Abstract
Heart failure (HF) with preserved left ventricular ejection fraction (LVEF) and diabetes commonly co-exist, but the impact of diabetes on HF outcomes in individuals with HF and preserved LVEF has not been well studied. We assessed the risk of HF death or hospitalization for worsening HF associated with diabetes by studying 987 patients with HF and preserved LVEF enrolled in the Digitalis Investigation Group (DIG) ancillary study. Diabetic individuals (n=285, 28.9%) were younger, had a larger body mass index, faster heart rate, and higher pulse pressure than non-diabetic individuals. Diabetic individuals were also more likely to be female, have a prior history of hypertension, ischemic etiology for HF, and were more likely to be treated with diuretics. During the mean follow-up of 37 months, 88 (30.9%) diabetic individuals and 133 (19.0%) non-diabetic individuals experienced the primary outcome of HF hospitalization or HF death. After adjustments for baseline differences, diabetes was associated with a 68% increased risk of HF hospitalization or HF death (adjusted HR 1.68, 95% CI 1.26 to 2.25, p<0.001). In conclusion, in individuals with HF and preserved LVEF, diabetes is associated with significantly increased risk of developing adverse HF outcomes.
Keywords: Diabetes, Diastolic Heart Failure, Prognosis
Diabetes mellitus is an important co-morbidity likely contributing to adverse heart failure (HF) outcomes in patients with HF and preserved left ventricular ejection fraction (LVEF). The prevalence of diabetes continues to increase in the general population1 and among community-based patients with HF.2 Although diabetes has been shown to be a predictor of adverse cardiovascular outcomes in patients with reduced LVEF,3 the impact of diabetes on adverse HF outcomes has not been well studied in individuals with HF and preserved LVEF. Given potential diabetes-associated effects on vascular compliance4 and LV systolic stiffness and potential effects on LV diastolic function,5 processes that have been implicated in the pathophysiology of HF with preserved LVEF, we hypothesized that diabetes would be associated with increased rates of hospitalization for worsening HF or HF death in this group of patients. Therefore, we examined the prognostic impact of diabetes on adverse HF outcomes in patients with preserved LVEF enrolled in the Digitalis Investigation Group (DIG) study.
METHODS
The database used for this study was a public-use copy of the Digitalis Investigation Group (DIG) study from the National Heart, Lung, and Blood Institute (Bethesda, Maryland). The design6 and primary analyses7 of the DIG study have been described in detail. Briefly, the DIG study tested the effects of effects of digoxin in mortality and hospitalization in patients with heart failure. The diagnosis of HF was based on current or past clinical symptoms, signs, or radiologic evidence of pulmonary congestion. Important exclusion criteria included recent myocardial infarction or unstable angina (less than 4 weeks from enrollment) and renal insufficiency (defined as a creatinine > 3.0 mg/dL). Patients were recruited from February 1991 through August 1993.7 Although the main results of the trial were published in the 6800 individuals with a LVEF ≤45%,7 the DIG study also included an ancillary trial of 988 individuals with HF and a LVEF >45%. The results of digoxin therapy on cardiovascular outcomes in the patients with HF and preserved LVEF of the DIG ancillary trial were recently published.8 Our analyses are limited to these individuals with HF and LVEF >45% who were enrolled in the DIG ancillary trial.
Baseline demographics were obtained at entry into the study. The classification of diabetes was based on a reported history of diabetes at study entry. Data on baseline diabetes were available in 987 participants; 1 individual did not have data available for the classification of diabetes and was excluded from the study. Diabetic treatment or classification between type 1 and type 2 diabetes was not available.
The primary outcome was time to hospitalization for worsening HF or HF death. Secondary outcomes included the individual outcomes of times to hospitalization for worsening HF, HF mortality, all-cause mortality, and cardiovascular mortality, and the time to the combined outcome of cardiovascular mortality and hospitalization for worsening HF. Differences in baseline variables were compared using chi-square tests for categorical variables and t-tests for continuous variables. Two-sided p-values < 0.05 were considered statistically significant. Univariate and multivariable Cox proportional hazards models were used to assess the relationship between diabetes and the outcomes of interest. Covariates included variables identified as important predictors of the combined outcome of HF death or hospitalization for worsening HF, and variables with p-values <0.10 by univariate analyses were entered into the multivariable model. The final model included age, gender, LVEF, cardiothoracic ratio, heart rate, diastolic blood pressure, brachial pulse pressure, number of signs or symptoms of HF, New York Heart Association (NYHA) classification, history of myocardial infarction, ischemic heart failure etiology, previous digoxin use, non-potassium sparing diuretic use, glomerular filtration rate (GFR) and body mass index (BMI). GFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study Group equation.9 Brachial pulse pressure was calculated as the difference between the brachial systolic and diastolic blood pressure. Tests for interaction between diabetes and subgroups (gender, age, ischemic etiology of HF, obesity, and NYHA classification) were performed by adding a cross-product term of these variables on the combined primary outcome of HF death or hospitalization for worsening HF; the test for a statistical interaction between diabetes and subgroup were performed prior to other variables being entered into the model. Statistical analysis was performed using STATA 9.2 (College Station, Texas).
RESULTS
Of the total 987 HF individuals with a LVEF >45% included in this analysis, 285 (28.9%) individuals had a documented history of diabetes. The baseline characteristics according to diabetic status are demonstrated in Table 1. At baseline, diabetic individuals were younger and were more likely to be women, have a prior history of hypertension, have ischemic etiology for HF and were more likely to be treated with diuretics and nitrates. Diabetic individuals also had a larger BMI, faster heart rate, had a higher systolic blood pressure and pulse pressure, and were more likely to have peripheral edema and radiologic evidence of pulmonary congestion than non-diabetic individuals at baseline.
Table 1. Baseline Characteristics by Diabetic Status.
| Variable | Diabetes Mellitus |
p-value | |
|---|---|---|---|
| Yes (n=285) |
No (n=702) |
||
| Age (years) | 64.8 ± 9.8 | 67.6 ± 10.4 | <0.001 |
| Female | 48.8 % | 38.0 % | 0.002 |
| Non-white | 16.5 % | 12.8 % | 0.13 |
| Body mass index (kg/m2) | 30.8 ± 7.3 | 27.7 ± 5.5 | <0.001 |
| Left ventricular ejection fraction (%) | 55.1 ± 7.9 | 55.6 ± 8.2 | 0.37 |
| Heart Rate (beats per minute) | 77.7 ± 12.4 | 75.0 ± 11.7 | 0.002 |
| Systolic blood pressure (mm Hg) | 141.4 ± 21.8 | 136.0 ± 20.9 | <0.001 |
| Diastolic blood pressure (mm Hg) | 76.5 ± 12.0 | 77.0 ± 11.1 | 0.58 |
| Brachial pulse pressure (mm Hg) | 64.8 ± 18.5 | 59.0 ± 18.1 | <0.001 |
| Signs and/or symptoms of HF | |||
| Rales | 74.7 % | 73.2 % | 0.62 |
| Elevated jugular venous pressure | 50.5 % | 46.3 % | 0. 23 |
| Peripheral edema | 69.5 % | 55.0 % | <0.001 |
| S3 | 31.9 % | 33.5 % | 0.64 |
| Radiologic evidence of pulmonary congestion |
68.4 % | 60.0 % | 0.01 |
| Cardiothoracic ratio | 0.52 ± 0.08 | 0.52 ± 0.08 | 0.50 |
| Estimated glomerular filtration rate (ml/minute/1.73m2) |
60.0 ± 20.8 | 62.4 ± 20.4 | 0.09 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 4.3 ± 0.5 | 0.007 |
| Ischemic heart failure etiology | 64.6 % | 53.1 % | 0.001 |
| New York Heart Association Class | 24.2 % | 21.1 % | 0.29 |
| III-IV | |||
| Prior myocardial infarction | 54.4 % | 47.6 % | 0.05 |
| Current angina | 33.7 % | 28.2 % | 0.09 |
| Hypertension | 70.9 % | 55.1 % | <0.001 |
| Previous digoxin use | 32.0 % | 36.6 % | 0.17 |
| Medication | |||
| Non-potassium sparing diuretic | 81.8 % | 73.7 % | 0.007 |
| Potassium-sparing diuretic | 7.4 % | 8.3 % | 0.64 |
| Angiotensin converting enzyme. inhibitors |
88.8 % | 85.2 % | 0.14 |
| Nitrates | 44.6 % | 37.3 % | 0.04 |
| Digoxin | 47.4 % | 50.7 % | 0.34 |
Continuous variables are presented as means ± standard deviations; categorical variables presented as percentages.
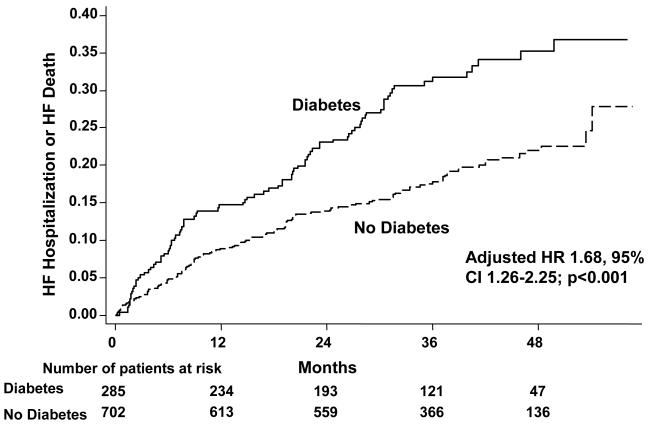
During the mean follow-up of 37 months, diabetic individuals were more likely than non-diabetic individuals to experience the primary outcome of HF mortality or hospitalization for worsening HF [unadjusted hazard ratio (HR) 1.79, 95% CI 1.37 to 2.35; p<0.001] (Table 2, Figure 1). After adjusting for potential confounders, including age, gender, LVEF, cardiothoracic ratio, heart rate, diastolic blood pressure, brachial pulse pressure, number of signs or symptoms of HF, NYHA classification, history of myocardial infarction, ischemic heart failure etiology, previous digoxin use, non-potassium sparing diuretic use, GFR, and BMI, the adjusted hazard ratio for the combined primary outcome of HF death or hospitalization for worsening HF was 1.68 (95% CI 1.26 to 2.25, p<0.001).
Table 2. Cardiovascular Outcomes by Diabetic Status.
| Variable | Diabetes Mellitus |
|
|---|---|---|
| Yes (n=285) |
No (n=702) |
|
| Heart Failure Death or Hospitalization for Worsening HF |
88 (30.9%) | 133 (19.0%) |
| Unadjusted HR (95% CI) | 1.79 (1.37, 2.35, p<0.001) | Reference |
| Adjusted HR (95% CI)† | 1.68 (1.26, 2.25, p<0.001) | Reference |
| Hospitalization for Worsening Heart Failure | 81 (28.4%) | 116 (16.5%) |
| Unadjusted HR (95% CI) | 1.88 (1.42, 2.50, p<0.001) | Reference |
| Adjusted HR (95% CI) | 1.76 (1.30, 2.39, p<0.001) | Reference |
| Heart Failure Death | 21 (7.4 %) | 43 (6.1 %) |
| Unadjusted HR (95% CI) | 1.26 (0.75, 2.12, p=0.39) | Reference |
| Adjusted HR (95% CI) | 1.40 (0.78, 2.50, p=0.26) | Reference |
| Total Mortality | 81 (28.4 %) | 150 (21.4%) |
| Unadjusted HR (95% CI) | 1.38 (1.06, 1.81, p=0.02) | Reference |
| Adjusted HR (95% CI) | 1.48 (1.10, 1.99, p=0.009) | Reference |
| Cardiovascular Mortality | 58 (20.3%) | 104 (14.8%) |
| Unadjusted HR (95% CI) | 1.43 (1.03, 1.97, p=0.03) | Reference |
| Adjusted HR (95% CI) | 1.54 (1.08, 2.18, p=0.02) | Reference |
| Cardiovascular Mortality or Hospitalization for Worsening Heart Failure |
116 (40.7%) | 180 (25.6%) |
| Unadjusted HR (95% CI) | 1.76 (1.39, 2.22, p<0.001) | Reference |
| Adjusted HR (95% CI) | 1.69 (1.32, 2.17, p<0.001) | Reference |
Model includes age, gender, left ventricular ejection fraction, cardiothoracic ratio, heart rate, diastolic blood pressure, brachial pulse pressure, number of signs or symptoms of heart failure, New York Heart Association classification, history of myocardial infarction, ischemic heart failure etiology, previous digoxin use, non-potassium sparing diuretic use, glomerular filtration rate and body-mass index.
Figure 1.
Kaplan-Meier event curves for the combined outcome of HF death or hospitalization for worsening HF by diabetic status. p-value (log-rank) <0.001
Other cardiovascular endpoints are shown in Table 2.
To assess the potential confounder of interim myocardial infarction on HF outcomes, analyses were performed excluding patients (n=54) who were hospitalized for acute myocardial infarction during the follow-up period. After exclusion of these individuals, the hazard of the primary outcome of HF mortality or hospitalization for worsening HF remained higher in diabetic individuals compared with non-diabetic individuals (adjusted HR 1.63, 95% CI 1.21 to 2.21, p=0.002).
Hospitalization for worsening HF was also markedly increased in diabetic individuals compared with non-diabetic individuals (adjusted HR 1.76, 95% CI 1.30 to 2.39, p<0.001). In addition, hospitalization admissions for cardiovascular causes occurred in 155 (54.4%) diabetic individuals and 311 (44.3%) non-diabetic individuals (p<0.01).
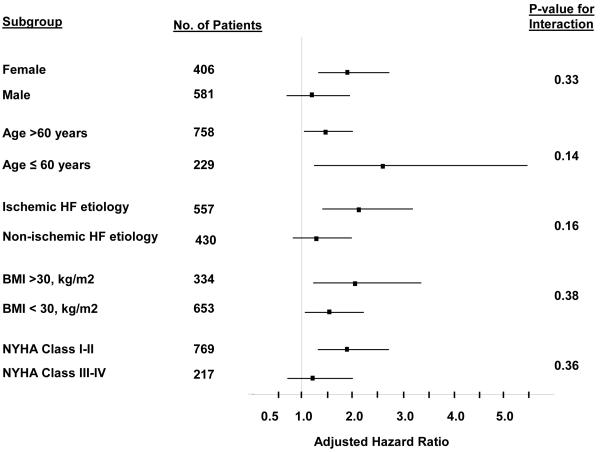
Further exploratory analyses were also performed examining the potential interaction of diabetes and gender on the combined primary outcome of HF death or hospitalization for worsening HF. The prevalence of diabetes was higher in women than in men (34.2% vs. 25.1%, respectively, p<0.001). Of the 406 women enrolled in the study, 56 (40.3 %) diabetic women and 64 (24.0%) non-diabetic women experienced the primary outcome of HF death or HF hospitalization (adjusted HR 1.94, 95% CI 1.31 to 2.87, p<0.01). Similarly, in the 581 men in the study, 32 (21.9%) diabetic men and 69 (15.9%) non-diabetic men experienced the primary outcome of HF death or HF hospitalization (adjusted 1.37, 95% CI 0.87 to 2.15, p=0.18). Although the hazard ratio associated with diabetes was larger in women than in men, the interaction of gender and diabetes was not statically significant (p-value for interaction=0.33). Additional exploratory subgroup analyses (figure 2) did not demonstrate a statistical interaction between diabetes and the subgroups of age greater than 60 years, ischemic HF etiology, obesity, and NYHA classification.
Figure 2.
Effect of diabetes on the adjusted hazard ratios for the outcome of HF death or HF hospitalization in selected subgroups.
DISCUSSION
Previous studies have established diabetes as a risk factor for the development of adverse events in individuals with reduced LVEF,3 however, only one prior study has examined the prognostic impact of diabetes on cardiovascular outcomes in patients with HF and preserved LVEF.10 In this study of individuals with HF and preserved LVEF enrolled in the DIG ancillary study, we demonstrate that diabetes was present in approximately 30% of individuals. At baseline, individuals with diabetes had increased rates of peripheral edema, radiologic evidence of pulmonary congestion, and diuretic use supporting the hypothesis that diabetic patients may be more prone to problems of congestion and volume retention. Importantly, the presence of diabetes was associated with significantly increased risk of hospitalization for worsening HF or death attributed to HF. The increased morbidity and mortality persisted even after adjustments for potential confounders such as hypertension, obesity, renal insufficiency, and ischemic heart disease. Although a previous analysis of this cohort has demonstrated that diabetes is an independent risk factor for mortality,11 our study adds to the existing body of literature regarding HF with preserved LVEF by demonstrating that diabetes is associated with a nearly 70% increased hazard of HF death or hospitalization for worsening HF. This hazard is similar in magnitude to a recent analysis of the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program which demonstrated that the presence of diabetes was associated with a doubling in risk of cardiovascular death or HF hospitalization.10
There are several mechanisms through which diabetes may be contributing to the pathophysiology and adverse outcomes in patients with HF and preserved LVEF. Associated co-morbid conditions and complications such as hypertension, accelerated atherosclerosis, and renal insufficiency are likely contributing to the adverse outcomes, but the increased hazard seen with diabetes persists despite adjusting for these comorbidities. One important mechanism linking diabetes and HF with preserved LVEF may be increased arterial load in individuals with diabetes and subsequent matched increases in LV systolic stiffness (ventricular-arterial coupling).12-16 Although ventricular-arterial coupling appears beneficial in maintaining optimal cardiovascular performance,13,14 marked increases of arterial load matched with increased LV systolic stiffness may have detrimental cardiovascular effects, including increased sensitivity to volume shifts and decreased exercise capacity.12 This process may be particularly relevant to individuals with diabetes, as studies have suggested that diabetes results in increased arterial stiffness,4 a process likely related to effects of advanced glycation end products (AGEs). The higher brachial pulse pressure, an indirect surrogate of vascular compliance, in diabetic participants of this analysis is consistent with a potential pathogenic role of increased vascular stiffness.
In addition to abnormalities of LV systolic stiffness and arterial stiffness, abnormalities of LV diastolic function (impaired LV relaxation and increased passive LV stiffness) are also likely contributing to the greater HF burden in diabetic patients with HF and preserved LVEF. Previous non-invasive studies of diabetic patients without HF have demonstrated that abnormalities of diastolic function are common and are independent of LV mass, systolic function, hypertension, or other comorbidities.5 Potential mechanisms contributing to increased diastolic abnormalities in diabetic patients include abnormalities of myocardial fibrosis,17 direct and indirect effects of AGEs,18 abnormal myocardial metabolism,19 and increased arterial stiffness.20 Importantly, increased abnormalities of LV diastolic function have been shown to play an important role in the pathophysiology of HF with preserved LVEF.16,21,22
Several limitations of this exploratory study should be noted. Participants for the DIG ancillary trial were recruited from 1991-1993. Although the prevalence of diabetes in this clinical trial was approximately 29% and was similar to a recent analysis from the CHARM study 10, the prevalence of diabetes has continued to increase among community HF patients over time,2 and prevalence estimates from a clinical trial may underestimate the true prevalence of diabetes in individuals with HF and preserved LVEF in the general community. Indeed, in a contemporary registry of patients presenting with acute decompensated HF and preserved LVEF, the prevalence of diabetes was 45%.23 In addition, glycometabolic measures, such as glucose or glycosylated hemoglobin, or specific diabetic therapy were not available. Thus, the degree of glycemic control in the diabetic population in the DIG trial could not be assessed. Finally, the rates of beta-blocker use were not available in this study.
Acknowledgement
The Digitalis Investigation Group (DIG) study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the Digitalis Investigation Group (DIG) Investigators. This manuscript was not prepared in collaboration with investigators of the Digitalis Investigation Group (DIG) and does not necessarily reflect the opinions or views of the Digitalis Investigation Group (DIG) or the National Heart, Lung, and Blood Institute.
This study was supported in part by NIH Mentored Career Development Award (5K01-HL092585-02) to Dr. David Aguilar.
Footnotes
No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 3.De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Van Belle E, Bauters C. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 5.Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–1949. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 6.Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 7.The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr., Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 11.Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. J Am Coll Cardiol. 2004;44:1025–1029. doi: 10.1016/j.jacc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 13.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264:H1817–1824. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 19.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 20.Sharman JE, Haluska BA, Fang ZY, Prins JB, Marwick TH. Association of arterial wave properties and diastolic dysfunction in patients with type 2 diabetes mellitus. Am J Cardiol. 2007;99:844–888. doi: 10.1016/j.amjcard.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 21.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 22.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]