Abstract
Purpose
To assess for activation of the unfolded protein response in corneal endothelium of Fuchs endothelial corneal dystrophy patients.
Design
Retrospective comparative case series of laboratory specimens
Methods
Corneal specimens of patients with Fuchs dystrophy and controls with corneal pathologies other than Fuchs dystrophy were evaluated by transmission electron microscopy (TEM) to evaluate for structural changes of the rough endoplasmic reticulum in corneal endothelium. TEM images were evaluated for alterations of rough endoplasmic reticulum as sign of unfolded protein response. Normal autopsy eyes, Fuchs dystrophy, and keratoconus corneas were used for immunohistochemistry. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections of patient corneas for three unfolded protein response markers (GRP78, phospho-eIF2α, CHOP) and two apoptosis markers (Caspase 3 and 9). Immunohistochemistry signal quantitation of corneal endothelium for evaluation of marker expression was performed using automated software. Corneal sections were assessed quantitatively for levels of immunohistochemistry marker expression.
Results
TEM showed enlargement of rough endoplasmic reticulum in corneal endothelium of all Fuchs dystrophy specimens. Immunohistochemistry quantitation demonstrated a significant increase in mean signal in corneal endothelium from Fuchs dystrophy patients for markers GRP78, phospho-eIF2α, CHOP and caspase 9, compared with non- Fuchs dystrophy corneas (p < 0.05).
Conclusions
Results of both TEM and immunohistochemistry indicate activation of unfolded protein response in Fuchs dystrophy. Unfolded protein response activation leads to endothelial cell apoptosis in Fuchs dystrophy and may play a central pathogenic role in this disease.
Introduction
Fuchs endothelial corneal dystrophy (Fuchs dystrophy) is a primary disease of the corneal endothelium characterized by loss of endothelial cells (CECs) and abnormalities of Descemet membrane. These pathologic changes lead progressively to corneal edema and loss of vision, occurring approximately 20 years after disease onset 1. Fuchs dystrophy has recently been characterized as having an early onset form with diagnosis as early as the first decade of life 2, 3 and a late onset form with diagnosis typically between the 3rd and 4th decades of life. Previous studies of Fuchs dystrophy pathogenesis indicate increased endothelial cell apoptosis 3-5. However, early disease stages are asymptomatic, and studies of Fuchs dystrophy tissues are limited to failed patient corneas undergoing corneal transplant surgery. Thus, the cellular pathophysiology of Fuchs dystrophy remains poorly understood.
Protein folding is critical for cellular functioning, and all cells have mechanisms to ensure proper protein folding and disposal of irreversibly misfolded proteins 6. Accumulation of misfolded proteins can result in endoplasmic reticulum stress, a condition which is toxic to cells 7. To counteract this stress, cells initiate the unfolded protein response which is a comprehensive program to reduce the accumulation of toxic unfolded proteins 7. Endoplasmic reticulum stress and unfolded protein response have been shown to play important roles in the pathogenesis of multiple human diseases including diabetes, Alzheimer and Parkinson diseases, and atherosclerosis 6. In addition, endoplasmic reticulum stress and unfolded protein response have been implicated in a variety of ophthalmologic diseases including cataract 8, retinitis pigmentosa 9, and glaucoma 10.
The unfolded protein response consists of three effector arms mediated by three endoplasmic reticulum transmembrane receptors: pancreatic endoplasmic reticulum kinase (PKR)-like endoplasmic reticulum kinase (PERK); activating transcription factor 6 (ATF6); and inositol requiring enzyme 1 (IRE1) 7. These effectors are maintained in an inactive state by binding to the intraluminal endoplasmic reticulum chaperone protein GRP78 7. Additional important markers of unfolded protein response activation include the α subunit of eukaryotic initiation factor 2 (phospho-eIF2α) which is phosphorylated by PERK under endoplasmic reticulum stress conditions and C/EBP homologous protein (CHOP) 11. Levels of GRP78, phospho-eIF2α, and CHOP are increased in cells undergoing unfolded protein response. Failure to alleviate endoplasmic reticulum stress by unfolded protein response can lead to cellular apoptosis 7. The complex molecular mechanisms involved in the apoptotic death of endoplasmic reticulum stressed cells have not been fully elucidated 11. However, downstream effectors of unfolded protein response mediated apoptosis in humans may include caspase 4, caspase 3, and caspase 9 12,13 (Fig. 1).
Fig. 1.
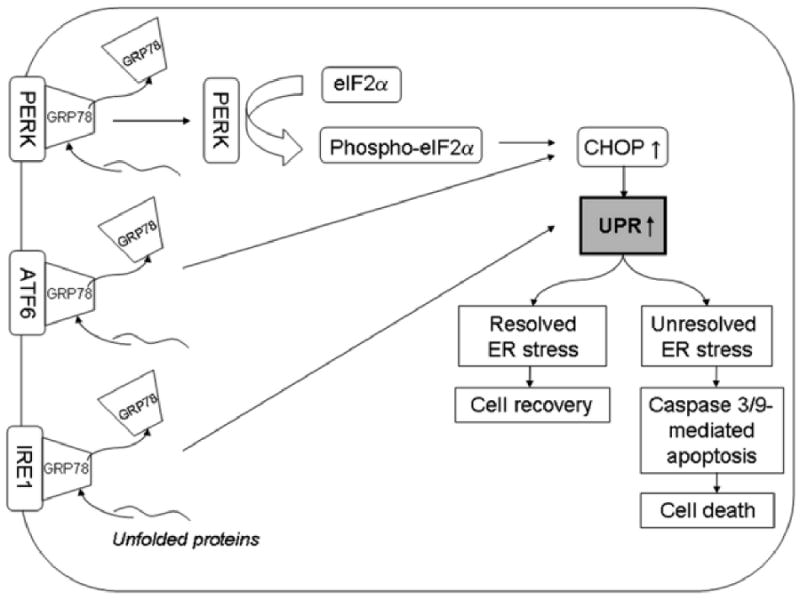
Simplified diagram of the unfolded protein response. Three major effectors of unfolded protein response, IRE1, ATF6, and PERK are maintained in an inactive, membrane bound form by binding with GRP78. Binding of unfolded proteins to GRP78 causes release of GRP78 from the three unfolded protein response effector proteins and results in their activation. Activated ATF6 and IRE1 lead to increased activity of CHOP protein which stimulates unfolded protein response. Activated PERK also activates CHOP via phosphorylation of eIF2α. Depending on the magnitude and duration of unfolded protein stress, unfolded protein response results in either cell recovery or cell death via apoptosis mediated by caspase 3 and 9. GRP78: glucose response protein 78; PERK: pancreatic ER kinase (PKR)-like ER kinase; ATF6: activating transcription factor 6; IRE1: inositol requiring enzyme 1; phospho-eIF2α: phosphorylated eukaryotic initiation factor 2α; CHOP: C/EBP homologous protein.
We hypothesized that unfolded protein response is activated in Fuchs dystrophy by the abundant production of misfolded protein as indicated by thickening of Descemet membrane and the accumulation of wide-spaced collagen aggregates which are hallmark findings in Fuchs dystrophy14-16. We tested this hypothesis by electron microscopic assessment of endoplasmic reticulum in Fuchs dystrophy patients to evaluate for structural evidence of unfolded protein response activation. In addition, we performed immunohistochemistry of Fuchs dystrophy corneal endothelium for three unfolded protein response markers (GRP78, phospho-eIF2α and CHOP) and two apoptosis markers (caspase 3 and 9).
Materials and Methods
Patients and Controls
The diagnosis of Fuchs dystrophy and keratoconus was made by fellowship trained corneal specialists. For Fuchs dystrophy specimens, diagnosis was based on the presence of confluent endothelial guttae in both eyes and presence of corneal edema (stromal and/or epithelial) in the operative eye. Diagnosis of keratoconus was based upon corneal topography along with the presence of standard clinical signs. Corneas from autopsy cases with no history of corneal disease or pathologic corneas with diagnoses other than Fuchs dystrophy served as controls. Patient and control characteristics are summarized in Table 1 (TEM experiments) and Table 2 (immunohistochemistry).
Table 1.
Presence of prominent rough endoplasmic reticulum in the corneal endothelium of Fuchs dystrophy and control corneas assessed by transmission electron microscopy
| Controls | prominent RERa +/- | Diagnosis |
|---|---|---|
| 1 | - | granular dystrophy |
| 2 | - | lattice dystrophy |
| 3 | + | macular dystrophy |
| 4 | +/- | lattice dystrophy |
| 5 | - | macular dystrophy |
| 6 | - | lattice dystrophy |
| 7 | - | macular dystrophy |
| 8 | +/- | granular dystrophy |
| 9 | - | granular dystrophy |
| Fuchs Dystrophy | prominent RER +/- | Diagnosis |
| 1 | + | Fuchs dystrophy |
| 2 | + | Fuchs dystrophy |
| 3 | + | Fuchs dystrophy |
| 4 | + | Fuchs dystrophy |
| 5 | + | Fuchs dystrophy |
| 6 | + | Fuchs dystrophy |
| 7 | + | Fuchs dystrophy |
| 8 | + | Fuchs dystrophy |
| 9 | + | Fuchs dystrophy |
| 10 | + | Fuchs dystrophy |
rough endoplasmic reticulum
Table 2.
Patient information for corneas used for immunohistochemistry
| Autopsy corneas | Age | Cause of death | Corneal findings | Gender | Death to preservation time (h) |
|---|---|---|---|---|---|
| 19 | cystic fibrosis | normal | male | 16 | |
| 54 | larynx carcinoma | normal | female | 11 | |
| 70 | liver cirrhosis | normal | male | 44 | |
| 92 | aspiration pneumonia | normal | male | 49 | |
| 58 | human immunodeficiency virus | normal | male | 13 | |
| 65 | pneumonia | normal | female | 58 | |
| 59 | adenocarcinoma gall bladder | normal | female | 15 | |
| 53 | multisystem organ failure | normal | male | 21 | |
| 6 | cerebral infarctions | normal | male | 23 | |
| 63 | metastatic carcinoma | normal | male | 52 | |
| 75 | aneurysm rupture | normal | female | 53 | |
| 80 | aspiration pneumonia | normal | male | 46 | |
| 47 | lung cancer | normal | male | 59 | |
| 60 | necrotizing pancreatitis | normal | male | 7 | |
| 45 | pulmonary failure, liver transplant rejection | normal | male | 63 | |
| 51 | metastatic melanoma | normal | male | 24 | |
| 71 | adenocarcinoma colon | normal | male | 30 | |
| 67 | gastrointestinal hemorrhage | normal | male | 43 | |
| Mean | 58 | 35 | |||
| SDa | 20 | 19 | |||
| Fuchs dystrophy corneas | Age | Clinical diagnosis | Pathological Diagnosis | Gender | |
| 38 | Fuchs dystrophy | Fuchs dystrophy | female | ||
| 60 | Fuchs dystrophy | Fuchs dystrophy | male | ||
| 89 | Fuchs dystrophy | Fuchs dystrophy | female | ||
| 68 | Fuchs dystrophy | Fuchs dystrophy | male | ||
| 34 | pseudophakic bullous keratopathy | Fuchs dystrophy | male | ||
| 68 | Fuchs dystrophy | Fuchs dystrophy | male | ||
| 74 | Fuchs dystrophy | Fuchs dystrophy | female | ||
| 67 | Fuchs dystrophy | Fuchs dystrophy | male | ||
| Mean | 62 | ||||
| SD | 18 | ||||
| Keratoconus corneas | Age | Clinical diagnosis | Pathological Diagnosis | Gender | |
| 16 | keratoconus | keratoconus | male | ||
| 34 | keratoconus | keratoconus | female | ||
| 18 | keratoconus | keratoconus | female | ||
| 15 | keratoconus | keratoconus | male | ||
| 14 | keratoconus | keratoconus | male | ||
| Mean | 19 | ||||
| SD | 8 | ||||
standard deviation
Transmission Electron Microscopy (TEM)
Specimens for TEM were fixed in buffered 1% glutaraldehyde and postfixed with phosphate buffer (0.1 M) and 1% osmium tetroxide solution. Standard dehydration of the specimen was performed before it was embedded in epoxy resin, sectioned, and stained with paraphenylenediamine. Ultra-thin sections were cut and stained with uranyl acetate–lead citrate and were examined using an electron microscope (Hitachi H7600, Ibaraki, Japan). Ten cases of human corneas of Fuchs dystrophy patients and 9 control corneas of patients with corneal dystrophies other than Fuchs dystrophy were randomized and evaluated for shape and size of the rough endoplasmic reticulum in a blinded fashion by a fellowship trained eye pathologist (CGE).
Immunohistochemistry
Normal autopsy eyes, Fuchs dystrophy, and keratoconus corneas were formalin-fixed and paraffin-embedded by the Johns Hopkins Pathology Laboratory. Unstained sections (5 μm) were deparaffinized and processed for immunohistochemistry using Elite ABC Peroxidase Kit (Vector Labs, Burlingame, CA) following the manufacturer's instructions. Heat-induced epitope retrieval was performed in a buffer solution containing 10 mM Tris, 1 mM EDTA and 0.05% Tween 20 (Sigma, St. Louis, MO), pH 9.0. Sections were stained with diaminobenzedine substrate (DAB) (Vector Labs) with addition of nickel solution and counterstained with hematoxylin solution (Mayer's). Negative control slides using no primary antibody and non-specific, species and isotype matched antibodies were included. Antibodies (dilutions in brackets) included: rabbit polyclonal anti-cleaved caspase 3 (1/200), rabbit polyclonal anti-phosphorylated eIF2α (1/50; both from Cell Signaling, Danvers, MA), rabbit polyclonal anti-cleaved caspase 9 (1/1000; Imgenex, San Diego, CA), rabbit polyclonal anti-CHOP (1/50), rabbit polyclonal anti-GRP78 (1/50), normal rabbit IgG (1/50; all from Santa Cruz Biotechnology, Santa Cruz, CA) and biotinylated anti-rabbit IgG (1/200; Vector Labs).
Image acquisition and processing
Light microscopy images were obtained using an Olympus IMT-2 microscope (Olympus, Center Valley, PA) coupled to a ProgRes C5 digital camera system (Jenoptik, Jena, Germany). Images at 200× magnification were used. For assessment of immunohistochemistry staining, a representative frame with intact endothelium from the central cornea was imaged of each slide.
Immunohistochemistry quantitation
Quantitative assessment of immunohistochemistry staining was performed using FRIDA Software (FRamework for Image Dataset Analysis, ©2007 The Johns Hopkins University, http://bui2.win.ad.jhu.edu/frida/) 17. This software provides a pixel color threshold mask as well as a freehand mask to mark regions of interest. A range of positive immunohistochemistry color signal is specified, and the software subsequently quantitates all pixels with the selected range of colors within an area of interest. The entire corneal endothelium in a single immunohistochemistry stained section across the central cornea was selected as the region of interest. The same pixel color mask was applied to all samples being analyzed for a given antibody marker.
Statistical Analysis
P-values were calculated using the nonparametric Mann–Whitney test (Instat, Graphpad Software, San Diego, CA). P-values < 0.05 were considered statistically significant.
Results
Transmission electron microscopy
Evaluation of corneal endothelium showed markedly enlarged endoplasmic reticulum in all (n = 10) Fuchs dystrophy patients (Table 1, Fig. 2) compared with controls. Rough endoplasmic reticulum contained a fine granular material and exhibited a marked increase in ribosomes. In addition, Fuchs dystrophy specimens showed typical findings, including a substantially thickened posterior collagenous layer and focal posterior excrescences (guttae). Control corneas (n = 9) exhibited prominent rough endoplasmic reticulum in one case (Table 1). In two control cases, the rough endoplasmic reticulum was labeled as “indeterminate”. The remaining 6 control specimens did not show alterations in the structure of rough endoplasmic reticulum (Table 1, Fig. 2).
Fig. 2.
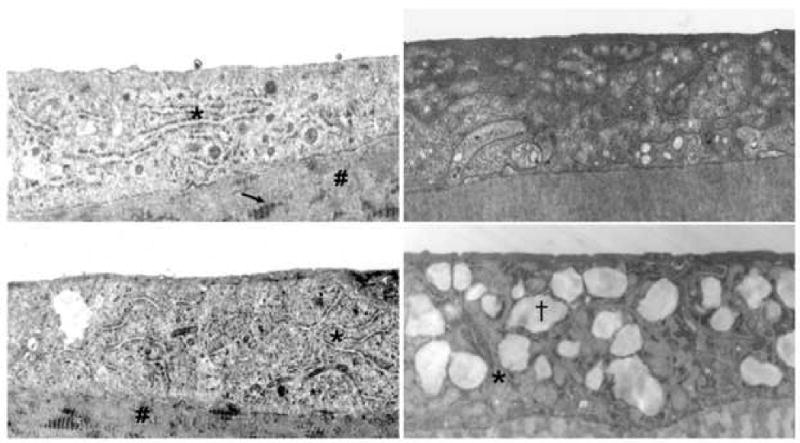
Electron microscopy images of corneal endothelium of patients with Fuchs dystrophy (upper and lower left images), granular corneal dystrophy (upper right image), and macular corneal dystrophy (lower right image). Comparison shows prominent and enlarged rough endoplasmic reticulum in the upper and lower left images as compared to the upper and lower right images. Asterisks indicate rough endoplasmic reticulum in the upper/lower left and the lower right images. # indicates posterior collagenous layer (upper/lower left) with wide-spaced collagen (arrow, upper left), typical of Fuchs dystrophy. (†) indicates intracytoplasmic vacuole typical of macular corneal dystrophy (lower right) 29. Original magnification (upper left: 15000×; lower left: 8000×; upper right: 15000×; lower right: 15000×).
A total of 18 autopsy, 8 Fuchs dystrophy and 5 keratoconus corneas were studied (Table 2). Autopsy corneas were from individuals with an average age of 58 ± 20 years (mean ± standard deviation), and Fuchs dystrophy corneas were from individuals with an average age of 62 ± 18 years (p > 0.05 compared to autopsy corneas). Keratoconus corneas were from individuals with an average age of 19 ± 8 years (p < 0.05 compared to Fuchs dystrophy corneas). All patient corneas had been previously diagnosed with Fuchs dystrophy or keratoconus as described in the Methods. All control corneas had been assessed as unremarkable by an ophthalmic pathologist in the Wilmer Eye Pathology Laboratory.
Immunohistochemistry quantitation for the unfolded protein response marker GRP78 in Fuchs dystrophy corneal endothelium demonstrated a 4.7 fold increase in signal compared with normal autopsy corneal endothelium (16,311 ± 9,722 vs. 3,438 ± 3,776, mean ± standard deviation) (p < 0.05, Fig. 3A). Keratoconus corneal endothelium exhibited a significantly lower GRP78 signal (425 ± 511) than Fuchs dystrophy patients (p < 0.01) and autopsy eyes (p < 0.05) (Fig. 3A). The unfolded protein response marker phospho-eIF2α demonstrated a 4.4 fold increase in signal in Fuchs dystrophy corneal endothelium compared with normal corneal endothelium (5,170 ± 4,672 vs. 1,186 ± 788, p < 0.05) (Fig. 3B). Keratoconus corneal endothelium exhibited a significantly lower phospho-eIF2α signal (396 ± 397) than Fuchs dystrophy patients (p < 0.05) and autopsy eyes (p < 0.01) (Fig. 3B). The unfolded protein response marker CHOP demonstrated a 2.6 fold increase in signal in Fuchs dystrophy corneal endothelium compared with normal corneal endothelium (13,012 ± 6,798 vs. 4,915 ± 5,077, p < 0.05) (Fig. 3C). The apoptosis marker caspase 9 demonstrated a 2.5 fold increase in signal in Fuchs dystrophy corneal endothelium compared with normal corneal endothelium (10,555 ± 6,291 vs. 4,284 ± 2,283, p < 0.05) (Fig. 3D). The apoptosis marker caspase 3 demonstrated a 2.0 fold increase in signal in Fuchs dystrophy corneal endothelium compared with normal corneal endothelium (3,768 ± 3,139 vs. 1,932 ± 1,374) (Fig. 3E).
Fig. 3.
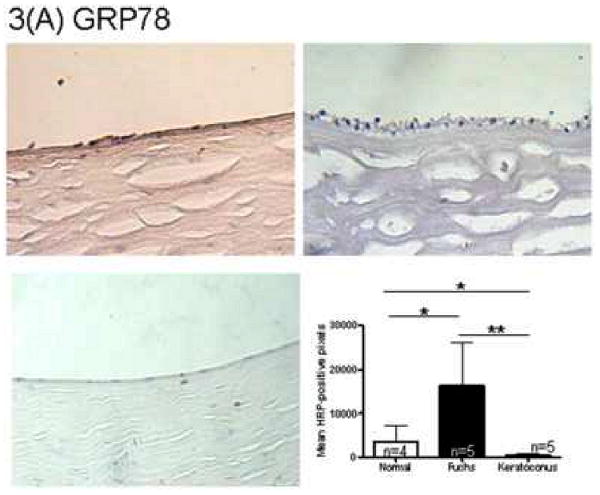
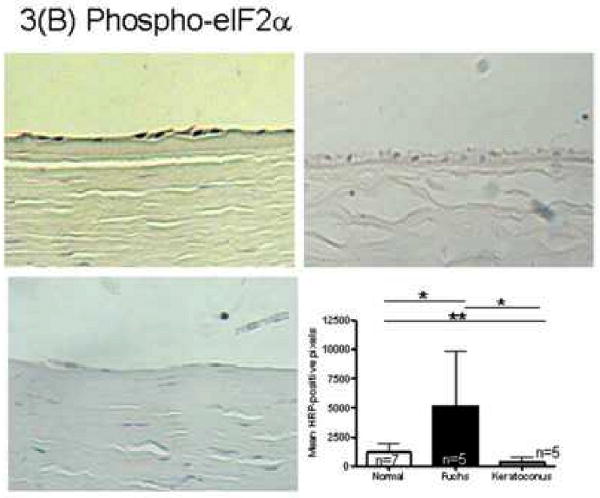
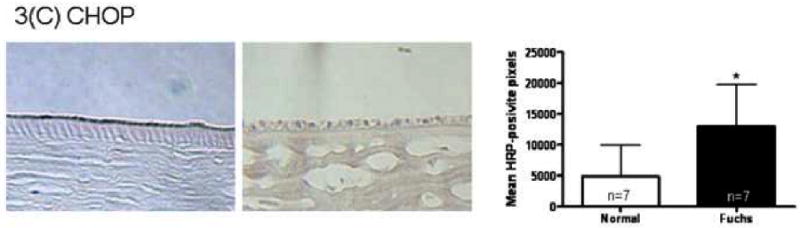
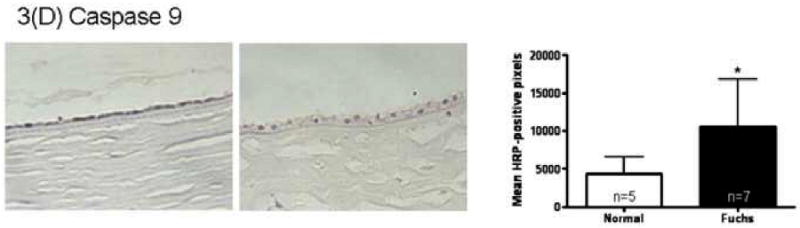
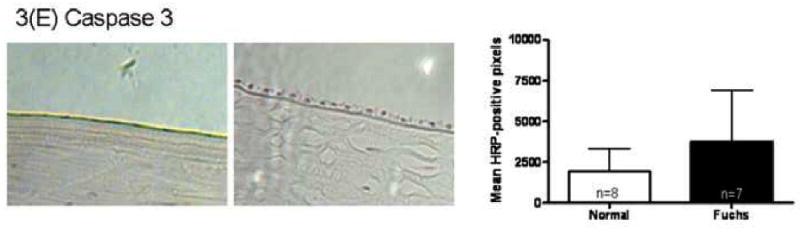

(A-E): Immunohistochemistry for unfolded protein response markers (GRP78, phospho-eIF2α, CHOP) and apoptosis markers (caspase 3 and 9) in Fuchs dystrophy (A, B: upper left, C-E: left), normal human corneas (A, B: upper right, C-E: middle) and keratoconus corneas (A, B: lower left). Horseradish peroxidase (HRP) positive signal was quantitated as described in the Methods. Error bars indicate standard deviation. * indicates p < 0.05, ** indicates p < 0.01, by Mann-Whitney U test.
3(F) Fuchs dystrophy patient corneas were stained with no primary antibody (left) and non-immune, normal IgG (middle). Quantitation of HRP positive signal was compared with Fuchs dystrophy patient corneas stained with primary antibody to GRP78. Original magnification for all panels = 200×.
Negative control staining with non-immune, normal rabbit IgG as primary antibody or no primary antibody showed 8.2 fold and 78 fold decreases in signal compared with GRP78 (2,011 ± 1748 vs. 16,311 ± 9,722 and 209 ± 554 vs. 16,311 ± 9,722, respectively) (p < 0.01 for both comparisons) (Fig. 3F).
Discussion
Throughout nature, proper folding of proteins is required for normal cellular functioning. Thus, all cells have mechanisms to initiate and maintain proper protein folding. In addition, the unfolded protein response is a highly conserved mechanism in eukaryotic cells to respond to endoplasmic reticulum stress caused by the accumulation of misfolded proteins. The overall goal of the unfolded protein response is to decrease the demand and increase the capacity for protein folding within the cell 18. Strategies to decrease protein folding demand include reduced production of secretory proteins 19. Strategies to increase protein folding capacity include increased production of folding-promoting (chaperone) proteins in the endoplasmic reticulum 20 and increased size of the endoplasmic reticulum to dilute the unfolded protein load 18. However, if the imbalance between protein folding demand and capacity cannot be resolved, the unfolded protein response switches from a pro-survival to a pro-apoptotic program leading to cell death 7.
Our work shows marked increases in rough endoplasmic reticulum in Fuchs dystrophy corneal endothelium which is a hallmark sign of endoplasmic reticulum stress and unfolded protein response. The present study confirms the observation of increased rough endoplasmic reticulum containing fine granular material as described earlier by Hogan et al. 21. In addition, we demonstrate significantly increased levels of GRP78, phosphorylated eIF2α, and CHOP in the corneal endothelium of Fuchs dystrophy patients. These three proteins play a major role in the unfolded protein response (Fig. 1). We have also shown increased levels of caspase 9 and caspase 3 in Fuchs dystrophy corneal endothelium. These proteins are major effectors of cellular apoptosis and are implicated in the cell death arm of the unfolded protein response when endoplasmic reticulum stress is inadequately resolved 12, 13. These findings strongly suggest a role for unresolved endoplasmic reticulum stress leading to unfolded protein response-mediated endothelial cell apoptosis as a pathogenic mechanism in Fuchs dystrophy.
Multiple additional lines of evidence further support the role of unfolded protein response as a central pathogenic mechanism in Fuchs dystrophy. Mutations in two genes have been shown to cause familial Fuchs dystrophy. The first gene encodes the alpha 2 subunit of collagen VIII (COL8A2) which is a basement membrane collagen produced by corneal endothelial cells and is a major component of Descemet membrane. Mutations producing amino acid substitutions at positions 450 and 455 within the protein lead to Fuchs dystrophy 2, 22, 23. While it remains unclear how these altered proteins lead to endothelial cell death in Fuchs dystrophy, it should be noted that collagen production is highly dependent on protein modification and folding occurring in the endoplasmic reticulum. Furthermore, similar mutations in genes encoding collagens I, II, and X lead to endoplasmic reticulum stress, unfolded protein response, and human diseases including osteogenesis imperfecta, spondyloepiphyseal dysplasia, and Schmid metaphyseal chondrodysplasia 24-26. Thus, Fuchs dystrophy mutations in COL8A2 may inhibit protein folding and assembly to activate unfolded protein response in a similar manner. Further supporting this hypothesis is work by Zhang et al. which showed accumulation of COL8A2 within the rough endoplasmic reticulum of the corneal endothelium from a patient with Fuchs dystrophy caused by a leucine to tryptophan substitution at amino acid position 450 within the COL8A2 protein 3, 27. This finding can be explained by delayed processing of the mutant COL8A2 protein through the endoplasmic reticulum which is consistent with the induction of endoplasmic reticulum stress and unfolded protein response.
The second gene associated with familial Fuchs dystrophy is SLC4A11 which encodes a membrane bound sodium borate co-transport protein produced by the corneal endothelium 28. Mutant forms of the SLC4A11 protein produced in cell culture lines showed abnormal accumulation of protein within the cytoplasm and reduced levels of normal trafficking to the cell membrane 28. Furthermore, these mutant forms of SLC4A11 showed abnormalities of post-translational modification with carbohydrates (glycosylation). Both of these abnormalities are consistent with altered processing and trafficking of proteins within the endoplasmic reticulum consistent with endoplasmic reticulum stress and unfolded protein response activation.
Multiple studies have demonstrated apoptosis in CECs of advanced stage Fuchs dystrophy corneas 4,5. However, little data exist on the early stages of the disease and how progression towards apoptosis occurs. Vithana et al. suggest a mixed pathophysiologic mechanism involving chronic partial loss of SLC4A11 protein function and gradual accumulation of aberrant misfolded protein 28. However, Vithana et al. stop short of proposing a role for unfolded protein response-mediated apoptosis as a unified pathogenic mechanism for Fuchs dystrophy resulting from mutations in SLC4A11.
Based on the available evidence supporting the presence of unfolded proteins and endoplasmic reticulum stress resulting from Fuchs dystrophy mutations in the COL8A2 and SLC4A11 genes, we propose that unfolded protein response plays a central and early role in the pathogenesis of Fuchs dystrophy. This mechanism involves the production of mutant proteins which undergo altered folding and processing within the endoplasmic reticulum. The presence of unfolded proteins induces release of GRP78, an inhibitor of unfolded protein response, from the three unfolded protein response effectors, IRE1, ATF6, and PERK (Fig. 1). Release of GRP78 leads to activation of these three effectors, which in turn stimulates the unfolded protein response. The chronic production and accumulation of these unfolded proteins result in unremitting endoplasmic reticulum stress which over prolonged periods cannot be fully compensated by unfolded protein response. Endothelial cell death via caspase dependent apoptosis pathways ensues, resulting in the clinical manifestations of Fuchs dystrophy.
Mutations in COL8A2 account for less than 10% of Fuchs dystrophy patients, and none of the Fuchs dystrophy patients included in this study had known Fuchs dystrophy mutations in COL8A2. The clear finding of unfolded protein response activation in our series suggests that this pathogenic pathway may have broader relevance to Fuchs dystrophy patients with mutations in other genes yet to be described as risk factors for Fuchs dystrophy. Thus, the expression of multiple different gene mutations in distinct Fuchs dystrophy pedigrees or sporadic cases may converge on unfolded protein response as a final common pathway of endothelial cell death.
Although this proposed mechanism is an attractive explanation of Fuchs dystrophy pathogenesis, our findings are still limited by the end-stage nature of the Fuchs dystrophy tissues studied. In addition, alternate approaches to quantify unfolded protein response markers such as Western blotting or quantitative reverse transcriptase polymerase chain reaction would be valuable to support our findings. However, these techniques are not feasible for individual patient specimens given the small amount of endothelial tissue obtained at keratoplasty. Further confirmation of this hypothesis awaits ongoing experimental studies using cell culture and animal models of Fuchs dystrophy in our laboratory.
Acknowledgments
Acknowledgements/ Disclosures
a. Funding/Support (including none): Supported by grants from the Eye Bank Association of America (CE), the Medical Illness Counseling Center (Chevy Chase, Maryland, ASJ), National Institutes of Health EY015523 (ASJ), Research to Prevent Blindness (ASJ), and the Wilmer Scholars Research Fund (ASJ).
The funding organizations had no role in the design or conduct of this research.
No authors have any financial/conflicting interests to disclose.
b. Financial Disclosures: The authors indicate no financial conflict of interest.
c. Contributions to Authors in each of these areas: Involved in design of study (C.E., C.K., C.L.S., A.R.S., C.G.E., A.S.J.); conduct of study (C.E., C.K., C.L.S., A.R.S., C.G.E., A.S.J.); collection of data (C.E., A.R.S.);management (C.E., C.K., C.L.S., C.G.E., A.S.J.) analysis (C.E., A.R.S., C.G.E., A.S.J.), and interpretation of data (C.E., C.K., C.L.S., C.G.E., A.S.J); preparation, review, or approval of the manuscript (C.E., C.K., A.R.S., C.L.S., C.G.E., A.S.J).
d. Statement about Conformity with Author Information: The study adhered to the Declaration of Helsinki. Johns Hopkins Institutional Review Board approval was obtained.
e. Other Acknowledgments: none
Biographies

Albert S. Jun, M.D., Ph.D., is Associate Professor of Ophthalmology at the Wilmer Eye Institute, Johns Hopkins School of Medicine. Dr. Jun's clinical and research interests include Fuchs dystrophy and endothelial keratoplasty. Dr. Jun completed residency at the Wilmer Institute and cornea fellowship at Moorfields Eye Hospital. Dr. Jun's professional activities have been recognized with grants and awards from the NIH, the Heed Foundation, the Eye Bank Association of America, and the Association of University Professors of Ophthalmology.

Christoph Engler, M.D., graduated from the University of Freiburg, Germany. He is currently working as a post-doctoral fellow in corneal research at the Wilmer Eye Institute, Johns Hopkins School of Medicine, in the laboratory of Dr. Albert Jun. Dr. Engler was awarded the Research Grant Award of the Eye Bank Association of America in 2008. His research interests include endothelial cell keratoplasty and pathophysiology of Fuchs corneal dystrophy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sundin OH, Jun AS, Broman KW, et al. Linkage of late-onset Fuchs corneal dystrophy to a novel locus at 13pTel-13q12.13. Invest Ophthalmol Vis Sci. 2006;47(1):140–145. doi: 10.1167/iovs.05-0578. [DOI] [PubMed] [Google Scholar]
- 2.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10(21):2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 3.Gottsch JD, Sundin OH, Liu SH, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46(6):1934–1939. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 4.Borderie VM, Baudrimont M, Vallee A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2000;41(9):2501–2505. [PubMed] [Google Scholar]
- 5.Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119(11):1597–1604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein responsein lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47(9):3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 9.Lin JH, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone MA, Ayroles JF, Yamamoto A, et al. Overexpression of myocilin in the Drosophila eye activates the unfolded protein response: implications for glaucoma. PLoS ONE. 2009;4(1):e4216. doi: 10.1371/journal.pone.0004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 12.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277(37):34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 13.Hitomi J, Katayama T, Eguchi Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson SE, Bourne WM. Fuchs' dystrophy. Cornea. 1988;7(1):2–18. [PubMed] [Google Scholar]
- 15.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Bell WR, Sundin OH, et al. Immunohistochemistry and electron microscopy of early-onset Fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation. Trans Am Ophthalmol Soc. 2006;104:85–97. [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin AC, Jadallah S, Toubaji A, et al. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68(7):766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(12):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 20.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 21.Hogan MJ, Wood I, Fine M. Fuchs' endothelial dystrophy of the cornea. 29th Sanford Gifford Memorial lecture. Am J Ophthalmol. 1974;78(3):363–383. doi: 10.1016/0002-9394(74)90224-4. [DOI] [PubMed] [Google Scholar]
- 22.Gottsch JD, Sundin OH, Liu SH, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46(6):1934–1939. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 23.Mok JW, Kim HS, Joo CK. Q455V mutation in COL8A2 is associated with Fuchs' corneal dystrophy in Korean patients. Eye. 2008 doi: 10.1038/eye.2008.116. [DOI] [PubMed] [Google Scholar]
- 24.Chessler SD, Byers PH. BiP binds type I procollagen pro alpha chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J Biol Chem. 1993;268(24):18226–18233. [PubMed] [Google Scholar]
- 25.Hintze V, Steplewski A, Ito H, Jensen DA, Rodeck U, Fertala A. Cells expressing partially unfolded R789C/p.R989C type II procollagen mutant associated with spondyloepiphyseal dysplasia undergo apoptosis. Hum Mutat. 2008;29(6):841–851. doi: 10.1002/humu.20736. [DOI] [PubMed] [Google Scholar]
- 26.Wilson R, Freddi S, Chan D, Cheah KS, Bateman JF. Misfolding of collagen X chains harboring Schmid metaphyseal chondrodysplasia mutations results in aberrant disulfide bond formation, intracellular retention, and activation of the unfolded protein response. J Biol Chem. 2005;280(16):15544–15552. doi: 10.1074/jbc.M410758200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006;172(172):69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- 28.Vithana EN, Morgan PE, Ramprasad V, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17(5):656–666. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- 29.Snip RC, Kenyon KR, Green WR. Macular corneal dystrophy: Ultrastructural pathology of corneal endothelium and Descemet's membrane. Invest Ophthalmol. 1973;12(2):88–97. [PubMed] [Google Scholar]


