Abstract
Introduction
Many physicians recommend that patients receive follow-up chest imaging after the diagnosis of pneumonia to ensure that a pulmonary malignancy is not missed. However there is little research evidence to support this practice. Our aims were to assess the frequency of the diagnosis of pulmonary malignancy, and to identify risk factors for pulmonary malignancy following hospitalization for pneumonia.
Methods
By excluding patients with a prior diagnosis of pulmonary malignancy, we examined the incidence of a new pulmonary malignancy diagnosis in inpatients ≥65 years with a discharge diagnosis of pneumonia in fiscal years 2002–2007, and at least one year of Department of Veterans Affairs outpatient care prior to the index admission.
Results
Of 40,744 patients hospitalized with pneumonia, 3,760 (9.2%) patients were diagnosed with pulmonary malignancy after their index pneumonia admission. Median time to diagnosis was 297 days with only 27% diagnosed within 90-days of admission. Factors significantly associated with a new diagnosis of pulmonary malignancy included history of chronic pulmonary disease, any prior malignancy, white race, being married, and tobacco use. Increasing age, Hispanic ethnicity, need for intensive care unit admission, and a history of congestive heart failure, stroke, dementia, or diabetes with complications were associated with a lower incidence of pulmonary malignancy.
Conclusion
A small, but clinically important, proportion of patients are diagnosed with pulmonary malignancy post-hospitalization for pneumonia. Additional research is needed to examine whether previously undiagnosed pulmonary malignancies may be detected at admission, or soon after, for those hospitalized with pneumonia.
Keywords: pneumonia, cancer, incidence
Introduction
Pneumonia is the second leading cause of hospitalization (after child birth) in the United States with > 1.2 million hospitalizations in 2006 [1]. In the United States (US), pneumonia (along with influenza) is the eighth leading cause of death and the number one infectious cause of death [2]. In the last two decades, patients aged 65–84 have shown a 20% increase in the number of pneumonia-related hospitalizations.
Although many physicians recommend that patients receive follow-up chest imaging after the diagnosis of pneumonia to ensure that a pulmonary malignancy is not missed, there is little research evidence to support this practice. Few studies have examined the incidence of diagnosed pulmonary malignancy after hospitalization for pneumonia [3, 4]. These studies showed only a small number (<2%) of pneumonia patients received a new diagnosis of pulmonary malignancy but were limited by a short follow-up period (< 3 months after initial hospitalization) and inclusion of very young patients (> 18 years of age) who are at low risk for pulmonary malignancies.
The aims of our study were to assess the frequency of the diagnosis of pulmonary malignancy, either primary or metastatic, after hospitalization for outpatient-acquired pneumonia in subjects ≥ 65 years of age, and to identify risk factors for post-hospitalization pulmonary malignancy.
Methods
For this study we used data from the administrative databases of the Department of Veterans Affairs (VA) Health Care System. These databases are the repositories of clinical data from all of the VA hospitals and outpatient clinics [5]. The Institutional Review Board of the University of Texas Health Science Center at San Antonio approved this study under expedited review.
Inclusion and Exclusion Criteria
Patients included in this study:
Were age 65 or older on the date of admission. This age cut off was chosen due to the design of the primary study.
Had at least one outpatient clinic visit in the year preceding the index admission.
Received at least one active and filled outpatient medication within 90-days of admission.
Were hospitalized during fiscal years 2002–2007 (Oct 2001-Sep 2007).
Had a previously validated discharge diagnosis of pneumonia/influenza (ICD-9 codes 480.0-483.99 or 485-487) [6].
Received antimicrobial therapy within the first 48 hours of admission.
As our primary aim was to examine the incidence of new pulmonary malignancy, we excluded patients with pre-existing pulmonary malignancies (assessed October 2000 forward) from our analyses. We assessed only the first hospitalization for pneumonia during the study period and excluded patients with an admission for pneumonia in the previous 12 months (one-year clean-out period).
Data
We used demographic, utilization, and comorbidity data from the National Patient Care Database, pharmacy data from the VA Decision Support System National Data Extracts and Pharmacy Benefits Management, and vital status information from VA's Vital Status file which incorporates data from veterans' death benefits claims, inpatient deaths, Medicare Vital Status files, and the Social Security Administration death master file. Encrypted patient identifiers linked the information across these databases.
We obtained demographic information (age, sex, race, marital status) from inpatient and outpatient data. Race categories included white, black, Hispanic, and other/unknown. ICD-9 codes for tobacco use (305.1, V15.82), smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence (Zyban, nicotine replacement, or varenicline) were used to infer tobacco use.
We also obtained information on comorbid conditions from inpatient and outpatient administrative data. Alcohol abuse was defined by ICD-9 codes 291, 303, 305.0, and illicit drug use by ICD-9 codes 292, 304, 305 excluding 305.1. We used Charlson’s comorbidity methodology to classify other preexisting comorbid conditions, both individually and as a composite score [7, 8]. Charlson’s comorbidity system includes 19 comorbid conditions, which are classified using ICD-9 codes from prior outpatient and inpatient codes [9].
Outcomes
We used a new diagnosis of either primary lung cancer or pulmonary metastasis after index hospitalization as the primary outcome for this study. ICD-9 codes were used to identify primary or metastatic pulmonary cancers. These ICD-9 codes included 162, 163, 165, 165.0, 165.8, 165.9, 176.4, 197.0, 197.1, 197.2, 197.3, 198, 199, 199.0, 199.1, 230, 231, 231.1, 231.2, 231.8, 231.9, 234, 235, 235.6, 235.7, 235.8, 235.9, and 238. Prior research has validated the use of ICD-9 codes to identify pulmonary malignancies [10].
Secondary outcomes included 30- and 90-day mortality, long-term survival, and length of hospital stay for the index admission. Mortality was assessed through October 1, 2007 using the VA vital status file. Previous studies have demonstrated that this methodology has a sensitivity of ~98% for veterans’ deaths [11].
Statistical Analyses
Bivariate statistics were used to test the association of sociodemographic and clinical characteristics with all-cause 30-day mortality. Categorical variables were analyzed using the chi-square test and continuous variables were analyzed using Student’s t-test. Due to the number of statistical tests and the large sample size of this database we set statistical significance at p ≤0.001.
To evaluate risk factors for a post-pneumonia hospitalization diagnosis of pulmonary malignancy, baseline patient sociodemographic and clinical characteristics were used as independent variables in an extended Cox model [12]. The baseline variables included comorbid conditions that were significantly associated with pulmonary malignancy, age group at admission (65–74, 75–84, 85+), race/ethnicity, intensive care unit (ICU) admission, tobacco use, and marital status. Due to failure of the proportional hazards assumption in the standard Cox models, two variables, age group at admission and history of metastatic cancer, were included as time varying covariates. Grambsch and Therneau’s method was used to evaluate the proportional hazards assumption [13], and our final model satisfied these criteria.
To analyze time-to-diagnosis of pulmonary malignancy, a graph was created using Kaplan-Meier estimated probabilities. To examine the impact of a new diagnosis of pulmonary malignancy on mortality over the entire period of follow-up, we used Cox proportional hazard models to estimate and graph the baseline survivor functions after adjusting for variables associated with pulmonary malignancy, which were stratified into 2 time periods (0 to 90 days and 90 to 1500 days). We broke these 2 time periods into separate graphs due to an inversion of risk at ~90 to 100 days.
All analyses were performed using STATA 9 (College Station, Texas).
Results
We identified 40,744 patients who met the inclusion criteria and who did not have a diagnosis of pulmonary malignancy prior to the pneumonia hospitalization. The mean age was 77.7 years with a standard deviation (SD) of 6.8 years; 53.0% of subjects were married, and 98.1% were male. In this cohort, 10.5% were black, 66.6% were white, 1.5% were Hispanic, 4.6% were other, and 16.8% were of unknown race/ethnicity. Regarding mortality, 12.9% (N= 5270) of the subjects died within 30-days of admission, and 20.7% (N=8451) died within 90-days of admission. In our cohort, 3,760 (9.2%) patients were diagnosed with pulmonary malignancy after hospitalization for pneumonia.
Table 1 shows descriptive demographics and comorbid conditions by subsequent diagnosis of pulmonary malignancy. Factors significantly associated with increased risk of diagnosis of pulmonary malignancy in univariate analyses included lower age, white race, being married, current tobacco use, chronic obstructive pulmonary disease, prior malignancy and metastatic solid tumor (both excluding prior pulmonary malignancies). Factors associated with lower risk of diagnosis of pulmonary malignancy include being black or Hispanic, requiring ICU admission during the hospitalization, requiring mechanical ventilation, congestive heart failure, prior stroke, dementia, diabetes with complications, diabetes without complications, and renal disease.
Table 1.
Characteristics of patients (N=40,744) hospitalized with pneumonia by diagnosis of pulmonary malignancy
| Variables | Post- hospitalization Pulmonary Malignancy (N= 3760) |
No Pulmonary Malignancy (N=36984) |
P-value |
|---|---|---|---|
| Demographics | |||
| Age (mean, SD) | 76.5 (6.3) | 77.8 (6.8) | <0.001 |
| Men | 3711 (99) | 36272 (98) | 0.007 |
| Race | |||
| White | 2656 (71) | 24489 (66) | <0.001 |
| Black | 325 (9) | 3957 (11) | <0.001 |
| Hispanic | 41 (1) | 745 (2) | <0.001 |
| Other | 153 (4) | 1939 (5) | 0.002 |
| Missing | 606 (16) | 6246 (17) | 0.23 |
| Married | 2100 (56) | 19603 (53) | 0.001 |
| Characteristics of hospitalization | |||
| ICU admission | 285 (8) | 5471 (15) | <0.001 |
| Use of mechanical ventilation | 105 (3) | 2627 (7) | <0.001 |
| Comorbid Conditions | |||
| Current tobacco use | 1700 (45) | 12670 (34) | <0.001 |
| Alcohol abuse | 163 (4) | 1478 (4) | 0.3 |
| Illicit drug abuse | 39 (1) | 399 (1) | 0.8 |
| Myocardial infarction | 244 (6) | 2647 (7) | 0.14 |
| Congestive heart failure | 872 (23) | 9778 (26) | <0.001 |
| Peripheral vascular disease | 527 (15) | 5552 (15) | 0.68 |
| Chronic obstructive pulmonary disease | 2129 (57) | 18685 (51) | <0.001 |
| Stroke | 561 (15) | 7011 (19) | <0.001 |
| Peptic ulcer | 135 (4) | 1227 (3) | 0.38 |
| Rheumatologic disease | 124 (3) | 979 (3) | 0.02 |
| Mild liver disease | 14 (0.4) | 136 (0.4) | 0.97 |
| Dementia | 101 (3) | 2182 (6) | <0.001 |
| Diabetes without complications | 1120 (30) | 12273 (33) | <0.001 |
| Diabetes with complications | 310 (8) | 3832 (10) | <0.001 |
| Moderate Liver disease | 22 (0.6) | 286 (0.8) | 0.2 |
| Hemiplegia | 43 (1) | 613 (2) | 0.02 |
| Renal disease | 382 (10) | 4766 (13) | <0.001 |
| Any prior malignancy* | 846 (23) | 5270 (14) | <0.001 |
| Metastatic solid tumor* | 33 (0.9) | 100 (0.3) | <0.001 |
| AIDS | 13 (0.4) | 81 (0.2) | 0.12 |
| Outcomes | |||
| Mortality at 30-days | 48 (1) | 5222 (14) | <0.001 |
| Mortality at 90-day | 267 (7) | 8184 (22) | <0.001 |
| Length of stay, mean (SD) | 6.21 (7.4) | 8.1 (13.3) | <0.001 |
Excluding prior pulmonary malignancies
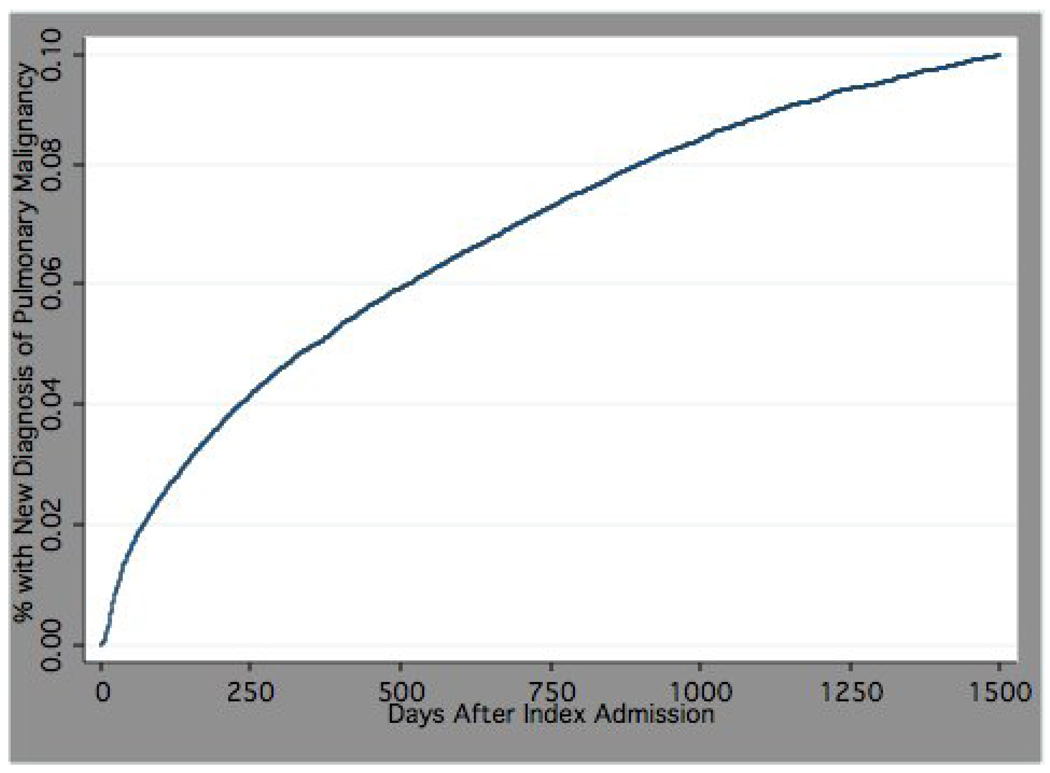
Median time to diagnosis of pulmonary malignancy was 297 days (mean 452.1, SD 442.7), and only 27% were diagnosed within 90-days of admission. Figure 1 depicts time to diagnosis of pulmonary malignancy.
Figure 1.
Time to new diagnosis of pulmonary malignancy for patients ≥ 65 years hospitalized with pneumonia
Table 2 presents the results of the extended Cox model. In this model, factors significantly associated with post-pneumonia pulmonary malignancy included history of chronic obstructive pulmonary disease (hazard ratio [HR] 1.12, 95% confidence interval [CI], 1.04–1.20), any prior malignancy (1.69, 1.56–1.83) or metastatic solid tumor (3.33, 2.10–5.12), (both excluding those with prior pulmonary malignancy), white race (1.15, 1.06–1.24), being married (1.10, 1.03–1.17), and current tobacco use (1.37, 1.28–1.46). Increasing age group (0.88, 0.82–0.95), Hispanic ethnicity (0.60, 0.44–0.82), need for ICU admission (0.48, 0.43–0.55), having a history of congestive heart failure (0.92, 0.85–0.99), stroke (0.80, 0.74–0.88), dementia (0.53, 0.43–0.65), renal disease (0.88, 0.79–0.88), and diabetes with complications (0.88, 0.87–0.99) were associated with a lower incidence of diagnosis.
Table 2.
Results of the extended Cox model for time until diagnosis of pulmonary malignancy
| Variable | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Age group (65–74, 75–84, 85+) | 0.88 | 0.82–0.95 |
| Men | 1.37 | 1.02–1.81 |
| Race/ethnicity | ||
| White | 1.15 | 1.06–1.24 |
| Black | 0.92 | 0.80–1.04 |
| Hispanic | 0.60 | 0.44–0.82 |
| Missing/other | 1.0 | |
| Married | 1.10 | 1.03–1.17 |
| Current tobacco use | 1.37 | 1.28–1.46 |
| Intensive care unit admission during index hospitalization | 0.48 | 0.43–0.55 |
| Congestive heart failure | 0.92 | 0.85–0.99 |
| Stroke | 0.80 | 0.74–0.88 |
| Dementia | 0.53 | 0.43–0.65 |
| Diabetes without complications | 0.93 | 0.86–1.01 |
| Diabetes with complications | 0.88 | 0.77–0.99 |
| Chronic obstructive pulmonary disease | 1.12 | 1.04–1.20 |
| Renal disease | 0.88 | 0.79–0.98 |
| Any malignancy* | 1.69 | 1.56–1.83 |
| Metastatic solid tumor* | 3.33 | 2.1–5.22 |
Excluding prior pulmonary malignancies
As a sub-analysis we excluded all subjects who died within 90-days of hospital admission and re-ran the survival analyses. We found factors still associated with new diagnosis of pulmonary malignancy included current tobacco use (HR 1.18, 95% CI, 1.15–1.23), any malignancy (1.13, 1.08–1.18), metastatic solid tumor (7.46, 4.9–11.4), chronic obstructive pulmonary disease (1.12, 1.08–1.16), congestive heart failure (1.16, 1.12–1.20), and male gender (1.26, 1.11–1.41).
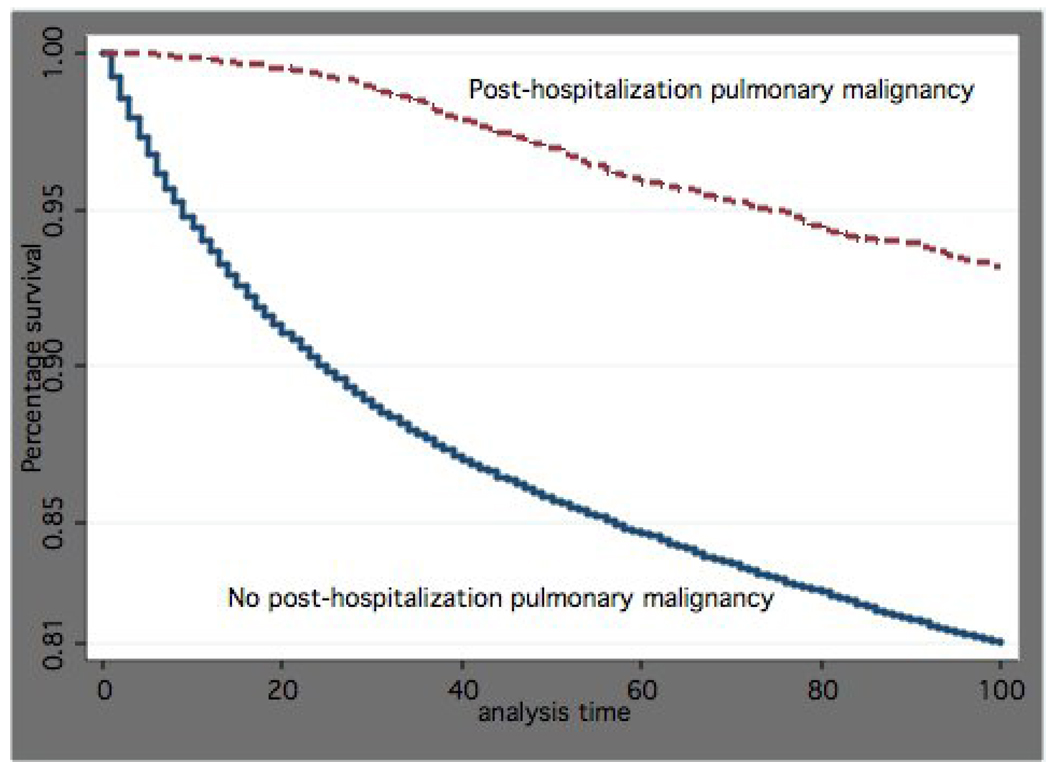
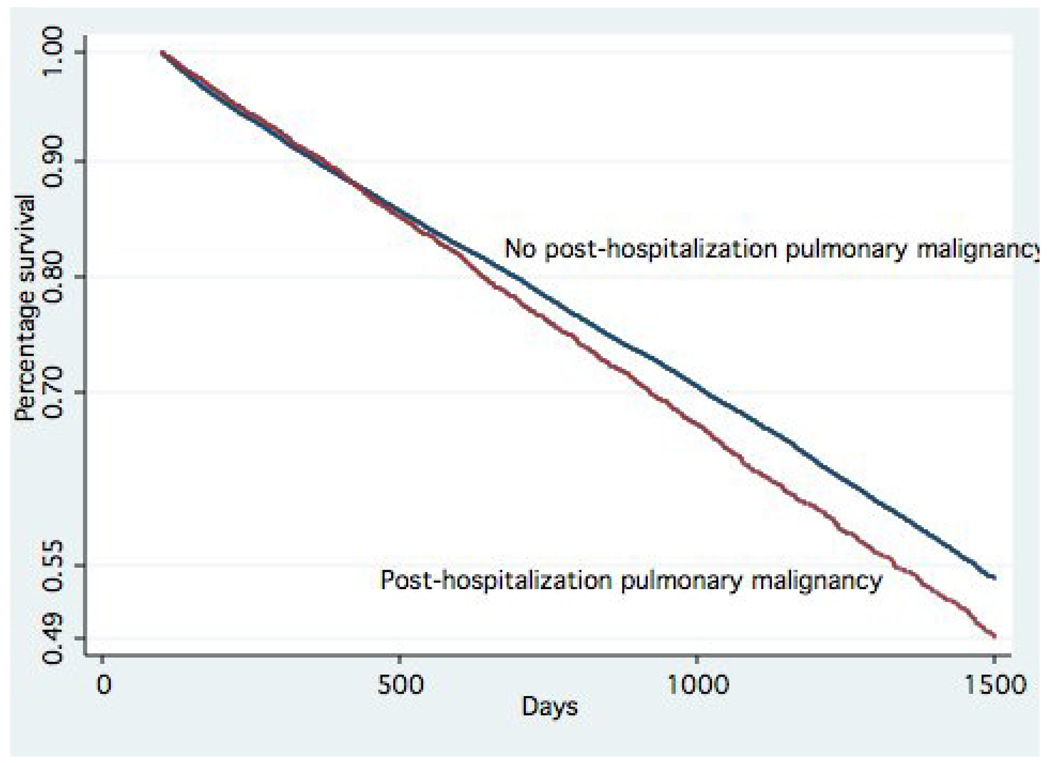
Figure 2 and Figure 3 are plots of survival time by whether patients received post-hospitalization diagnosis of pulmonary malignancy. Figure 2, which covers 0 to 90-days post admission, demonstrates that patients with a subsequent diagnosis of pulmonary malignancy had significantly lower short-term mortality as compared to those who did not (p<0.001). Figure 3, which covers 90+ days after the pneumonia admission, shows patients with a diagnosis of pulmonary malignancy had significantly higher long-term mortality as compared to those who did not (p<0.0001).
Figure 2.
Proportion surviving within the first 90-days by post-hospitalization diagnosis of pulmonary malignancy in patients with pneumonia (p<0.0001)
Figure 3.
Proportion surviving after 90-days by post-hospitalization diagnosis of pulmonary malignancy in patients with pneumonia (p<0.0001)
Discussion
We found that a small, but clinically important, number of elderly patients with pneumonia are diagnosed with post-hospitalization pulmonary malignancy. Our results support the concept of follow-up imaging for elderly patients with pneumonia. However as this is retrospective study we are unable to comment on the appropriate time frame or imaging modality (e.g., chest x-ray, computed tomography of the chest).
In our study we found that there were significant differences in several comorbid conditions and demographics between those subjects who were subsequently diagnosed with pulmonary malignancies and those who were not. Many of these are quite clearly related to increased incidence of pulmonary malignancy (e.g., tobacco use, chronic obstructive pulmonary disease, history of cancer or metastatic cancer). However others are not as clear (e.g., history of stroke, renal disease, or dementia), and we hypothesize that these are "protective" against pulmonary malignancy due to increased mortality associated with these conditions so that many of these patients die prior to developing clinically significant pulmonary malignancies. In addition, we found that increasing age in our cohort was associated with lower incidence of pulmonary malignancy. This corresponds to data from the Surveillance Epidemiology and End Results (SEER) cancer registries that reported the median age of diagnosis is 70 years of age, which is on the lower side of the age range of our cohort.
Interestingly we found significant differences in outcomes (30-day and 90-day mortality, long-term survival, and length of stay) between the patients with post-pneumonia pulmonary malignancy and those without, and in factors such as ICU admission. It is noteworthy that patients subsequently diagnosed with lung cancer had improved survival for the first 90 days post-pneumonia hospitalization, however lower survival after 90 days. It appears that patients with pulmonary malignancy have survivals < 2 years, which is consistent with previous literature [14]. This suggests that there are significant differences between the two groups even at the time of pneumonia hospitalization, and that even though there is a substantial delay until the initial diagnosis of pulmonary malignancy, the pulmonary malignancy is at least partially responsible for their initial hospitalization.
In this study we found a much higher incidence of pulmonary malignancy as compared to previous papers. Marrie [3] found in a prospective study of 1269 patients with community-acquired pneumonia that only 25 (1.97%) had lung cancer while Holmberg and Kargsbjerg [4] found that 1.3% of patients with community-acquired pneumonia had a previously undiagnosed pulmonary malignancy. Both studies had significant limitations including a short, and unclear, follow-up period (< 3 months), and inclusion of very young patients (> 18 years of age), who are at very low risk for pulmonary malignancies. We believe that the differences in the results between these studies and ours was due to our older population that is at much higher risk for pulmonary malignancy and the much longer follow-up (up to 5 years).
Our study has several limitations. Although our study was a large database analysis and subject to the recognized limitations of such studies, we carefully assembled our cohort from complete patient discharge data to avoid ascertainment bias. However due to the large number of subjects in our study routine definitions of statistical significance (p<0.05) are meaningless. Therefore we based our decisions on which variables to include in the survival analysis on clinical relevance. Our sample was predominantly men due to our use of VA administrative data, and further research is needed to examine these issues in women. Although we relied upon ICD-9 codes to define both pneumonia and pulmonary malignancies, previous studies have validated the use of both [10, 15–17]. In addition, due to the design of the primary study we only examined patients ≥ 65 years of age. Future studies should also examine other age groups. Also we were unable to examine severity of illness at presentation using previously validated measures such as the pneumonia severity index [18] due to the lack of several variables (e.g., systolic blood pressure). Next, data on when and where radiographic testing was done is not available in these databases so we are unable to examine these issues. Finally, as this is not a prospective cohort study, we are unable to identify when these malignancies would be able to be first identified. Therefore we are unable to address whether there is a true “physiologic” delay between hospitalization and diagnosis, or as we suspect many of these patients did not get repeat chest imaging until they developed additional symptoms. Further research is needed to determine what would be the appropriate timing of follow-up testing, and what that testing would be.
In conclusion, our study finds that a small, but clinically important, number of elderly patients are diagnosed with pulmonary malignancy post-hospitalization for pneumonia. Post-hospitalization follow-up is important for those previously hospitalized with pneumonia especially for those at higher risk for malignancy. Further research is needed to examine other groups, such as those < 65 years of age and women, and to examine whether strategies to detect previously undetected pulmonary malignancy at time of admission, or soon after, are associated with improved outcomes for patients hospitalized with pneumonia.
Acknowledgments
The project described was supported by Grant Number R01NR010828 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. Dr Copeland is funded by Merit Review Entry Program grant MRP-05-145 from the VA Health Services Research and Development program. Dr. Restrepo is funded by a KL2 of the National Institutes of Health and the University of Texas Health Science Center at San Antonio. The funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.AHRQ News and Numbers. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Pneumonia Most Common Reason for Hospitalization. [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005. vol 56. Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 3.Marrie TJ. Pneumonia and carcinoma of the lung. J Infect. 1994;29(1):45–52. doi: 10.1016/s0163-4453(94)95060-1. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg H, Kragsbjerg P. Association of pneumonia and lung cancer: the value of convalescent chest radiography and follow-up. Scand J Infect Dis. 1993;25(1):93–100. [PubMed] [Google Scholar]
- 5.Brown SH, Lincoln MJ, Groen PJ, Kolodner RM. VistA--U.S. Department of Veterans Affairs national-scale HIS. Int J Med Inform. 2003;69(2–3):135–156. doi: 10.1016/s1386-5056(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 6.Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084. [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MackKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SK, Brooks SE, Mullins CD, Baquet CR, Merchant S. Use of ICD-9 coding as a proxy for stage of disease in lung cancer. Pharmacoepidemiology and Drug Safety. 2002;11(8):709. doi: 10.1002/pds.759. [DOI] [PubMed] [Google Scholar]
- 11.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therneau TM, Grambsch PM. Statistics for biology and health. New York: Springer; 2000. Modeling survival data : extending the Cox model. [Google Scholar]
- 13.Therneau TM, Grambsch PM, Fleming TR. Martingale hazards regressions and the analysis of censored survival data. Biometrika. 1990;77:147–170. [Google Scholar]
- 14.van Rens MT, de la Riviere AB, Elbers HR, van Den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117(2):374–379. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]
- 15.Marrie TJ, Durant H, Sealy E. Pneumonia--the quality of medical records data. Med Care. 1987;25(1):20–24. doi: 10.1097/00005650-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Whittle J, Fine MJ, Joyce DZ, et al. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual. 1997;12(4):187–193. doi: 10.1177/0885713X9701200404. [DOI] [PubMed] [Google Scholar]
- 17.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]