Abstract
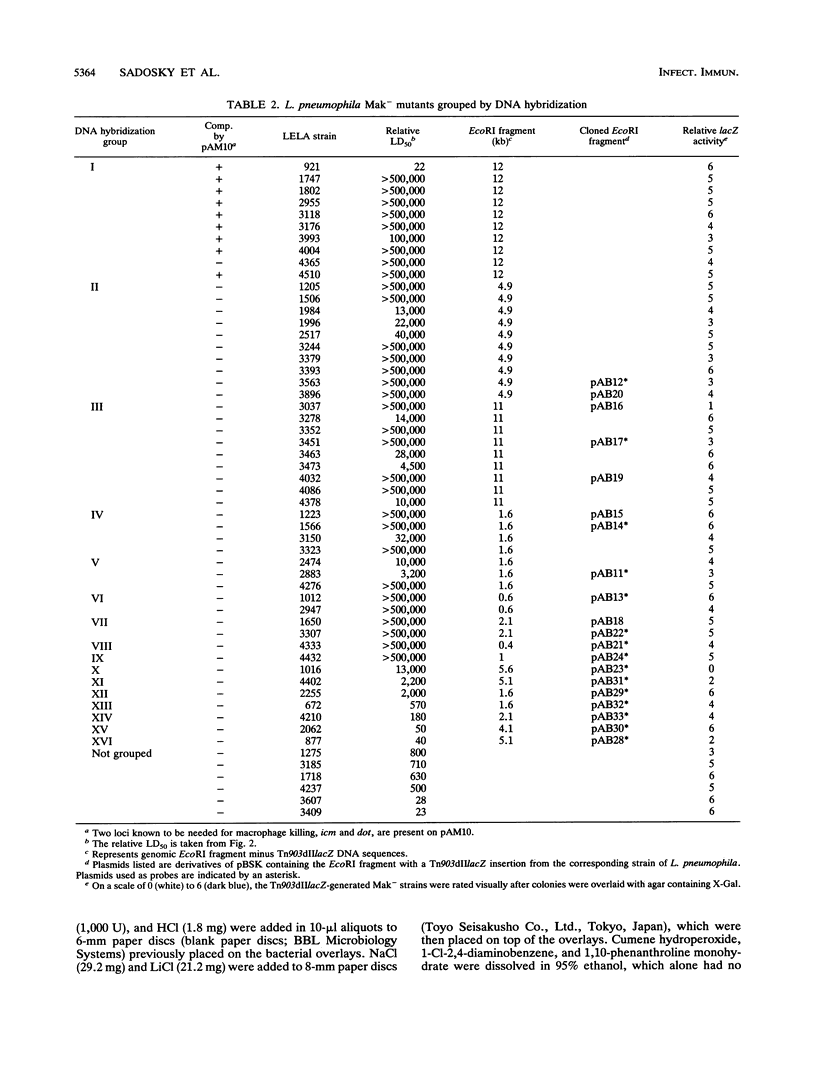
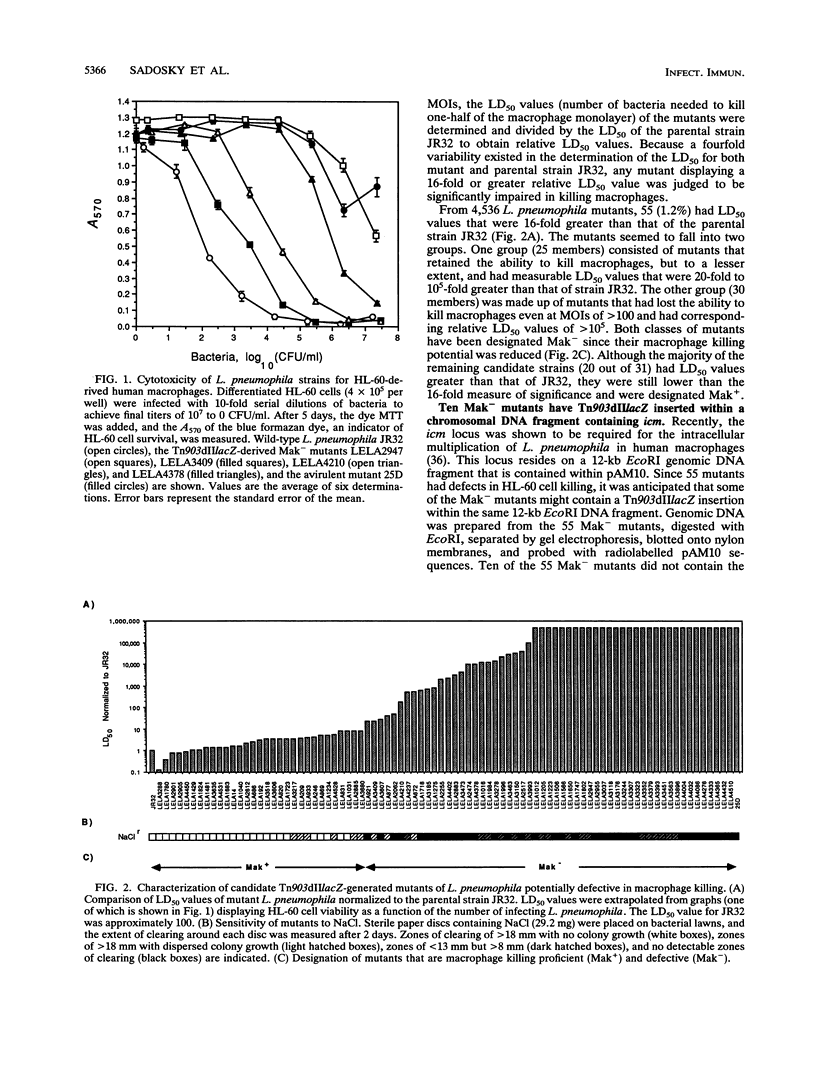
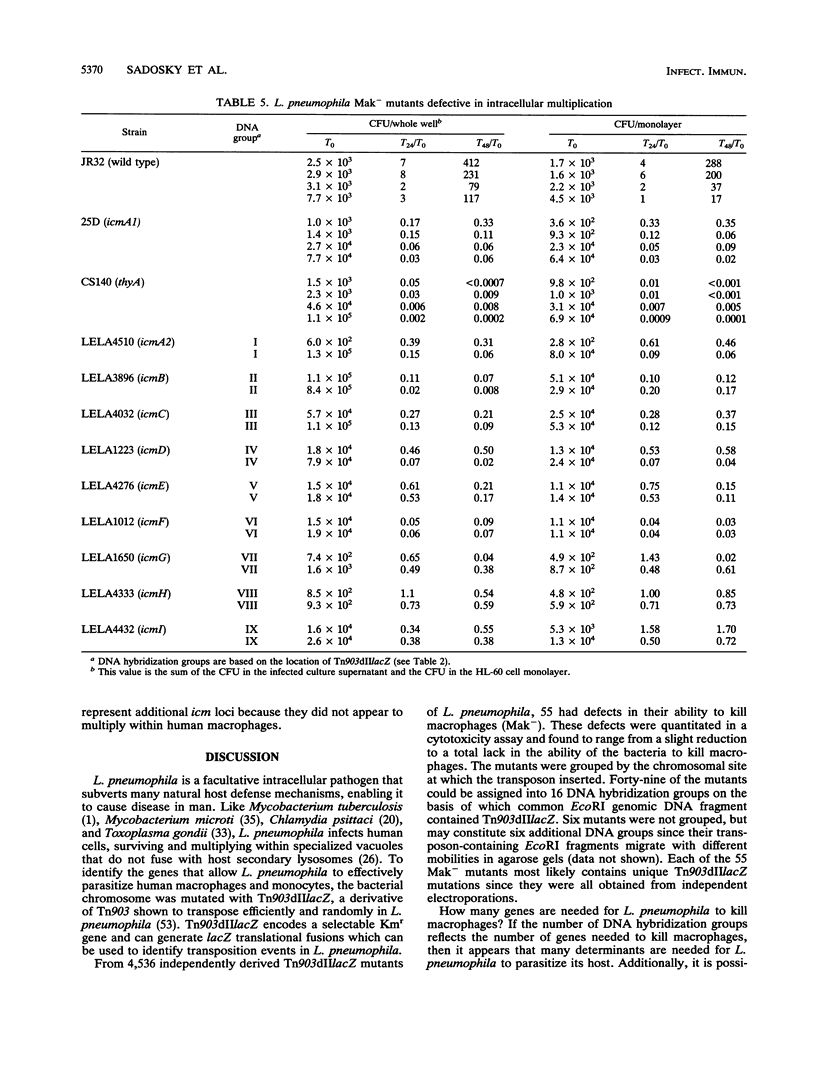
Legionella pneumophila was mutagenized with Tn903dIIlacZ, and a collection of mutants was screened for defects in macrophage killing (Mak-). Of 4,564 independently derived mutants, 55 (1.2%) showed a reduced or complete lack in the ability to kill HL-60-derived human macrophages. Forty-nine of the Mak- mutants could be assigned to one of 16 DNA hybridization groups. Only one group (9 of the 10 members) could be complemented for macrophage killing by a DNA fragment containing icm and dot, two recently described L. pneumophila loci that are required for macrophage killing. Phenotypic analysis showed that none of the mutants were any more sensitive than the wild type to human serum, oxidants, iron chelators, or lipophilic reagents nor did they require additional nutrients for growth. The only obvious difference between the Mak-mutants and wild-type L. pneumophila was that almost all of the Mak- mutants were resistant to NaCl. The effects of LiCl paralleled the effects of NaCl but were less pronounced. Resistance to salt and the inability to kill human macrophages are linked since both phenotypes appeared when Tn903dIIlacZ mutations from two Mak- strains were transferred to wild-type backgrounds. However, salt sensitivity is not a requisite for killing macrophages since a group of Mak- mutants containing a plasmid that restored macrophage killing remained resistant to NaCl. Mak- mutants from groups I through IX associated with HL-60 cells similarly to wild-type L. pneumophila. However, like the intracellular-multiplication-defective (icm) mutant 25D, the Mak- mutants were unable to multiply within macrophages. Thus, the ability of L. pneumophila to kill macrophages seems to be determined by many genetic loci, almost all of which are associated with sensitivity to NaCl.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baine W. B. Cytolytic and phospholipase C activity in Legionella species. J Gen Microbiol. 1985 Jun;131(6):1383–1391. doi: 10.1099/00221287-131-6-1383. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Horwitz M. A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990 Oct 1;172(4):1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. H., Isberg R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993 Jan;7(1):7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich C. E., Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989 Jun;57(6):1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich C. E., Johnson W. Virulence conversion of Legionella pneumophila: a one-way phenomenon. Infect Immun. 1988 Dec;56(12):3121–3125. doi: 10.1128/iai.56.12.3121-3125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto N. P., Eisenstein B. I., Mody C. H., Engleberg N. C. A mutation in the mip gene results in an attenuation of Legionella pneumophila virulence. J Infect Dis. 1990 Jul;162(1):121–126. doi: 10.1093/infdis/162.1.121. [DOI] [PubMed] [Google Scholar]
- Cianciotto N. P., Eisenstein B. I., Mody C. H., Toews G. B., Engleberg N. C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989 Apr;57(4):1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto N., Eisenstein B. I., Engleberg N. C., Shuman H. Genetics and molecular pathogenesis of Legionella pneumophila, an intracellular parasite of macrophages. Mol Biol Med. 1989 Oct;6(5):409–424. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. N., Saha A. K., Glew R. H. Virulence factors of the family Legionellaceae. Microbiol Rev. 1992 Mar;56(1):32–60. doi: 10.1128/mr.56.1.32-60.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fields B. S., Barbaree J. M., Shotts E. B., Jr, Feeley J. C., Morrill W. E., Sanden G. N., Dykstra M. J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986 Sep;53(3):553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Blake M., Niles W. D., Horwitz M. A. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol. 1985 Apr;162(1):85–91. doi: 10.1128/jb.162.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Joyce C. M. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7176–7180. doi: 10.1073/pnas.77.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund K. W. Legionella toxin. Pharmacol Ther. 1981;15(1):123–130. doi: 10.1016/0163-7258(81)90021-8. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Ripley M., Weeratna R. Cloning and nucleotide sequence of a gene (ompS) encoding the major outer membrane protein of Legionella pneumophila. J Bacteriol. 1992 Feb;174(3):914–920. doi: 10.1128/jb.174.3.914-920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ainsworth T., Trust T. J., Kay W. W. Congo red agar, a differential medium for Aeromonas salmonicida, detects the presence of the cell surface protein array involved in virulence. J Bacteriol. 1985 Dec;164(3):1233–1237. doi: 10.1128/jb.164.3.1233-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Reed J. W., Hanks J. F., Hirsch A. M., Walker G. C. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987 Nov 20;51(4):579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- Marra A., Blander S. J., Horwitz M. A., Shuman H. A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A., Horwitz M. A., Shuman H. A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990 Apr 1;144(7):2738–2744. [PubMed] [Google Scholar]
- Marra A., Shuman H. A. Genetics of Legionella pneumophila virulence. Annu Rev Genet. 1992;26:51–69. doi: 10.1146/annurev.ge.26.120192.000411. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984 Jan;43(1):397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C. Virulent to avirulent conversion of Legionnaires' disease bacterium (Legionella pneumophila)--its effect on isolation techniques. J Infect Dis. 1979 Jun;139(6):707–711. doi: 10.1093/infdis/139.6.707. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Chen J. X., Shuman H. A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988 Jun;56(6):1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Jiwa A. H., Engleberg N. C., Eisenstein B. I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988 Aug;5(2):87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990 Apr 26;344(6269):882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., LaMarco K. L., Das S., Remaley A. T., Olomu N., Pope M. T., Glew R. H. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985 Nov 15;243(1):150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock D. M., Crowther P. J., Doherty J., Jefferson S., DeCruz E., Noyer-Weidner M., Smith S. S., Michael M. Z., Graham M. W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989 May 11;17(9):3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]