Abstract
Background
The study was conducted to determine whether a phosphodiesterase (PDE) 3 inhibitor has potential as a novel contraceptive in primates.
Methods
Regularly cycling adult female cynomolgus macaques of proven fertility (n=16) were treated for 7 months with placebo (controls) or the PDE 3 inhibitor ORG 9935 as a daily food treat (150 mg/kg), or as a weekly depot subcutaneous injection (150 mg/kg). After one month, a male of proven fertility was introduced into each group. Females underwent weekly monitoring of progesterone (P) and ultrasound evaluation for pregnancy if P remained elevated (1.0 ng/mL) > 3 weeks. ORG 9935 values were evaluated using high-performance liquid chromatography.
Results
Overall, the pregnancy rate in ORG 9935-treated monkeys (4/8, 50%) did not differ from controls (7/8, 88%, p = 0.5). However, no animal became pregnant in a cycle when the serum level of ORG 9935 exceeded 300 nmol/L. Moreover, 2 treated monkeys who mated throughout the treatment phase and did not conceive became pregnant within 4 cycles after stopping ORG 9935. The other 2 animals were discontinued prematurely from the protocol.
Conclusions
These results demonstrate that ORG 9935 may prevent pregnancy in primates at serum concentrations above 300 nmol/L, and that the effect is reversible.
Keywords: oocyte, meiosis, contraception, phosphodiesterase inhibitor, macaque
1. Introduction
Nonuse of contraception contributes to an unacceptably high rate of unintended pregnancy, and according to data from the 2002 National Survey of Family Growth, 7.4% of sexually active couples in the United States use no method of contraception, an increase of 2.2% from the last survey of 1995 [1]. Unintended pregnancy also results from inconsistent or incorrect use or failure of existing contraceptive method with approximately half of all unintended pregnancy occurring as a result of contraceptive failure [2]. While highly effective when used correctly, real or perceived side effects of hormonal methods limit their acceptability and likely contribute to contraceptive failure [3]. Therefore, to increase the acceptability of contraception and reduce the number of unintended pregnancies, unwanted births, and abortions, new non-hormonal contraceptive strategies are needed.
In female contraception, the oocyte looms large as an obvious target. In women and other mammals, the preovulatory surge in gonadotropins triggers reinitiation of oocyte meiotic maturation, such that a fertilizable metaphase II-stage oocyte is available at the time of ovulation [4]. An essential event occurring in the signal transduction pathway leading to the resumption of meiosis is a decrease in intracellular cyclic adenosine monophosphate (cAMP), and the enzyme responsible for the decline in cAMP is phosphodiesterase (PDE) 3A [5, 6]. Divergence of PDE isoform expression exists in the primate ovary with PDE3 (oocyte) and PDE4 (somatic cells) the primary isoforms expressed within the follicle [4, 5, 7]. Therefore, selective treatment with a PDE3 inhibitor should result in ovulation of a non-fertilizable, immature oocyte without affecting the development or rupture of the follicle, subsequent development of a functional corpus luteum, or normal menstrual cyclicity.
The effect on oocyte maturation and contraceptive potential of PDE3 inhibitors was initially demonstrated in the rodent [8]. Subsequent studies have confirmed that the PDE3 inhibitor ORG 9935, a carboximidamide derivative, selectively blocks the spontaneous resumption of meiosis that occurs in vitro in rhesus macaque [9] and in human [10] oocytes. ORG 9935 has also been shown to inhibit oocyte maturation without affecting ovulation or the development of the corpus luteum of multiple follicles when given to macaques during controlled ovarian stimulation (COS) gonadotropin cycles [11] and in the naturally-selected dominant follicle during controlled ovulation (COv) of the naturally-selected dominant follicle [12].
To determine whether a PDE3 inhibitor has potential use as a human contraceptive agent, we designed an experiment to test the hypothesis that chronic administration of the PDE3 inhibitor ORG 9935 to regularly cycling fertile cynomolgus macaques co-housed with a male in a breeding group will prevent pregnancy.
2. Materials
2.1. Establishment of breeding groups
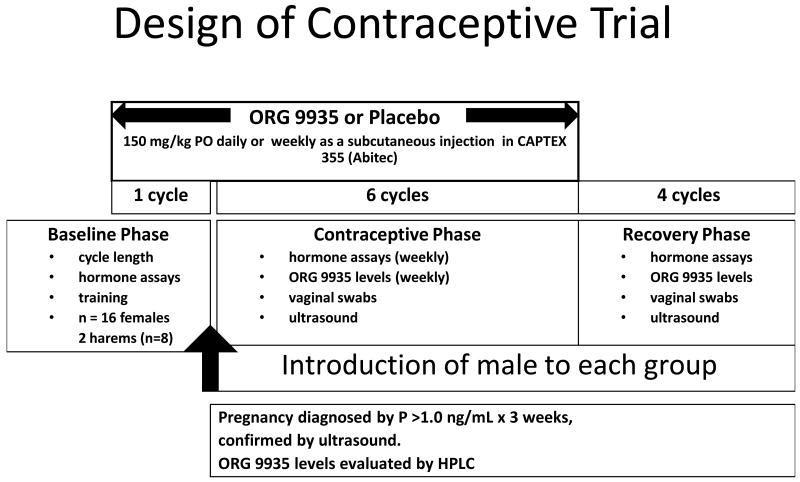
The general care and feeding of macaques at the Oregon National Primate Research Center (ONPRC) has been described previously [13]. The ONPRC Institutional Animal Care and Use Committee approved all study protocols and experiments prior to initiation. Adult female cynomolgus macaques of proven fertility (n=16) were divided into two equal groups and co-housed in identically sized adjacent spaces of approximately 168 square feet (12′ wide × 14′ deep) in a climate-controlled indoor environment. Females were socialized to the group, and trained to enter a tunnel where they could be individually identified and examined daily for evidence of menses and mating. Females received a food treat after examination, and were returned to the group housing area. During this time period, menstrual records and hormone levels were obtained to confirm normal cycles. The overall design of the experiment is illustrated in Fig. 1.
Fig. 1.
Design of the contraceptive study.
2.2. Verification of ovulatory cycles and mating
On a daily basis, females underwent examination that included vaginal insertion of a cotton-tipped swab if visible menstrual bleeding was not grossly evident. Later in the study, in animals where cyclicity was well established, swabs were performed only if expected menses was not seen and during midcycle to confirm mating. Animals underwent venipuncture and a brief physical exam after sedation with intramuscular ketamine (1 mg/kg) on a weekly basis. Serum concentrations of estradiol and progesterone (P) were determined by specific electrochemoluminescent assays using a DPC Immulite 2000 (Siemens Healthcare Diagnostics, Deerfield, Ill) clinical assay platform by the Endocrine Technology Core Laboratory, ONPRC [14].
Mating was confirmed by microscopic examination of a smear of the vaginal swab on a glass slide at 100×. The presence of sperm on at least one smear during a cycle was considered as evidence of mating. If this occurred in a cycle where serum P was > 1.0 ng/mL on a least one occasion, the cycle was considered a cycle at risk for pregnancy. The normal P level in the follicular phase of the macaque cycle is < 0.2 ng/mL. Values above 0.2 ng/mL are considered detectable by the Immulite assay, and typically occur after the midcycle gonadotrophin surge. While levels vary widely in the luteal phase, a single value above 1.0 ng/mL is presumptive evidence of ovulation, with normal luteal phase values between 1-6 ng/mL[15].
2.3 Treatment with the PDE3 inhibitor ORG 9935
Female monkeys either received no further treatment (controls, n=8), or the PDE3 inhibitor ORG 9935 (n=8). To reduce the possibility of error, all animals in one harem received the same treatment (either ORG 9935 or placebo). ORG 9935-treated animals received the compound as a daily oral food treat (150 mg/kg) or as a subcutaneous injection (150 mg/kg) in Captex oil (Abitec, Columbus, OH) once per week at the same time as the weekly blood draw. Animals were switched from oral to subcutaneous dosing if they stopped voluntarily accepting the food treats. By the fourth month of the contraceptive trial, all of the ORG 9935-treated animals were receiving injections of the drug. This dose regimen had been established as effective in prior studies conducted in rhesus macaques [9, 11, 12], and other preliminary studies conducted in our lab using cynomolgus monkeys (unpublished data). Since prior studies had demonstrated a delay between dosing and optimal effect in inhibition of oocyte maturation [11], the introduction of male monkeys was delayed until females had completed at least 30 days on treatment.
The planned treatment period for all females was six ovulatory cycles with confirmed mating.
2.4. Introduction of males
During the period of acclimation and training of the female monkeys, two healthy adult male cynomolgus monkeys of proven fertility were housed in adjacent cages where they could maintain olfactory and visual contact with their respective harems. A mesh insert prevented mating. Once dosing had been established in the female harems, a single male was introduced into each group.
2.5. Verification of pregnancy
If P levels exceeded 1.0 ng/mL for three consecutive weeks, animals were sedated with ketamine (1 mg/kg) and examined with ultrasound using a GE Healthcare (Seattle, WA) Model SP10-16 ultrasound equipped with a 4.5 mHz linear array abdominal transducer. An intrauterine pregnancy was diagnosed on the basis of an intrauterine gestational sac, or a gestational sac with a yolk sac or fetus. If the ultrasound appearance was ambiguous, or if P remained elevated without evidence of an intrauterine pregnancy, serum samples were sent to the California National Primate Research Center for detection of monkey chorionic gonadotropin using a validated assay [Tarantal, 1997]. All pregnancies were terminated.
2.6. Reversibility
Both control and ORG 9935-treated animals that did not become pregnant during the treatment phase were followed for up to 4 additional cycles to determine if pregnancy would occur after the treatment was stopped. The ORG 9935-treated animals continued to be co-housed with the same male used during the treatment phase. The two control females that did not become pregnant during the treatment phase were housed with another male of proven fertility during the reversibility period of the study.
2.7 ORG 9935 levels
An assay for ORG 9935 was developed by Dr. Dennis Koop, Director of the Bioanalytical Shared Resource/Pharmacokinetics Core Facility at OHSU. Monkey serum samples were stored at -20°C in aliquots and analyzed in batches at the conclusion of the study. Naïve serum was provided for the generation of spiked standards.
ORG 9935 levels were determined by high pressure liquid chromatography (HPLC) using structurally related quazinone as the internal standard. Serum samples were thawed and a 100-μL aliquot was removed. The internal standard, quazinone (100 ng in 5 μL methanol), was added to each sample and proteins were precipitated by the addition of 400 μL of acetonitrile at room temperature. The samples were mixed and the protein removed by centrifugation at 12,000 xg for 10 min. The supernatant was removed and placed in an autosampler vial for analysis. For each set of samples, a standard curve was prepared in naïve serum at the same time using 100, 200, 500, 1000 and 2000 ng/mL ORG 9935.
Samples were analyzed using an Agilent 1100 HPLC system (Aligent Technologies, Santa Clara, CA) with a diode array detector. The compounds were resolved from other plasma components using a 4.6 × 150 mm, 5 μm Hypersil Gold HPLC column with a 5 μm 4.6 × 12.5 mm Hypersil Gold guard column (ThermoFisher, Waltham, MA) maintained at 35°C. The HPLC mobile phase consisted of water with 0.1% ammonium hydroxide (solvent A) and acetonitrile with 0.1% ammonium hydroxide (solvent B) delivered at a flow rate of 1.0 mL/min. The column was equilibrated with 20% solvent B and then increased to 90% solvent B in 20.0 min, held for 2 min and returned to 20% solvent B and equilibrated for 8 min. The injection volume was 25 μL. ORG 9935 and quazinone eluted at 8.2 and 4.9 min, respectively. ORG 9935 was monitored at 330 nm and quazinone at 250 nm. Linear least-square regression of the plasma concentrations and measured peak area ratios was used for the quantification. Data acquisition and quantitative processing were accomplished with ChemStation (Agilent Technologies Inc, Wilmington, DE) software.
The assay was sensitive to detect ORG 9935 to a level of 100 nmol/L. Laboratory staff that performed and analyzed the ORG 9935 results were blinded to the outcomes of the contraceptive experiment.
2.8 Telemetry
To investigate potential cardiovascular effects of PDE3 inhibitors in primates, three additional female rhesus macaques (not involved in the contraceptive study) were fitted with small (37 gm, 25 mm3), circular, data transmission implants (TA11PA-D70, Data Sciences International, St Paul, MN). These devices allow for continuous data collection with remote data sampling in unrestrained animals without artifacts induced by handling or the presence of observers. The transmitter were placed subcutaneously through a small incision on the back, with a small catheter tunneled subcutaneously from the implant and inserted into the femoral artery, and two wire leads tunneled to the anterior chest wall to continuously monitor physiologic parameters such as blood pressure, temperature, heart rate, and activity. A receiver placed in the cage transmitted this information to a dedicated computer outside of the animal area that received and recorded these data.
Heart rate and blood pressure measurements were obtained after initiation of short-term (up to 3 days) treatment with ORG 9935 at several doses (200 mg/kg once/d, 200 mg/kg BID, 400 mg/kg once/d, 50 mg/kg q 6h) and compared with the baseline averages.
2.9. Outcomes and statistical analysis
Power analysis for a binomial distribution for Fisher's Exact test was performed to determine the minimum number of females necessary for the experiment (http://www.stat.ucla.edu/calculators/). For a primary outcome of pregnancy rate, we used a one-tailed test as previous work in rodents demonstrated a reduction in pregnancy rates and did not suggest a fertility enhancing effect. With a probability of success (pregnancy) in control animals of 90% (based upon previous experience at ONPRC) [16], an α of 0.05, and β of 0.2 (80% power), and assuming that at least a 70% reduction in pregnancy (probability of pregnancy in treated animals = 20%) must be achieved for the method to be practical, a total of 8 female animals was required in each treatment group. In practical terms, observing a 70% reduction in pregnancy would require all eight control animals (100%) to become pregnant and only 2/8 (25%) pregnancies in the treated group, or 7 (88%) pregnancies in controls and only 1 (13%) among treated animals. A 50% reduction in pregnancy (e.g. from 90% to 50%) would provide an initial validation of the approach, and be achieved with 7/8 (88%) and 3/8 (38%) pregnancies in control and treated animals respectively.
The telemetry data were recorded as continuous variables. Since recordings were made every 1 min, a large number of data points were available from each animal. The mean values of pre- and post-treatment were compared using ANOVA.
3. Results
Of the 16 females that began the contraceptive study, 8 control and 6 ORG 9935-treated animals experienced six ovulatory cycles during the treatment phase.
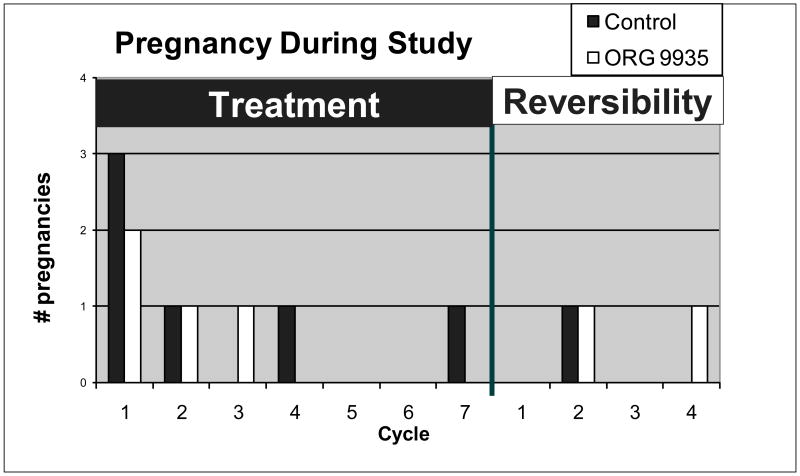
In the control group, 7/8 (88%) of the females became pregnant during the course of the study (Fig. 2). Six of these pregnancies occurred during the planned 6-month treatment phase. One control female was continued for seven cycles during the treatment phase because vaginal swabs did not confirm mating during three of the first six cycles. Therefore, the pregnancy occurred during the fourth cycle of mating. One control animal was never mated during 6 ovulatory cycles, but become pregnant when she was co-housed with another male during the first cycle that mating was confirmed. The other female had evidence of ovulation and documented mating in eight cycles and failed to conceive. She also failed to conceive after exposure to a different male for an additional three cycles and was presumed infertile.
Fig. 2.
Pregnancies during the treatment and recovery cycle. One control animal was never mated during 6 ovulatory cycles. She became pregnant when she was co-housed with another male during the reversibility phase during the first cycle that mating was confirmed.
The overall rate of pregnancy among the ORG 9935-treated monkeys (4/8, 50%) did not differ significantly from the control group (p= 0.14). All pregnancies occurred early within the first 3 cycles in the treated group (Fig. 2). Two ORG 9935-treated females demonstrated evidence of normal ovulation and mating during six cycles but did not become pregnant. Both of these animals became pregnant after discontinuation of treatment (see Fig. 2), one each after the 2nd and 4th cycles of exposure to the same male used during the contraceptive trial. Two of the eight females assigned to the ORG 9935-treated group were removed from the study early, one each after 4.5 and 5.5 cycles of exposure due to incompatibility in the group. Both of these animals became anovulatory prior to removal. Excluding these two females did not affect the overall pregnancy rate (4/6, 67%).
The serum samples obtained weekly during the treatment phase were analyzed for ORG 9935 using HPLC. Since these samples were obtained on a weekly basis immediately prior to the subcutaneous or oral dosing of ORG 9935, the values represent minimal levels circulating prior to the next drug administration. We used menstrual cycle records and the previously determined hormone samples to correlate each value to a cycle of exposure during the contraceptive and reversibility experiments. Significantly, no pregnancies occurred in animals with a trough level of ORG 9935 >300 nmol/L (see Fig. 3). Levels of ORG 9935 gradually decreased after dosing was discontinued (data not shown), and were below 300 nmol/L in the two ORG 9935-treated animals that became pregnant during the reversibility phase. Oral or subcutaneous administration of ORG 9935 produced wide variability in serum levels.
Fig. 3.
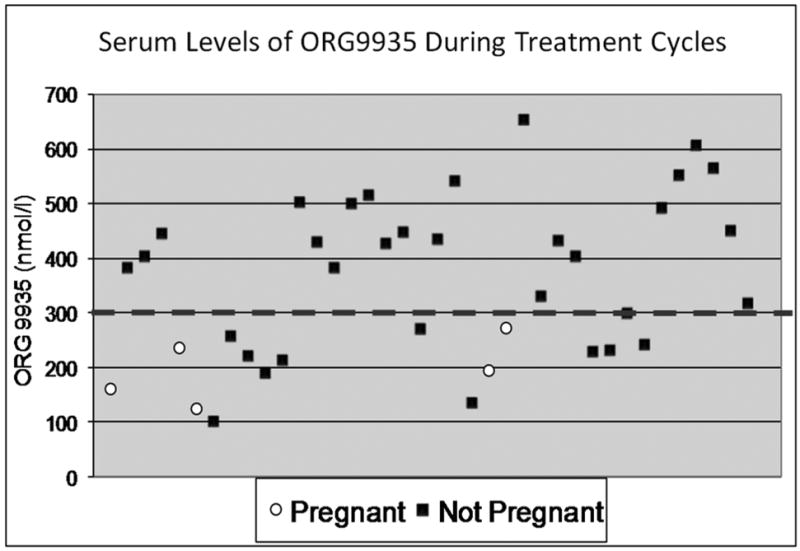
Scatterplot of ORG 9935 levels (nmol/L) during cycles where pregnancy did (white circles) and did not (black squares) occur. The dotted line shows the threshold (300 nmol/L above which pregnancy did not occur. There were 4 pregnancies in the treated animals. Two of the low values were samples obtained from one female during a pregnant cycle.
The telemetry data demonstrate that caged animals experience wide baseline fluctuations in heart rate (range 110 – 180 bpm, Fig. 4) in response to a number of confounding effects (e.g., light-dark cycle, activity in room, handling, feeding). Oral ORG 9935 doses of 200 or 400 mg/kg once/day or200 mg/kg twice/daily resulted in acute increases in mean heart rate recorded over 24 h (134 to 199 bpm, p <0.001)that returned to baseline upon discontinuation of the drug (Fig. 4). Mean systolic blood pressure (BP)decreased from 104 to 90 mm/hg in response to oral treatments with 200 mg/kg BID and 400 mg/kg once/day. Similar magnitudes of change were noted with diastolic and mean BP. The treated monkeys tolerated these changes without difficulty. Results from these experiments demonstrate that cardiovascular effects of PDE3 inhibitors following acute dosing in primates are dose-dependent, and reversible.
Fig. 4.
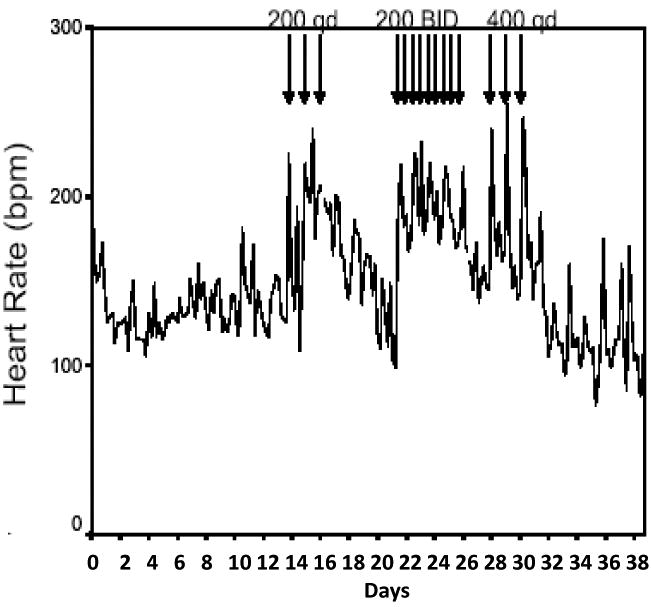
Heart rate response of a female rhesus macaque to oral treatment with Org 9935 200 mg/kg once/day (200 qd), 200 mg/kg twice/day (200 BID), and 400 mg/kg once/day (400 qd). Arrows indicate drug dosing. Note normal variations in daily heart rate in untreated animals, and that heart rate returns to pre-treatment levels following discontinuation of treatment.
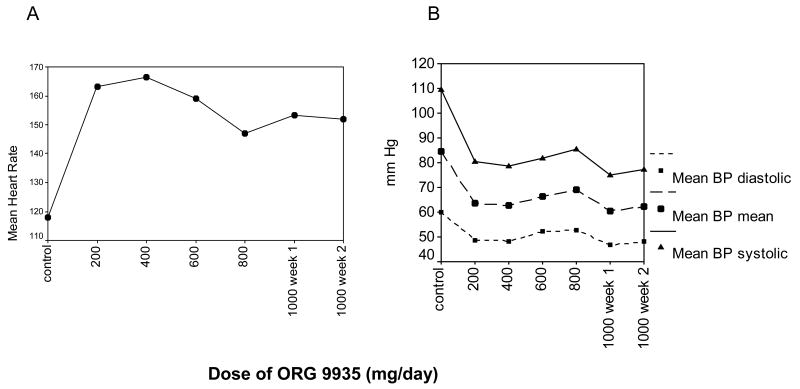
To explore whether chronic in-titration of the PDE3 inhibitor might reduce the effects observed with acute dosing, one monkey underwent continuous cardiovascular monitoring during a multi-week extended oral dosing trial of ORG 9935 involving a gradual increase in dose from 40 mg/kg/d to 200 mg/kg/d. Resting heart rate increased from a baseline of 117 to 166 bpm at 80 mg/kg/d of drug. Additional increases in dose, however, did not result in further elevations. In fact, although the mean heart rate (152 bpm) observed at a dose of 200 mg/kg/d was higher than the control value (117 bpm), it was much lower than that observed at the initial smaller doses (Fig. 5A), and this heart rate was maintained during a second week of treatment at the same dose. The mean heart rate on this high chronic dose (152 bpm) was much lower than that recorded in a different animal (195 bpm) treated with the same dose (200 mg/kg) acutely (Fig. 4). Similar trends were noted with respect to blood pressure (Fig. 5B). Again, the initial decline occurred with initiation of treatment and stabilized with increasing dose. The mean systolic BP decreased from a control value of 109 mm Hg to 80 mm Hg at a dose of 40 mg/kg/d and 77 mm Hg at a dose of 200 mg/kg/d. The magnitude and direction of change was similar for mean arterial pressure (84, 63, 62 mm Hg) and diastolic pressure (60, 49, 48 mm Hg).
Fig. 5.
Heart rate (A) and blood pressure (B) changes observed in a 5 kg rhesus macaque during long-term oral treatment with ORG 9935 at daily doses from 200 mg to 1000 mg/day. The dose (x axis) was increased every 7 days; 200 = 40 mg/kg.
4. Discussion
This is the first trial to evaluate whether an inhibitor of meiosis can prevent pregnancy in primates. The PDE3 inhibitor ORG 9935 has been shown to prevent primate oocyte maturation in vitro [9][ref], and in vivo during both COS [11] and COv cycles in rhesus macaques [12]. While a PDE3 inhibitor has been shown to prevent pregnancy in rodents [8], our results further these observations and establish that inhibition of meiotic maturation using a PDE3 inhibitor might represent the basis for a novel non-hormonal strategy in women.
Although the overall pregnancy rates among the ORG 9935-treated animals did not differ significantly from the control group, our HPLC analysis of ORG 9935 verified that all these pregnant animals had low serum levels of the PDE3 inhibitor in the cycle they became pregnant. Of note, no pregnancies occurred when the lowest serum level of ORG 9935 exceeded 300 nmol/L. Although pregnancy did not occur during every exposure when the ORG 9935 level was below this threshold, this would not be expected since pregnancy only occurs if a fertilizable metaphase II oocyte is available at ovulation, and our samples were taken weekly. Final meiotic maturation occurs during the periovulatory interval triggered by the midcycle luteinizing hormone (LH) surge. The concept of a threshold drug level to achieve an effect is well established for hormonal contraceptive methods; a minimal concentration necessary to inhibit ovulation is a feature of contraceptive progestagens [17].
In contrast to hormonal contraception, treatment with a PDE 3 inhibitor prevents final meiotic maturation of the oocyte without affecting ovulation and normal function of the corpus luteum [12]. A PDE 3 inhibitor could therefore represent a novel non-hormonal method that would not affect normal menstrual cycle events. In addition, the mechanism is entirely preconceptual. If oocyte maturation occurs (due to low drug levels), our data would suggest that the pregnancy is unaffected.
Our results also suggest that the contraceptive effect of ORG 9935 is reversible upon discontinuation of treatment. Both of the animals that did not conceive maintained ORG 9935 levels above 300 nmol/L during treatment. When treatment was discontinued, they both became pregnant within 4 cycles, and this correlated with a decline in the ORG 9935 level below the threshold.
Hormonal contraceptives are highly effective but unacceptable side effects occur in some users, and others (e.g., women with thrombophilias or hormone sensitive cancers) have absolute contraindications to their use [18]. To be a practical method of contraception in women, a novel method must have an acceptable side effect profile. Potential concerns regarding PDE3 inhibitors as anti fertility agents include the effects of the agents on non-ovarian tissues. The PDE3 isoform is expressed in respiratory smooth muscle [19], platelets, cardiac ventricular myocytes and coronary smooth muscle [20], pancreatic islet cells [21], and adipocytes [22]. In rodents, treatment with ORG 9935 induced a significant increase in heart rate [8]. Our telemetry studies confirm an increase in heart rate and decrease in blood pressure with treatment, but that cardiovascular effects diminish and stabilize with extended treatment. These results are consistent with the tachyphylaxis to cardiovascular effects observed during human treatment of cardiomyopathy with PDE3 inhibitors [23]. Although this change could also reflect an increase in metabolism, there is no evidence that cilostazole, a PDE3 inhibitors approved for long-term use for intermittent claudication, induces hepatic microenzymes (package insert Pletal (cilostazole), Otsuka America). We did not observe significant changes in hepatic enzymes or renal function in our animals treated up to 6 months with ORG 9935 (data not shown).
We observed poor tolerance of treatment during some of experiments with ORG 9935. The drug is practically insoluble in water, and a large amount of the powder needed to be given either orally, or subcutaneously is present as a suspension. These same dosing methods produced wide variability in serum levels in treated animals (Fig. 3). Intolerance occurred at the highest drug levels (typically in excess of 1000 -2000 nmol/L) that were 3 to 6-fold higher than the level (300 nmol/L) needed for contraceptive protection. Further development of this concept will require a drug delivery system capable of producing stable blood levels. There also appears to be extensive alternative splicing found for most of the PDE genes [24-26]. Knowledge of these differences could potentially lead to the production of a PDE3A inhibitor with greater oocyte specificity.
Nonhuman primates represent a scarce and expensive animal resource, and we studied the minimum number of animals to achieve statistical power. We did not achieve a significant effect in our primary outcome (a 70% reduction in the number of pregnancies is our treated group). Still, our results do show a dose-dependent effect on fertility with ORG 9935 levels above 300 nmol/L, and that the effect is reversible upon discontinuation of treatment.
These experiments are the first studies that report the successful use of a meiotic inhibitor as a contraceptive in primates. Our results demonstrate that treatment of macaques with the PDE3 inhibitor ORG 9935 prevents pregnancy at serum concentrations above 300 nmol/L, and that the contraceptive effect is reversible upon discontinuation of treatment. Improved drug dosing and delivery systems and more specific or potent inhibitors are needed before human trials can be considered.
Acknowledgments
ORG 9935 was provided as a gift of N.V. Organon (now Schering Plough) through the kind assistance of Pieter M. Verbost, PhD. The authors wish to thank the staff of the Division of Animal Resources at ONPRC for excellent animal care, and David Hess, PhD and the Endocrine Services Core Laboratory for steroid assays, and Dr. Dennis Koop, the Director of the Bioanalytical Shared Resource/Pharmacokinetics Core Facility at OHSU, for the ORG 9935 assay. This research was supported by the Eunice Kennedy Shriver NICHD through R01-042710, and by U54 HD18185; U54 HD 031398, and NCRR RR00163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital and health statistics. 2005:1–160. [PubMed] [Google Scholar]
- 2.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 3.Peipert JF, Gutmann J. Oral contraceptive risk assessment: a survey of 247 educated women. Obstet & Gyneco. 1993;82:112–17. [PubMed] [Google Scholar]
- 4.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–9. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsafriri A, Chun SY, Zhang R, et al. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Develop Biol (Orlando) 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 6.Conti M, Andersen CB, Richard FJ, et al. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Molec Cell Endocrinol. 1998;145:9–14. doi: 10.1016/s0303-7207(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 7.Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–184. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- 8.Wiersma A, Hirsch B, Tsafriri A, et al. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–7. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen JT, Schwinof KM, Zelinski-Wooten MB, et al. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17:2079–84. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira D, Albano C, Adriaenssens T, et al. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–52. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JT, Zelinski-Wooten MB, Schwinof KM, et al. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception. 2005;71:68–73. doi: 10.1016/j.contraception.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JT, Zelinski MB, Stanley JE, et al. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77:303–7. doi: 10.1016/j.contraception.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf DP, Thomson JA, Zelinski-Wooten MB, et al. In vitro fertilization-embryo transfer in nonhuman primates: The technique and its applications. Mol Reprod Dev. 1990;27:261–80. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- 14.Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–40. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- 15.Zelinski-Wooten MB, Slayden OD, Chwalisz K, et al. Chronic treatment of female rhesus monkeys with low doses of the antiprogestin ZK 137 316: establishment of a regimen that permits normal menstrual cyclicity. Hum Reprod. 1998;13:259–67. doi: 10.1093/humrep/13.2.259. [DOI] [PubMed] [Google Scholar]
- 16.Zelinski-Wooten MB, Chwalisz K, Iliff SA, et al. A chronic, low-dose regimen of the antiprogestin ZK 137 316 prevents pregnancy in rhesus monkeys. Hum Reprod. 1998;13:2132–8. doi: 10.1093/humrep/13.8.2132. [DOI] [PubMed] [Google Scholar]
- 17.Lahteenmaki PL, Lahteenmaki P. Concentration-dependent mechanisms of ovulation inhibition by the progestin ST-1435. Fertil Steril. 1985;44:20–4. [PubMed] [Google Scholar]
- 18.World Health Organization. Improving access to quality care in family planning: medical eligibility criteria for contraceptive use. 3rd. Geneva: WHO; 2003. p. 176. [Google Scholar]
- 19.Billington CK, Joseph SK, Swan C, et al. Modulation of human airway smooth muscle proliferation by type 3 phosphodiesterase inhibition. Am J Physio. 1999;276:L412–419. doi: 10.1152/ajplung.1999.276.3.L412. [DOI] [PubMed] [Google Scholar]
- 20.Cone J, Wang S, Tandon N, et al. Comparison of the effects of cilostazol and milrinone on intracellular cAMP levels and cellular function in platelets and cardiac cells. J Cardiovas Pharmacol. 1999;34:497–504. doi: 10.1097/00005344-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 21.El-Metwally M, Shafiee-Nick R, Pyne NJ, et al. The effect of selective phosphodiesterase inhibitors on plasma insulin concentrations and insulin secretion in vitro in the rat. Eur J Pharmacol. 1997;324:227–32. doi: 10.1016/s0014-2999(97)00076-9. [DOI] [PubMed] [Google Scholar]
- 22.Moberg E, Enoksson S, Hagstrom-Toft E. Importance of phosphodiesterase 3 for the lipolytic response in adipose tissue during insulin-induced hypoglycemia in normal man. Horm Metabo Rese. 1998;30:684–8. doi: 10.1055/s-2007-978958. [DOI] [PubMed] [Google Scholar]
- 23.Movsesian MA, Alharethi R. Inhibitors of cyclic nucleotide phosphodiesterase PDE3 as adjunct therapy for dilated cardiomyopathy. Expert Opin Investig Drugs. 2002;11:1529–36. doi: 10.1517/13543784.11.11.1529. [DOI] [PubMed] [Google Scholar]
- 24.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiolo Rev. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 25.Manganiello VC, Taira M, Degerman E, et al. Type III cGMP-inhibited cyclic nucleotide phosphodiesterases (PDE3 gene family) Cell Signalling. 1995;7:44555. doi: 10.1016/0898-6568(95)00017-j. [DOI] [PubMed] [Google Scholar]
- 26.Conti M, Nemoz G, Sette C, et al. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endoc Rev. 1995;16:370–89. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]