10.1245/ASO.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 47.deSantos LA, Rosengren JE, Wooten WB, Murray JA. Osteogenic sarcoma after the age of 50: a radiographic evaluation. AJR Am J Roentgenol. 1978;131(3):481–4. doi: 10.2214/ajr.131.3.481. [DOI] [PubMed] [Google Scholar]
- 48.Weinfield MS, Dudley HR. Osteogenic sarcoma: a follow-up study of the ninety-four cases observed at the Massachusetts General Hospital from 1920–1960. J Bone Joint Surg. 1962;44:269–76. [PubMed] [Google Scholar]
- 49.Aegerter E, Kirkpatrick JA. Orthopedic disease. 4. Philadelphia: Saunders; 1975. [Google Scholar]
- 50.Dahlin DC, Coventry MB. Osteogenic sarcoma: a study of six hundred cases. J Bone Joint Surg. 1967;49-A:101–10. [PubMed] [Google Scholar]
- 51.Taylor WF, Ivins JC, Pritchard DJ, Dahlin DC, Gilchrist GS, Edmonson JH. Trends and variability in survival among patients with osteosarcoma: a 7-year update. Mayo Clin Proc. 1985;60:91–104. doi: 10.1016/s0025-6196(12)60293-6. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Current Opinion in Oncology. 2007;19:341–46. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- 53.Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M the EUROCARE Working Group. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol. 2005;23(16):3742–51. doi: 10.1200/JCO.2005.00.554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.