Abstract
Rps2/rpS2 is a well conserved protein of the eukaryotic ribosomal small subunit. Rps2 has previously been shown to contain asymmetric dimethylarginine residues, the addition of which is catalyzed by zinc-finger-containing arginine methyltransferase 3 (Rmt3) in the fission yeast Schizosaccharomyces pombe and protein arginine methyltransferase 3 (PRMT3) in mammalian cells. Here we demonstrate that despite the lack of a zinc-finger-containing homolog of Rmt3/PRMT3 in the budding yeast Saccharomyces cerevisiae, Rps2 is partially modified to generate asymmetric dimethylarginine and monomethylarginine residues. We find that this modification of Rps2 is dependent upon the major arginine methyltransferase 1 (Rmt1) in S. cerevisiae. These results are suggestive of a role for Rmt1 in modifying the function of Rps2 in a manner distinct from that occurring in S. pombe and mammalian cells.
Keywords: ribosomes, protein arginine methylation, posttranslational modification of proteins, methyltransferases, ribosomal assembly, asymmetric dimethylarginine
Introduction
The functional roles of many post-translational modifications are still being discovered. Protein arginine methyltransferases (PRMTs) have been found to act on many cellular proteins and tend to be well conserved between organisms [1-4]. In humans, there appear to be at least nine members of the PRMT family; fewer members are found in fungi, and no examples have been found to date in prokaryotes [1, 2, 5]. Enzyme family members can be distinguished by the number and configuration of methyl groups that they transfer to arginine residues, as well as by the presence of additional binding domains. The functional roles of this modification are varied and include regulation of transcription, splicing, DNA repair, protein translocation, and signaling [1-4].
We have been interested in the modification of ribosomal proteins by arginine methylation. Two sites of such modification have been described to date [6]. The large ribosomal protein Rpl12 is modified at an internal guanidino nitrogen atom in an arginine residue in fungal species by a unique enzyme Rmt2 [5, 7, 8]. The small ribosomal protein Rps2/rpS2 is modified at the terminal guanidine nitrogen atoms of one or more arginine residues to generate asymmetric dimethylated derivatives in both fungal and mammalian species [9-11].
The mammalian PRMT3 enzyme has been shown to catalyze the methylation of rpS2 [9]; its homolog Rmt3 in the fission yeast S. pombe has been shown to catalyze methylation of the ribosomal protein homolog Rps2 [10]. Notably, PRMT3 and Rmt3 have been found to physically associate with rpS2/Rps2 in mammalian cells and S. pombe and it appears that this ribosomal protein may be a major substrate of these enzymes [9-14]. Both of these enzymes contain an N-terminal addition of a zinc-finger domain that is required for the binding of enzyme and Rps2/rpS2 and for most or all of their methyltransferase activity with these substrates [9, 11]. Interestingly, it appears that at least one role of the physical association of enzyme and substrate is independent of methyltransferase activity since catalytically inactive forms of Rmt3 can rescue the ribosomal subunit ratio defect found in S. pombe cells lacking the enzyme [11].
However, the budding yeast S. cerevisiae does not have a homolog to PRMT3. Despite this, we have found one or more arginine residues in Rps2 are methylated in these cells. We now show that the zinc-fingerless PRMT1 homolog Rmt1 is required for the methylation of Rps2 in S. cerevisiae. We suggest that protein arginine methylation of Rps2 may play a distinct role in organisms lacking homologs of PRMT3/Rmt3 [2, 5].
Materials and methods
Yeast Strains
S. cerevisiae strains were obtained from the Saccharomyces Genome Deletion Project and included the parent strain BY4742 and the RMT1, RMT2, and HSL7 gene deletion strains in this background. Cells were grown at 30° C in YPD media (1% bacto-yeast extract, 2% bacto-peptone, 2% dextrose) to an optical density of 0.5 – 1.0 at 600 nm. The cells were subsequently harvested by centrifugation at 4° C for 5 min at 5,000 × g.
Isolation of ribosomal small subunit proteins
Cells were in vivo labeled with S-adenosyl-L-[methyl-3H]methionine ([3H]AdoMet), ribosomes were prepared from extracts, and ribosomal subunits were separated by centrifugation on a high salt sucrose gradient according to the protocol described in [15], except that buffer A contained proteinase inhibitors from the Roche Proteinase Inhibitor Cocktail Tablet and buffer B contained 750 mM KCl. Briefly, the ribosomal fraction re-suspended in buffer B was layered on top of a 10%-38% sucrose gradient made in the presence of buffer B and centrifuged for 60,000 × g for 18.5 h at 4° C (unless otherwise specified) using a Beckman type SW41 rotor. Selected fractions were combined and then ethanol and acetic acid extracted as described in [15] to precipitate protein and remove RNA. The post-dialysis sample was lyophilized and re-suspended in 100 μl water. Protein concentration was determined by precipitating an aliquot using 10% trichloroacetic acid by the Lowry method using bovine serum albumin as a standard. For SDS gel electrophoresis, samples were combined with 2× dye, heated at 2 min at 100° C, and loaded onto a 12.6% SDS-PAGE in the Lammeli buffer system [16]. The gels were rocked overnight submerged in Coomassie Brilliant Blue dye, and then de-stained to enable visualization of protein bands.
Mass Spectrometry
Analysis by mass spectrometry was performed on an Applied Biosystems Q-Star Elite instrument in an approach similar to that used previously [17]. Briefly, the small subunit proteins were injected into a PLRP-S polymeric column with pore size of 300Ǻ, bead size of 5 μm, and dimensions of 150 × 1.0 mm (Polymer Laboratories, Amherst, MA) which was maintained at 50° C for the duration of the run. The column was initially equilibrated with 5% B (0.05% trifluoroacetic acid in acetonitrile) and 95% A (0.05% trifluoroacetic acid in water), and the proteins were eluted at 50 μl/min with 5 min at starting conditions, followed by a 25 min gradient from 5% to 30% B, then a 45 min gradient from 30% to 60% B, and finally a 15 min gradient from 60% to 100% B. The Q-Star mass spectrometer was calibrated using Glufib and run at a mass window of 300-2200, accumulation time of 1.5, source position 13;+5, and a DP of 140.
Results
Rps2 from S. cerevisiae contains asymmetric dimethylarginine
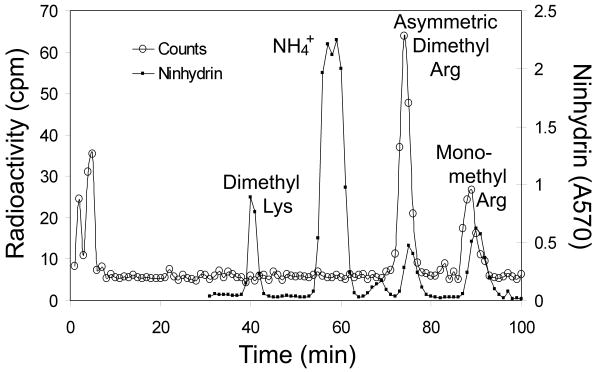
Previous studies have indicated that homologs of the Baker's yeast small subunit ribosomal protein 2 are asymmetrically dimethylated at arginine residues in S. pombe and in mammalian cells [9, 10]. To determine whether Rps2 of S. cerevisiae was also modified in this manner, we purified the wild type polypeptide by isolating ribosomes, preparing small subunit proteins, and separating the polypeptides by SDS-PAGE from cells labeled in vivo with [3H]AdoMet as a methyl donor. [3H]labeled Rps2 was excised from the gel, acid hydrolyzed, and the resulting amino acids analyzed by high resolution cation exchange chromatography (Fig. 1). We found that radioactivity eluted in the positions expected for asymmetric dimethylarginine (ADMA) and monomethylarginine (MMA); no radioactivity eluted in the position of methylated lysine residues (Fig. 1). This result is consistent with product formation of type I protein arginine methyltransferases [1].
Fig. 1.
Detection of asymmetric dimethylarginine and monomethylarginine residues in yeast Rps2. Amino acid analysis of the [3H]methylated ∼27 kDa polypeptide (Rps2) of the S. cerevisiae small ribosomal subunit. Cells (BY4742, RMT1+) were labeled in vivo with [3H]AdoMet and small ribosomal subunit proteins were isolated as described in Materials and methods. These proteins were fractionated by SDS gel electrophoresis as described in Materials and methods for 35 mA for 2.5 h, and then at 20 mA for 5 h. After Coomassie-staining, the band under the 31 kDa marker was excised, minced, and placed in a 6 × 50 mm flint glass tube. Acid hydrolysis and amino acid fractionation by ion exchange chromatography were performed as described in [16] with added standards of 1 μmol each of epsilon-dimethyllysine, NG,NG-dimethylarginine, and NG-monomethylarginine. The open circles show radioactivity from 200 μl of 1 min (1 ml) fractions collected. The closed circles show the absorbance at 570 nm of ninhydrin assays of 100 μl of the fractions for the added methylated amino acid standards. In this high-resolution chromatography, the tritiated methylated derivatives elute slightly before the non-isotopically labeled standards [19].
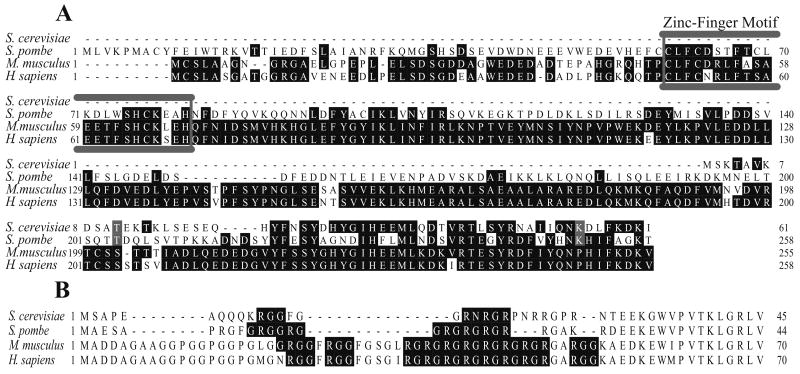
In S. pombe and mammalian cells, Rps2 homologs are substrates of a specific type I protein arginine methyltransferase (Rmt3 and PRMT3) that is characterized by an N-terminal domain including a zinc finger motif [9, 10, 18]. However, S. cerevisiae does not have such a protein arginine methyltransferase; the closest homolog is the Rmt1 species that is most similar to Rmt1 in S. pombe and PRMT1 in mammals (Fig. 2A). In spite of this difference, all of the Rps2 proteins of these organisms are characterized by N-terminal domains containing the canonical GRGG, RXR, and RGG recognition sequences for type I protein arginine methyltransferases, a group that includes both PRMT1 and PRMT3 (Fig. 2B).
Fig. 2.
Amino acid sequence conservation of Rmt1/Rmt3/PRMT3 and Rps2. (A) Aligned sequences for the N-terminal region of Rmt1 (S. cerevisiae, NP_009590), Rmt3 (S. pombe, NP_595572) and PRMT3 (M. musculus, NP_598501; H. sapiens, NP_005779). Identical residues are shaded, and the N-terminal zinc-finger motif is boxed in grey. (B) Aligned sequences for the N-terminus of Rps2 homologs, showing the RG-rich region (S. cerevisiae, NP_011392; S. pombe, NP_588435; M. musculus, NP_032529; H. sapiens, NP_002943). Protein arginine methyltransferase recognition motifs are shaded, including GRGG, RGG, and RXR [4, 34, 35].
Loss of methylation of yeast Rps2 upon deletion of the RMT1 gene
Although the S. cerevisiae genome does not encode a PRMT3 homolog, there are three known protein arginine methyltransferases in S. cerevisiae: Rmt1 (also known as Hmt1), Rmt2, and Hsl7. Of these enzymes, only Rmt1 has been shown to catalyze the formation of ADMA [8, 19, 20]. While, like PRMT3, these proteins all contain the conserved 7-β sheet AdoMet binding domain of Class I methyltransferases, none contain the zinc finger domain that typifies PRMT3 homologs and has been shown to be required for PRMT3's enzymatic activity [9, 11, 18]. We began by testing knockout strains of all three S. cerevisiae arginine methyltransferases and screening by intact mass spectrometry to determine if any showed a loss of methylation for Rps2 (Fig. 3).
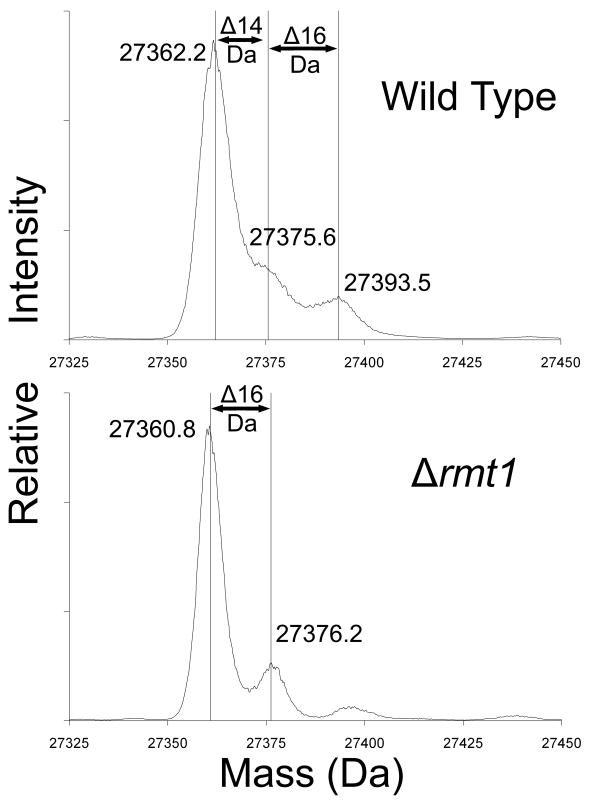
Fig. 3.
Loss of methylation of Rps2 in yeast cells lacking the Rmt1 protein arginine methyltransferase. Small subunits were isolated from the ribosomal fraction of RMT1+ cells (wild type) and of Δrmt1- cells prepared as described in Methods and materials but in 1 l of growth media without the [3H]AdoMet incubation. Due to greater cell pellet volumes, 4.5 ml buffer A was used to lyse each cell pellet and ribosomal pellets were individually re-suspended in 1 ml buffer B. An abbreviated gradient of 7%-27% sucrose made in buffer B was centrifuged for 16 h under the same conditions noted in Materials and methods. Small subunit proteins were prepared as described in Materials and methods except that dialysis was not performed and the sample supernatants after ethanol and acetic acid treatment were directly concentrated by vacuum centrifugation for about 35 min to bring the total volumes to approximately 100 μl. A final centrifugation of 20,000 × g for 5 min removed any particulate matter from solution, and 30 μl of each concentrated sample was analyzed by liquid chromatography/mass spectrometry using an Applied Biosystems Q-Star Elite instrument as described in Materials and methods. Data for the peak at 51 min containing Rps2 were de-convoluted using Analyst software and the resulting average masses of significant peaks are shown. Mass changes are indicated for 14 Da (methylation) as well as 16 Da (oxidation).
For Rps2 from wild type cells, the major peak at 27,362.2 Da corresponds to that of the unmodified protein lacking the initiator methionine residue and acetylated on the serine-2 residue as expected [21]. The calculated average mass is 27362.9; our experimental value is within 26 ppm, well within the expected error of 100 ppm. We also detected secondary peaks with masses that are about 13.4 and 31.3 Da larger that could correspond to the presence of substoichiometric levels of mono and dimethylated species (Fig. 3, top panel). The de-convoluted mass profile for Rps2 from cells lacking either the Rmt2 or the Hsl7 protein arginine methyltransferases was not different from that of the wild type cells shown in Fig. 3 (data not shown). However, for Rps2 purified from cells lacking the Rmt1 methyltransferase, we see a reduction in both of the secondary peaks (Fig. 3, bottom panel). These results suggest that Rmt1 catalyzes the methylation of Rps2; the presence of a species from the protein lacking Rmt1 at 16 Da greater than the N-acetylated serine form suggests the presence of an oxidized species. Some of the signal in the secondary species in the wild type sample may also reflect the presence of some oxidized material.
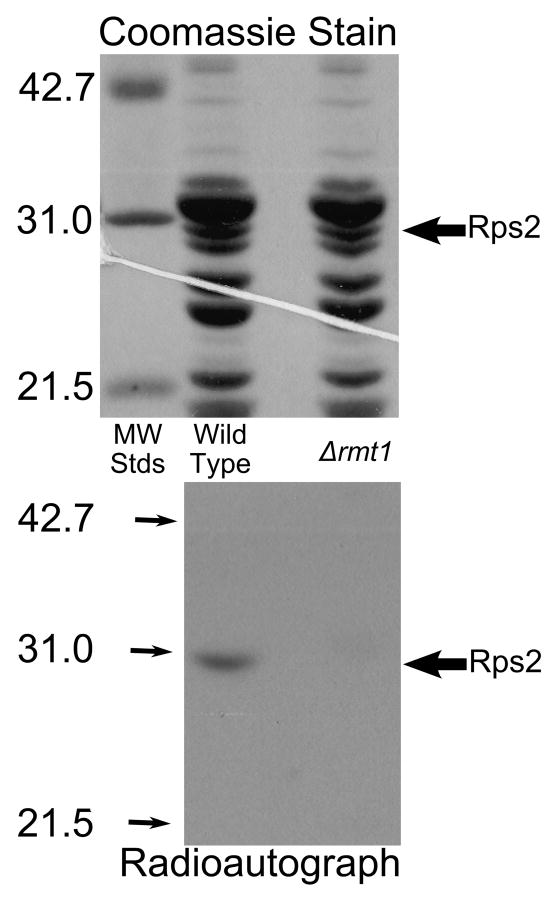
To confirm the identification of Rmt1 as the methyltransferase responsible for the modification of Rps2, we in vivo labeled both wild type and Δrmt1 strains to test whether Rmt1 was necessary for [3H]methyl addition to Rps2. Ribosomes were isolated by centrifugation, subunits separated by high salt sucrose gradient sedimentation, and the small subunit proteins pooled for each sample and further separated by SDS-PAGE. A protein band corresponding to Rps2 can be seen in both wild type and Δrmt1 samples (Fig. 4, top panel). However, the radioautograph clearly shows the loss of [3H]methylation of Rps2 in the Δrmt1 strain (Fig. 4, lower panel). This provides further evidence that Rps2 methylation is dependent upon the methyltransferase Rmt1 in S. cerevisiae.
Fig. 4.
Loss of methylation of Rps2 in Δrmt1 mutant cells. Intact cells were labeled with [3H]AdoMet as described in Materials and methods except that cells were grown initially in 1 l of medium and labeled with 2.0 nmol of [3H]AdoMet (Perkin Elmer Life Sciences, 83.3 Ci/mmol) in 40 ml of fresh YPD and the resulting cell pellets were lysed individually using 2 g beads and 4.5 ml buffer A for a total of ten cycles. Small ribosomal subunits were prepared by sucrose gradient centrifugation and the protein fraction isolated by ethanol/acetic acid treatment as described in Materials and methods. 50 μg of small subunit proteins were separated by SDS gel electrophoresis for 35 mA for 40 min and then 25 mA for 5 h with molecular weight standards (Bio-Rad Low Molecular Weight; hen ovalbumin, bovine carbonic anhydrase, and soybean trypsin inhibitor) in an adjoining lane. After protein staining (top panel), the gel was treated in EN3HANCE (Perkin Elmer Life Sciences) for 1 h, followed by a 20 min wash in water before being dried under vacuum for 2 h at 80° C followed by 1 h without heat. The gel was placed on Kodak BIOMAX XAR film for 3 months at -80° C (bottom panel). Large arrows indicate the position of the Rps2 band; small arrows indicate the position of the standards in the radioautograph.
Discussion
Protein arginine methylation of Rps2 in the fission yeast S. pombe and mammalian cells has been closely linked to a zinc-finger containing enzymes designated Rmt3 and PRMT3 [9-14]. The zinc-finger of the methyltransferase appears to be essential for the modification of Rps2 in mammalian cells [9]. We were thus surprised to see Rps2 protein arginine methylation in the budding yeast S. cerevisiae that lacks this member of the protein arginine methyltransferase family. We have shown here that the methylation of Rps2 in S. cerevisiae is catalyzed by the major Rmt1 enzyme which does not contain a zinc-finger.
Like many other ribosomal proteins, Rps2 has been implicated as playing roles in the ribosomal biosynthesis [22, 23]. Recent studies in S. pombe have indicated that Rps2 is essential for the proper processing of the A2 cleavage event that leads to the 18S rRNA [22]. In the absence of Rps2, synthesis of the 40S subunit is abolished [22]. Evidence has been presented that interaction of Rps2 in S. pombe with the Rmt3 protein arginine methyltransferase modulates the efficiency of 40S synthesis, as indicated by the altered 40S:60S ratio seen in cells lacking Rmt3 [10-12].
Studies of the role of Rps2 in rRNA processing in S. cerevisiae have shown that a depletion of Rps2 leads to export defects of the 20S rRNA [23]. This is interesting in light of the fact that Rmt1 has been shown to play a role in facilitating export for a number of its previously determined substrates, such as the mRNA-binding protein Npl3 [24, 25]. Thus methylation of Rps2 in S. cerevisiae may play a role in the export to the cytosol of ribosomal RNA species. Significantly, we have found that the methylation of Rps2 at arginine residues is substochiometric; the bulk of the protein is unmodified. Previous results have suggested that RNA binding may impede the ability of Rmt1 to methylate its substrates [26, 27]. We have observed varying levels of methylation of Rps2 dependent upon growth conditions (unpublished data), which may support a functional regulatory role for this modification. Due to the nuclear localization of at least a fraction of Rmt1 [28], it seems possible that Rps2 methylation occurs in the nucleus. This is distinct from the situation with S. pombe and mammalian cells, where the zinc-finger containing enzymes Rmt3 and PRMT3 appear to be wholly cytosolic. In these organisms, the modification of Rps2 may occur in the cytosol before it is imported into the nucleolus for its role in ribosomal assembly. The degree of modification of rpS2 in mammalian cells appears to be stoichiometric because protein purified from wild type cells is not a substrate for methylation by recombinant PRMT3 [13]. Additionally, mass spectrometric analysis suggests that the formation of eight dimethylarginine residues in Rps2 of S. pombe [11].
These results provide evidence that protein arginine methylation of Rps2 in budding yeast may have a distinct function than that in fission yeast or mammalian cells [22]. In mammalian cells, it has been suggested that PRMT3 and Rps2 form a complex together that leads to resistance to ubiquitination and degradation [14]. This does not appear to be the case in S. cerevisiae, since the protein is largely unmodified. In S. pombe, it has been suggested that there is a methylation-independent function of Rmt3 in regulating levels of 40S [11].
Rmt1 has been most extensively studied in terms of its role in transcription, rRNA processing, and mRNA export from the nucleus. The arginine-3 residue of histone H4 is methylated by Rmt1 leading to transcription repression [29, 30]. However, Rmt1 is also recruited to areas of high transcriptional activity for methylation of mRNA proteins involved in mRNA processing and export [31]. Rmt1 has also been demonstrated to methylate the nucleolar proteins Gar1, Nop1, and Nsr1 [32]. Although the functions of the modifications are unknown, these three proteins have previously been connected to rRNA processing. A number of the target proteins of Rmt1, including Npl3, Hrp1, and Nab2, function in shuttling mRNA to the cytosol. The methylation of these proteins by Rmt1 appears to promote their dissociation from nuclear factors and facilitate export from the nucleus [24, 25, 33]. Like with other Rmt1 substrates, it will be interesting to explore in the future whether methylation of Rps2 affects the protein-protein interactions that facilitate import and/or export of this RNA binding protein.
Acknowledgments
This work was funded by NIH Grant GM026020. R.S.L. was supported by the UCLA Cellular and Molecular Biology Training Program funded by NIH Grant GM007185. Mass spectrometry was performed in the UCLA Molecular Instrumentation Center supported by Grant S10RR024605 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachand F. Protein arginine methyltransferases: from unicellular eukaryotes to humans. Eukaryot Cell. 2007;6:889–898. doi: 10.1128/EC.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YH, Stallcup MR. Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation. Mol Endocrinol. 2009;23:425–433. doi: 10.1210/me.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation, Prog. Nucleic. Acid Res. Mol Biol. 1998;61:65–131. doi: 10.1016/s0079-6603(08)60825-9. [DOI] [PubMed] [Google Scholar]
- 5.McBride AE, Zurita-Lopez C, Regis A, Blum E, Conboy A, Elf S, Clarke S. Protein arginine methylation in Candida albicans: role in nuclear transport. Eukaryot Cell. 2007;6:1119–1129. doi: 10.1128/EC.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polevoda B, Sherman F. Methylation of proteins involved in translation. Mol Micro. 2007;65:590–606. doi: 10.1111/j.1365-2958.2007.05831.x. [DOI] [PubMed] [Google Scholar]
- 7.Chern MK, Chang KN, Liu LF, Tam TC, Liu YC, Liang YL, Tam MF. Yeast ribosomal protein L12 is a substrate of protein-arginine methyltransferase 2. J Biol Chem. 2002;277:15345–15353. doi: 10.1074/jbc.M111379200. [DOI] [PubMed] [Google Scholar]
- 8.Zobel-Thropp P, Gary JD, Clarke S. delta-N-methylarginine is a novel posttranslational modification of arginine residues in yeast proteins. J Biol Chem. 1998;273:29283–29286. doi: 10.1074/jbc.273.45.29283. [DOI] [PubMed] [Google Scholar]
- 9.Swiercz R, Person MD, Bedford MT. Ribosomal protein S2 is a substrate for mammalian PRMT3 (protein arginine methyltransferase 3) Biochem J. 2005;386:85–91. doi: 10.1042/BJ20041466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachand F, Silver PA. PRMT3 is a ribosomal protein methyltransferase that affects the cellular levels of ribosomal subunits. EMBO. 2004;23:2641–2650. doi: 10.1038/sj.emboj.7600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perreault A, Gascon S, D'Amours A, Aletta JM, Bachand F. A methyltransferase-independent function for Rmt3 in ribosomal subunit homeostasis. J Biol Chem. 2009;284:15026–15037. doi: 10.1074/jbc.M109.004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachand F, Lackner DH, Bähler J, Silver PA. Autoregulation of ribosome biosynthesis by a translational response in fission yeast. Mol Cell Bio. 2006;26:1731–1742. doi: 10.1128/MCB.26.5.1731-1742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swiercz R, Cheng D, Kim D, Bedford MT. Ribosomal protein rpS2 is hypomethylated in PRMT3-deficient mice. J Biol Chem. 2007;282:16917–16923. doi: 10.1074/jbc.M609778200. [DOI] [PubMed] [Google Scholar]
- 14.Choi S, Jung C, Kim J, Im D. PRMT3 inhibits ubiquitination of ribosomal protein S2 and together forms an active enzyme complex. Biochem Biophs Act. 2008;1780:1062–1069. doi: 10.1016/j.bbagen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Porras-Yakushi TR, Whitelegge JP, Miranda TB, Clarke S. A novel SET domain methyltransferase modifies ribosomal protein Rpl23ab in yeast. J Biol Chem. 2005;280:34590–34598. doi: 10.1074/jbc.M507672200. [DOI] [PubMed] [Google Scholar]
- 16.Miranda TB, Lowenson JD, Clarke S. A new type of protein methylation activated by tyrphostin A25 and vanadate. FEBS Let. 2004;577:181–186. doi: 10.1016/j.febslet.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 17.Webb KJ, Laganowsky A, Whitelegge JP, Clarke SG. Identification of two SET domain proteins required for methylation of lysine residues in yeast ribosomal protein Rpl42ab. J Biol Chem. 2008;283:35561–35568. doi: 10.1074/jbc.M806006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel A, Clarke S. PRMT3 is a distinct member of the protein arginine N-methyltransferase family. Conferral of substrate specificity by a zinc-finger domain. J Biol Chem. 2000;275:32974–32982. doi: 10.1074/jbc.M006445200. [DOI] [PubMed] [Google Scholar]
- 19.Gary JD, Lin WJ, Yang MC, Herschman HR, Clarke S. The predominant protein-arginine methyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1996;271:12585–12594. doi: 10.1074/jbc.271.21.12585. [DOI] [PubMed] [Google Scholar]
- 20.Sayegh J, Clarke SG. Hsl7 is a substrate-specific type II protein arginine methyltransferase in yeast. Biochem Biophys Res Commun. 2008;372:811–815. doi: 10.1016/j.bbrc.2008.05.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325:595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 22.Perreault A, Bellemer C, Bachand F. Nuclear export competence of pre-40S subunits in fission yeast requires the ribosomal protein Rps2. Nucleic Acids Res. 2008;36:6132–6142. doi: 10.1093/nar/gkn625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira-Cerca S, Pöll G, Gleizes PE, Tschochner H, Milkereit P. Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol Cell. 2005;20:263–275. doi: 10.1016/j.molcel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Shen EC, Henry MF, Weiss VH, Valentini SR, Silver PA, Lee MS. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev. 1998;12:679–691. doi: 10.1101/gad.12.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride AE, Cook JT, Stemmler EA, Rutledge KL, McGrath KA, Rubens JA. Arginine methylation of yeast mRNA-binding protein Npl3 directly affects its function, nuclear export, and intranuclear protein interactions. J Biol Chem. 2005;280:30888–30898. doi: 10.1074/jbc.M505831200. [DOI] [PubMed] [Google Scholar]
- 26.Frankel A, Clarke S. RNase treatment of yeast and mammalian cell extracts affects in vitro substrate methylation by type I protein arginine N-methyltransferases. Biochem Biophys Res Commun. 1999;259:391–400. doi: 10.1006/bbrc.1999.0779. [DOI] [PubMed] [Google Scholar]
- 27.Valentini SR, Weiss VH, Silver PA. Arginine methylation and binding of Hrp1p to the efficiency element for mRNA 3′-end formation. RNA. 1999;5:272–280. doi: 10.1017/s1355838299981633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry MF, Silver PA. A novel methyltransferase (Hmt1p) modifies poly(A)+-RNA-binding proteins. Mol Cell Biol. 1996;16:3668–3678. doi: 10.1128/mcb.16.7.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacoste N, Utley RT, Hunter JM, Poirier GG, Côte J. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J Biol Chem. 2002;277:30421–30424. doi: 10.1074/jbc.C200366200. [DOI] [PubMed] [Google Scholar]
- 30.Kuo MH, Xu XJ, Bolck HA, Guo D. Functional connection between histone acetyltransferase Gcn5p and methyltransferase Hmt1p. Biochim Biophys Acta. 2009;1789:395–402. doi: 10.1016/j.bbagrm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu MC, Bachand F, McBride AE, Komili S, Casolari JM, Silver PA. Arginine methyltransferase affects interactions and recruitment of mRNA processing and export factors. Genes Dev. 2004;18:2024–2035. doi: 10.1101/gad.1223204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Henry PA, Setya A, Henry MF. In vivo analysis of nucleolar proteins modified by the yeast arginine methyltransferase Hmt1/Rmt1p. RNA. 2003;9:746–759. doi: 10.1261/rna.5020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J Biol Chem. 2002;277:7752–7760. doi: 10.1074/jbc.M110053200. [DOI] [PubMed] [Google Scholar]
- 34.Smith JJ, Rücknagel KP, Schierhorn A, Tang J, Nemeth A, Linder M, Herschman HR, Wahle E. Unusual sites of arginine methylation in Poly(A)-binding protein II and in vitro methylation by protein arginine methyltransferases PRMT1 and PRMT3. J Biol Chem. 1999;274:13229–13234. doi: 10.1074/jbc.274.19.13229. [DOI] [PubMed] [Google Scholar]
- 35.Pahlich S, Bschir K, Chiavi C, Belyanskaya L, Gehring H. Different methylation characteristics of protein arginine methyltransferase 1 and 3 toward the Ewing Sarcoma protein and a peptide. Proteins. 2005;61:164–175. doi: 10.1002/prot.20579. [DOI] [PubMed] [Google Scholar]