This study provides evidence that low-pulse-energy femtosecond lasers can be used to alter the refractive index in living corneal tissue. These magnitude changes seem to occur without short-term cellular damage and can be increased with the enhancement of the two-photon absorption of the cornea using sodium fluorescein.
Abstract
Purpose.
To assess the effectiveness of intratissue refractive index shaping (IRIS) in living corneas and test the hypothesis that it can be enhanced by increasing the two-photon absorption (TPA) of the tissue.
Methods.
Three corneas were removed from adult cats and cut into six pieces, which were placed in preservative (Optisol-GS; Bausch & Lomb, Inc., Irvine, CA) containing 0%, 0.25%, 1%, 1.5%, or 2.5% sodium fluorescein (Na-Fl). An 800-nm Ti:Sapphire femtosecond laser with a 100-fs pulse duration and 80-MHz repetition rate was used to perform IRIS in each piece, creating several refractive index (RI) modification lines at different speeds (between 0.1 and 5 mm/s). The lines were 1 μm wide, 10 μm apart, and ∼150 μm below the tissue surface. The RI change of each grating was measured using calibrated, differential interference contrast microscopy. TUNEL staining was performed to assess whether IRIS or Na-Fl doping causes cell death.
Results.
Scanning at 0.1 mm/s changed the RI of undoped, living corneas by 0.005. In doped corneas, RI changes between 0.01 and 0.02 were reliably achieved with higher scanning speeds. The magnitude of RI changes attained was directly proportional to Na-Fl doping concentration and inversely proportional to the scanning speed used to create the gratings.
Conclusions.
IRIS can be efficiently performed in living corneal tissue. Increasing the TPA of the tissue with Na-Fl increased both the scanning speeds and the magnitude of RI changes in a dose-dependent manner. Ongoing studies are exploring the use of IRIS to alter the optical properties of corneal tissue in situ, over an extended period.
Ultraviolet nanosecond excimer lasers and near infrared (NIR) femtosecond lasers are two forms of laser technology that have been adopted in ophthalmic practice for the purpose of altering corneal optics and cutting corneal flaps. Ultraviolet excimer lasers use single-photon absorption to ablate corneal tissue, changing the cornea's thickness and curvature and thus its refractive state.1,2 This technology takes advantage of the fact that the cornea naturally absorbs ultraviolet light to photoablate the tissue. Femtosecond lasers use NIR light that is naturally transmitted through the cornea with little one-photon absorption. To affect the cornea, femtosecond laser light must be focused inside the tissue. The resultant increase in laser intensity at the focal point causes localized, nonlinear, multiphoton absorption, allowing for a range of modifications within the tissue; because the absorption is nonlinear, the surrounding tissue is left virtually unaffected.3–5 NIR femtosecond lasers are now used clinically for corneal flap cutting.6–12 Such lasers use low-repetition-rate (kilohertz to several megahertz range) pulses that induce photodisruption and optical breakdown of the corneal tissue, accompanied by high-density microplasma and bubbles. The layer of damaged tissue created by the laser can then be used to separate a tissue flap from the rest of the cornea.
In a very different set of applications, NIR femtosecond lasers have also gained popularity as powerful tools for micromachining patterns into different, mostly nonbiological materials.13–17 When the femtosecond pulses are tightly focused into transparent bulk materials, the resultant nonlinear absorption and highly localized energy deposition alter the material's properties, allowing for the fabrication of different components and devices such as gratings, waveguides, and photonic crystals.18–24 The changes seen in these materials are usually the result of laser-induced two-photon polymerization,18–24 and recent research has shown that this polymerization process can be enhanced by doping bulk materials with photoinitiators or chromophores that have large two-photon absorption (TPA) cross sections.18–20,25
In 2008, our group showed for the first time, that it is possible to use a low-pulse-energy femtosecond laser to induce low-scattering-loss RI changes in lightly fixed, postmortem corneal and lens tissues.26 These modifications were attained with a 27-fs pulsed laser in the NIR (800 nm) with pulse energies titrated to fall below the optical breakdown threshold of the tissue (<0.5 nJ). This process, termed intratissue refractive index shaping (IRIS), causes long-standing refractive index changes that range between 0.005 and 0.01 in the cornea and 0.015 and 0.021 in the lens.26 However, these changes are achievable only with a slow scanning speed of 0.7 μm/s, which makes realistic clinical application of this method unlikely. Recently, Ding et al.18 reported that both coumarin-1 and fluorescein can be used to enhance the scanning speed and magnitude of RI changes attainable during micromachining in hydrogels, which like the cornea, possess a relatively high water content. Fluorescein is of particular interest, because it is already commonly used in ophthalmic practice for the identification of corneal abrasions and epithelial defects.27 It has also been shown to be safe when injected systemically to visualize retinal vasculature leaks and other abnormalities.27
Thus, in the present study, our goal was to test two hypotheses: (1) that IRIS can be performed in living, unfixed corneas with greater effectiveness than in our previous report of this phenomenon in fixed postmortem corneas, and (2) that the effects of IRIS can be significantly enhanced if the TPA capability of the living tissue is increased.
Materials and Methods
Corneal Extraction and Preparation
Three corneas were excised from two normal, adult, domestic shorthair cats (felis catus) in accordance with the guidelines for the University of Rochester Committee on Animal Research, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the NIH Guide for the Care and Use of Laboratory Animals. The cats were first euthanatized with a lethal dose of pentobarbital sodium (Sleepaway; Fort Dodge Animal Health; Fort Dodge, IA). The corneas were surgically removed by cutting around the limbal edge, rinsed in chilled sterile saline solution, cut into six pie-shaped wedges and placed into chilled cornea preservative (Optisol-GS; Bausch & Lomb, Inc., Irvine, CA) containing 0%, 0.25%, 1%, 1.5% or 2.5% sodium fluorescein (Na-Fl; Sigma-Aldrich, St. Louis, MO) per volume for 2 hours.
Measuring Light Transmissivity
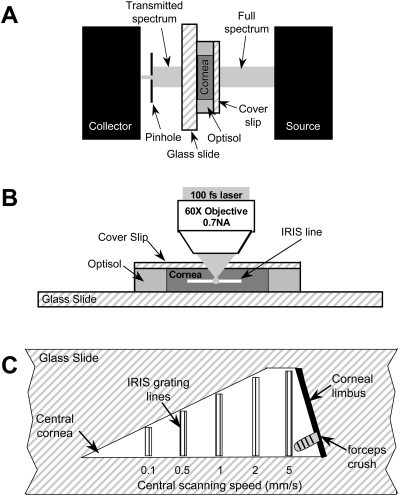
The light transmissivity of the undoped and doped corneal tissues was measured to assess the increased affinity to TPA and clarity of the tissue at the laser wavelength of 800 nm. A spectrometer (HR4000; Ocean Optics, Dunedin, FL) with a 2-mm-diameter pinhole in front of the collection lens (to accommodate the small size, 5–6 mm, of the tissue samples) was used. Each tissue sample was immersed in their respective preservative solutions and placed between a 1-mm-thick glass slide and thin (∼0.1 mm) glass coverslip. Full-spectrum light (200–1000 nm) from the spectrometer light source was shone through the coverslip, sample, and slide and directed to the collection lens through the pinhole (Fig. 1A). To account for light loss due to the glass coverslip and slide, we measured the transmissivity of these pieces, as well as the transmission of full-spectrum light through the preservative solution (without cornea), separately. A ring of 700-μm-thick hydrogel was placed on a glass slide and filled with the preservative before being covered with a thin glass coverslip. The hydrogel piece was used as a spacer to allow us to measure the transmissivity through a layer of preservative that approximated the thickness of the corneal pieces used.
Figure 1.
Experimental setups. (A) System used to measure light transmissivity in living corneal pieces. A full-spectrum (200–1000 nm) beam was emitted from the light source. After it passed through the coverslip, cornea, and glass slide, the transmitted light was then collected and measured by spectrometer. (B) IRIS micromachining apparatus. The live corneal wedges were placed on a glass slide with preservative solution. A thin coverslip was placed over each sample to help keep it flat and the slide-tissue-coverslip assembly was placed on a 3-D computer-controlled scanning stage under a long-working-distance microscope objective with 0.7 NA. (C) IRIS lines micromachined into each corneal piece. One long edge of each corneal piece was aligned with the edge of a glass slide and five sets of lines were micromachined. The two outer lines of each line set were machined at 0.1 mm/s, whereas the central lines were machined at the scanning speeds listed below each set.
IRIS in Undoped and Doped Living Cornea
The pieces of living cornea were micromachined one at a time with an 800-nm Ti:Sapphire femtosecond laser with 100-fs pulse duration, 80-MHz repetition rate, and 120-mW average power (Mai Tai; Newport Spectra-Physics, Mountain View, CA). The tissue pieces were placed endothelial side down on histologic glass slides and bathed with an adequate amount of their respective solutions. A thin (0.1-mm-thick) coverslip was placed over the tissue to keep it flat and prevent solution evaporation. The sample was then placed under the microscope objective (Fig. 1B) on a 3-D scanning platform formed by three linear servo stages (VP-25XA; Newport Spectra-Physics).28 A 60 × 0.70-NA long-working-distance microscope objective (LUCPlanFLN; Olympus, Tokyo, Japan) was used to focus the laser beam in the corneal stroma, ∼150 μm below the tissue surface. Five sets of RI modification lines (Fig. 1C), each consisting of five to six lines spaced 10 μm apart, were machined at 0.1, 0.5, 1, 2, and 5 mm/s. In addition, one to three damage lines were purposely machined at 0.1 mm/s on either side of the RI modification line sets, to aid in their identification for histology purposes.
After the micromachining process, the RI change within each IRIS region was measured by a DIC microscope relative to a micromachined calibration standard consisting of diffraction gratings written into hydrogels, whose diffraction efficiencies had already been measured.26,28
TUNEL Assay
To assess whether IRIS and Na-Fl doping were toxic to the living corneas, we performed TUNEL staining. Studies have shown that TUNEL staining can label cells undergoing apoptosis as little as 4 hours after corneal surgeries, such as LASIK and PRK.29 To provide a positive control for this experiment, one side of each corneal wedge was manually crushed with a pair of forceps. IRIS micromachining was immediately performed, after which the living corneal tissue pieces were again placed into their respective preservative solutions and stored for another 4 to 6 hours. After this time, they were rinsed in 0.1 M PBS, drop-fixed in 1% paraformaldehyde in 0.1 M PBS (pH 7.4) for 10 minutes, and transferred to 30% sucrose in 0.1 M PBS at 4°C for 2 days. Each corneal piece was then mounted in OCT compound (Tissue Tek; Sakura Finetek, Torrance, CA), frozen and serial 20-μm-thick cross sections were cut on a cryostat (2800 Frigocut E; Leica, Deerfield, IL), mounted on microscope slides three sections at a time and stored at −20°C until ready to stain.
For TUNEL-staining, slides containing corneal sections were air dried and rinsed in 0.1 M PBS. The tissue was then processed according to the manufacturer's directions (S7165 ApopTag In Situ Apoptosis Detection Kit; Chemicon International-Millipore Inc., Temecula, CA). Briefly, the mounted sections were postfixed in precooled ethanol:acetic acid 2:1 for 5 minutes at −20°C and drained. After the slides were rinsed, equilibration buffer was applied to the sections and briefly incubated at room temperature. The sections were then incubated with terminal deoxynucleotidyl transferase enzyme for 1 hour in a humidified chamber. The reaction was halted by incubating the sections in stop/wash buffer for 10 minutes followed by another wash in PBS. Warmed anti-digoxigenin conjugate (rhodamine-labeled) was applied to the sections and incubated in a dark, humidified chamber for 30 minutes. After a final wash in PBS, the stained sections were coverslipped in antifade mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). The stained sections were imaged with fluorescence microscopy (AX70; Olympus), and the photomicrographs were acquired with a high-resolution digital camera (Microfire; OPtronics, Goleta, CA) interfaced with a computer running image-analysis software (ImagePro software; MediaCybernetics, Silver Spring, MD).
Results
Doping Living Corneas with Na-Fl
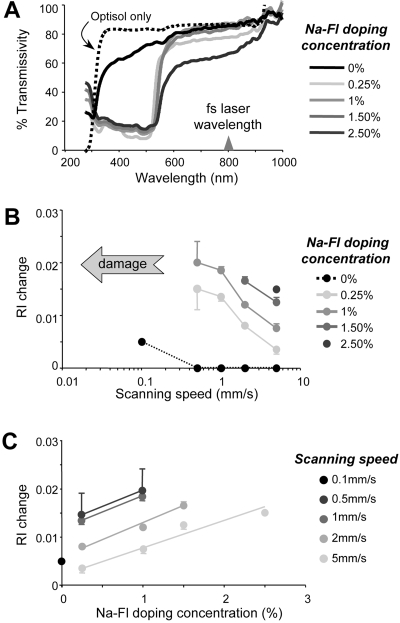
The preservative solution (Optisol-GS; Bausch & Lomb, Inc.) transmitted more than 80% of light for wavelengths ≥300 nm (Fig. 2A; dotted black line). Corneal pieces submerged in preservative only (with no Na-Fl) also transmitted light starting at 300 nm, but with slightly less efficiency at lower wavelengths, a likely consequence of their slight loss of transparency when excised from the eye (Fig. 2A, solid black line). Transmissivity rapidly increased to more than 80% at wavelengths above 600 nm (Fig. 2A). When live corneal pieces were submerged and allowed to absorb different concentrations of Na-Fl, strong absorption of light was noted up to a wavelength of ∼500 nm. This corresponds well to the light-absorption range of fluorescein (300–500 nm).27 All the doped corneal pieces were transparent near the 800-nm laser operation wavelength, indicating that single-photon absorption of the laser light at the working wavelength would have no adverse effect on the tissue.
Figure 2.
Effect of Na-Fl doping on light transmissivity and RI change in living corneal tissue. (A) Light transmissivity of the preservative solution (black dotted line) and corneal wedges stored in the preservative only (black line) or in preservative doped with different concentrations of Na-Fl (gray lines). Note the strong absorption of light in the range between 300 and 500 nm in Na-Fl-doped live corneal tissues. (B) Magnitude of RI change versus scanning speed attained in corneas doped with different concentrations of Na-Fl. (C) Magnitude of RI changes attained versus Na-Fl doping concentration at different scanning speeds. Note that at each scanning speed, the RI change increased monotonically with the Na-Fl doping concentration.
IRIS in Undoped, Living Corneal Tissue
At the lowest scanning speed of 100 μm/s, IRIS changed the RI of undoped living corneal tissue by 0.005 (Table 1, Figs. 2B, 2C). Increasing the scanning speed to 1, 2, or 5 mm/s caused RI changes of lesser magnitude, which fell below the lowest detectable limit of our system (Table 1).
Table 1.
Refractive Index Changes Induced by IRIS in Living Corneas
| Na-Fl Concentration (%) | Scanning Speed (mm/s) |
|||||
|---|---|---|---|---|---|---|
| Fixed Cornea |
Living (Unfixed) Cornea |
|||||
| 0.0007 | 0.1 | 0.5 | 1 | 2 | 5 | |
| 0 | 0.00826(0.002) | 0.005(0.0001) | No detectable change | |||
| 0.25 | NA | Damage | 0.015(0.004) | 0.014(0.001) | 0.008(0.0001) | 0.004(0.001) |
| 1 | Damage | 0.020(0.004) | 0.019(0.001) | 0.012(0.0001) | 0.008(0.001) | |
| 1.5 | Damage | Damage | Damage | 0.017(0.001) | 0.013(0.001) | |
| 2.5 | Damage | Damage | Damage | Damage | 0.015(0.0001) | |
Corresponding values previously obtained in fixed corneas26 are shown for comparison. Values are the means (SD).
Na-Fl Doping and the Magnitude of RI Changes: Dose Dependence and Effect on Scanning Speed
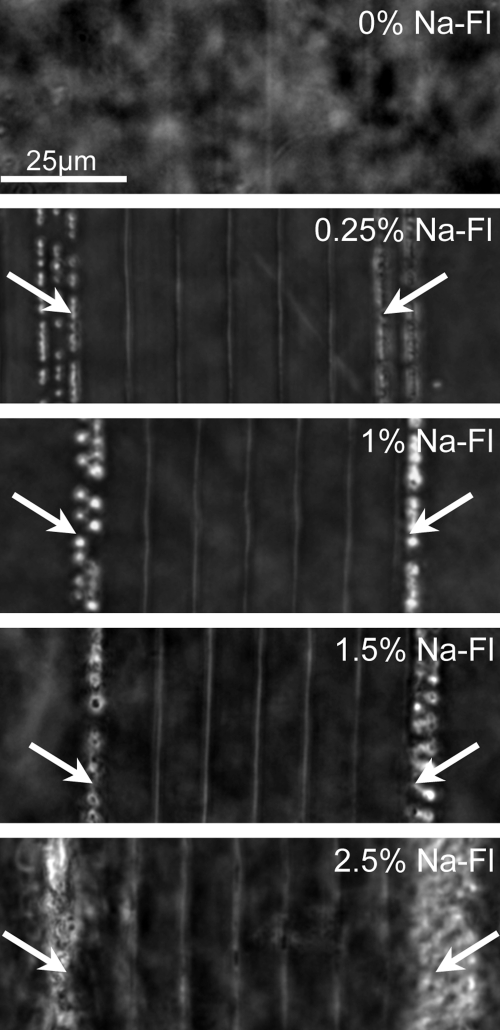
Doping the live corneal pieces with Na-Fl induced RI changes up to four times larger than those attained in undoped corneas (Table 1). However, scanning speed was critical: for any given concentration of Na-Fl, the magnitude of RI change induced was inversely related to scanning speed (Fig. 2B). Lower scanning speeds induced the largest RI changes, although there was a clear threshold above which strong plasma luminescence was observed. These changes were followed by the appearance of spots of tissue destruction and bubbles along the micromachined lines (Fig. 3, arrows). Such damage was always observed at 0.1 mm/s. However, when corneas were doped with 1.5% and 2.5% Na-Fl, damage occurred at faster speeds as well (Table 1). This finding suggests that Na-Fl significantly increases the laser energy absorption within the tissue exposed to the femtosecond laser and that it does so in a dose-dependent manner. Indeed, for a given scanning speed, RI changes increased monotonically with Na-Fl doping concentration (Fig. 2C).
Figure 3.
DIC photomicrographs of IRIS lines created at 0.1 mm/s (outer lines; arrow) and 2 mm/s (inner five lines) in living corneal tissue doped with different concentrations of Na-Fl in preservative solution. In undoped cornea (0% Na-Fl), the IRIS lines were barely visible, even at 0.1 mm/s, suggesting little or no RI change. By increasing the TPA of the tissue with Na-Fl doping, significant and visible RI changes were induced at high scanning speeds (such as 2 mm/s). However, low scanning speeds (0.1 mm/s) caused plasma luminescence and bubble formation (arrow).
TUNEL Staining of Doped, IRIS-Treated Corneas
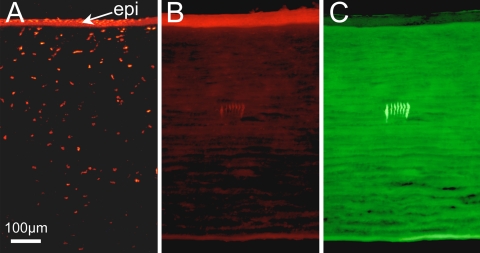
To provide a positive control for this experiment, live corneal tissue was manually crushed with forceps immediately before IRIS was performed. After IRIS, the tissue was replaced into the preservative for another 4 hours before fixation. The result was thinning and disruption of the corneal epithelium and the appearance of many TUNEL-positive cells in both the epithelium and stroma at the crush site (Fig. 4A). In contrast, there was a complete absence of TUNEL-positive cells around nearby micromachined lines (Fig. 4B). Having the IRIS lines in the same corneal sections as the crush sites was an excellent internal control for the efficacy of the TUNEL assay and of crushing as a means of inducing cell death within the experimental time frame in which IRIS was performed. Even the flanking lines inscribed at the slower scanning speed of 0.1 mm/s, which caused small bubbles of tissue destruction, did not cause the appearance of TUNEL-positive cells within this time frame (Fig. 4B). Fluorescence microscopy confirmed that our doping method resulted in complete, even labeling of the corneal stroma with Na-Fl (Fig. 4C). It also allowed us to determine that, at least over the 4- to 6-hour experimental period, incorporation of Na-Fl into living corneal tissue did not, by itself, cause apoptotic cell death of stromal keratocytes, epithelial cells, or endothelial cells (Fig. 4B).
Figure 4.
TUNEL assay of cat cornea infused with 1.5% Na-Fl in preservative. (A) Photomicrograph of peripheral portion of a corneal piece showing the segment crushed with a pair of forceps. Note the marked thinning and disruption of the epithelium (epi), along with multiple TUNEL-positive (red fluorescent) cells in both the epithelium and stroma at the crush location. (B) Central portion of the same corneal piece showing TUNEL staining around the seven IRIS lines inscribed ∼150 μm below the epithelium. The two most peripheral lines were inscribed at 0.1 mm/s. The middle five lines were inscribed at 2 mm/s. Note the complete absence of TUNEL-positive cells within and around the IRIS lines. (C) Portion of cornea imaged in (B) but shown under 480-nm fluorescent illumination to illustrate that Na-Fl (which fluoresces green under such excitation settings) had fully penetrated the corneal tissue, labeling the stroma uniformly. In contrast, the epithelium shows minimal uptake and fluorescence.
Discussion
The present study demonstrates that IRIS can be performed in living corneal tissue with much greater effectiveness than previously reported in fixed, postmortem corneas.26 It also shows that incorporation of Na-Fl, a chromophore known to enhance two-photon absorption, into living corneal tissue potentiates IRIS, causing significantly greater changes in RI at much greater scanning speeds, in a dose-dependent manner. Finally, in the short-term, neither IRIS nor Na-Fl doping appeared to be toxic to stromal keratocytes.
IRIS in Living Corneas: Comparison with IRIS in Fixed Corneas
When IRIS was performed in paraformaldehyde-fixed cat corneal tissue in a prior study, it induced RI changes that ranged between 0.005 ± 0.001 and 0.01 ± 0.001 and averaged 0.008 ± 0.002.26 That long-term storage in an aqueous solution did not eliminate the micromachined lines or the RI changes induced suggests that IRIS must have significantly altered the molecular and/or structural composition of the corneal extracellular matrix. However, fixation with paraformaldehyde, which is largely intended to prevent postmortem tissue degradation, acts by cross-linking protein structures,30 a large component of the corneal extracellular matrix. Because the tissue modifications that lead to a change in RI after exposure to high-repetition-rate femtosecond laser pulses are due to cumulative, free-electron-mediated chemical and thermal effects and to photochemical bond breaking, our prediction was that IRIS micromachining would be more effective in living (unfixed) tissue, than in tissue stabilized by significant protein cross-linking (due to fixation). The present experiments tested this prediction by performing IRIS in living cat corneas, which were immediately transferred to preservative solution (Optisol-GS; Bausch & Lomb, Inc.) after excision from the eye to ensure their viability.
Optisol-GS is a storage medium normally used to preserve postmortem human donor corneal tissue for the purpose of transplantation.31,32 It maintains epithelial, stromal, and endothelial viability for up to 14 days after corneal extraction.31,32 A previous study of feline corneoscleral tissue found that Optisol-GS maintained a functional endothelial cell layer for up to 15 days postmortem,33 suggesting similar effectiveness for corneas from different species. However, to maximize corneal health in our experiments, IRIS was performed within a few hours of corneal removal. After IRIS, the tissue was immediately returned to its Optisol-GS solution for another 4 hours, after which we used TUNEL staining to assess cellular viability in all corneal layers. Given the lack of TUNEL staining (except at the crush sites) and the persistent clarity of the corneas throughout our experiment, we are quite certain that corneal viability was not compromised by surgical extraction, doping with Na-Fl or IRIS. Thus, to our knowledge, this constitutes the first reported investigation of IRIS in living, biological specimens.
Previously, to attain RI changes in the range of 0.005 to 0.01 in fixed corneas, IRIS had to be performed at an extremely slow scanning speed of 0.7 μm/s. 26 In contrast, when IRIS was performed on live (undoped) corneas stored in Optisol-GS, scanning at 100 μm/s induced an RI change of 0.005, a 143× increase in scanning speed for a similar RI change. The increased effectiveness of IRIS in living, relative to fixed, corneas was most likely the result of increased cross-linking between structural elements within the paraformaldehyde-fixed tissues.26,30 Indeed, to create lines of altered RI within fixed tissue, the femtosecond laser would first have to break the fixative-created bonds before photochemically altering the matrix. These additional breaks are likely to require significant additional energy, making the process less efficient than in fresh, unfixed tissue.
Effect of Na-Fl on TPA in Living Corneal Tissue
Having shown that low-pulse-energy femtosecond laser micromachining can change the RI of both fixed26 and unfixed corneal tissues, we want to address several remaining issues. First, the slow scanning speeds required to achieve significant RI changes (0.7 μm/s in fixed corneas and even 100 μm/s in live corneas) are not conducive to trials in situ because the length of time needed to create sufficiently large patterns (e.g., over a circular area 6 mm in diameter) would be many hours to days. However, Ding et al.18 recently demonstrated large enhancements in the speed of femtosecond laser micromachining in dye-doped hydrogels. Specifically, they used two chromophores, fluorescein and coumarin 1, to enhance the TPA efficacy of the hydrogels. The result was a 1000× increase in micromachining speed in addition to an increase in magnitude of the RI changes achieved.18
In the present study, we decided to use Na-Fl to enhance the TPA efficiency in living cat corneal tissue, since fluorescein is already commonly and safely used in ophthalmic practice to stain corneal abrasions and monitor blood vessel leakage in the eye.27 Fluorescein does not form a firm bond to any vital tissue because of its weak acidity.27,34 When Na-Fl appears to stain corneal ulcers, it is essentially freely diffusing into the bare stroma and, when mixed with blood (after intravenous injections), a high proportion of the injected fluorescein (10%–20%) remains free.27 Incorporation of Na-Fl into living corneas was achieved by immersing corneal pieces into solutions of cornea preservative (Optisol-GS; Bausch & Lomb, Inc.) containing different concentrations of dissolved Na-Fl (weight/volume). In all cases, the dye appeared to be uniformly absorbed across the entire corneal thickness, an observation that was confirmed histologically (Fig. 4C). Furthermore, although all corneal pieces remained transparent, increasing concentrations of Na-Fl caused the tissue to acquire a progressively deeper, dark orange color (under bright light illumination). As in the hydrogel experiment,18 incorporation of Na-Fl into the cornea allowed for a significant increase in the speed at which IRIS could be performed. Moreover, for any given speed, there was also an increase in the magnitude of RI changes achieved (see Fig. 2 and Table 1). This was not surprising, given that the number of photons absorbed during the TPA enhancement process is a function of dye concentration and illumination volume.35 Therefore, the increased dye concentration allowed for a greater number of photons to be absorbed per unit volume within the tightly focused 1-μm beam.
Of interest, the maximum RI change achievable at the different scanning speeds tested was ∼0.02. Beyond this, either increasing Na-Fl doping concentration or decreasing scanning speed only resulted in plasma luminescence, a hallmark of tissue damage and bubble formation. This result suggests a possible limitation to the magnitude of RI changes attainable with femtosecond laser scanning in the living cornea, although the reasons for such a limitation remain to be elucidated.
Finally, a potential clinical application that also emerges from our findings is the use of Na-Fl to enhance femtosecond laser flap creation during traditional laser refractive surgery. Since even low concentrations of Na-Fl incorporated into corneal tissue significantly enhance the two-photon absorption of the cornea and the speed and magnitude of material changes induced (in our case, refractive index change), it is conceivable that Na-Fl could also be used to enhance photodestruction of the tissue under femtosecond laser conditions designed for flap cutting. Our results now provide an incentive to test this hypothesis experimentally.
Short-Term Effect of IRIS and Na-Fl Doping and Apoptosis
Understanding the cellular response to IRIS is an important step in assessing the potential clinical applications of this procedure. In the present study, we ascertained that IRIS did not induce the appearance of TUNEL-positive cells in the corneal stroma, whereas a crush injury of an adjacent segment of the same cornea did. Previous work investigating corneal wound healing in mice and rabbits noted that the DNA fragmentation detected by the TUNEL assay was most prominent 4 hours after corneal injury.36,37 This observation was confirmed in our system by crushing the corner of each corneal piece, which caused marked apoptotic cell death of both keratocytes and epithelial cells at this time point. However, additional experiments are needed to evaluate more accurately the long-term cellular and biological response of the cornea to IRIS. In particular, we are interested in evaluating whether the natural turnover of the stromal extracellular matrix is capable of erasing IRIS patterns over a period of days, weeks, or months. Another important question is whether multiple IRIS lines inscribed across corneal cells (unlike the single lines used in the present study) can eventually kill these cells, perhaps not 4 hours after laser micromachining, but days, weeks, or months later. To predict whether this is likely, it is important to ascertain the exact mechanisms by which the energy transferred by femtosecond laser pulses into the corneal tissue alters its RI.
Thus, while our data demonstrate feasibility and lack of toxicity of IRIS for a short period after laser micromachining, it was not possible for us to assess the long-term effects of this procedure on excised corneas. The preservative solution (Optisol-GS; Bausch & Lomb, Inc.) used to keep corneal tissue alive in the present study can only do so effectively for up to 14 days, although signs of degradation may begin to appear 5 days after extraction. Thus, to assess the longevity and impact of IRIS on corneal biology and optics more accurately, we must perform IRIS in situ. With the improvements in scanning speed reported presently, this possibility becomes highly feasible.
Possible Molecular Substrates of Femtosecond IRIS
Based on current knowledge about the effects of femtosecond laser radiation on different materials, at least two mechanisms may underlie IRIS in the cornea. First, in hydrogels, femtosecond laser micromachining that locally increased the material's refractive index was shown to displace water molecules away from the laser focal region, most likely via localized heating of the material.38 In the cornea, an inverse relationship has been demonstrated both experimentally and theoretically between refractive index and stromal hydration, with dehydration leading to an increase in the refractive index of the stroma.39,40 In addition (and perhaps consistent with localized dehydration of the tissue), the refractive index changes seen in IRIS could be caused by the very high free-electron densities generated (on the order of 1018 cm−3) in and around the micromachined lines. Such high electron densities can cause a local temperature increase in the tissue, which in turn, could directly alter the chemical makeup and/or the organization and spacing of individual collagen fibrils in the region of the focused beam.41 Because collagen fibrils have a higher refractive index than the surrounding material,42 it follows that any changes in stromal organization (such as dehydration) that causes these fibrils to be more closely packed, would be associated with an increase in refractive index of the tissue.39,40,43 This could also be an explanation of the increased fluorescence seen in the micromachined lines of the corneal pieces (Fig. 4C). The potential chemical or structural alterations within the tissue may result in the Na-Fl either binding to local structures within the collagen matrix or concentrating in locations of reduced hydration. However, additional experiments need to be performed to conclusively assess the mechanisms by which IRIS takes place in the living cornea.
Conclusion
In the present study, we investigated the feasibility of IRIS laser micromachining in living corneas. When compared with our previous study of IRIS in fixed cat corneas,26 IRIS in the living cornea attained a similar magnitude of refractive index change at a scanning speed that was 143× higher. Increasing the two-photon absorption capabilities of the living cornea via Na-Fl doping increased the magnitude of refractive index changes attainable by two- to fourfold. It also increased the speed at which these changes could be induced by 2 to 50 times relative to undoped, living corneas. Finally, neither IRIS nor the process of increasing TPA was observed to cause cell death within the cornea, at least over the short-term. In summary, the present experiments are an important step in the ongoing exploration of the use of low-pulse-energy femtosecond lasers for the purpose of altering the refractive state of intact, transparent ocular tissues.
Acknowledgments
The authors thank Tracy Bubel and Jennifer Swanton for excellent technical assistance in performing the histology for the study.
Footnotes
Supported by an unrestricted grant to the University of Rochester's Department of Ophthalmology from the Research to Prevent Blindness Foundation; by National Institutes of Health Grant R01 EY015836 (KRH) and Core Grant 08P0EY01319F to the Center for Visual Science; and grants from Bausch & Lomb Inc., and from the University of Rochester's Center for Electronic Imaging Systems, a NYSTAR-designated Center for Advanced Technology.
Disclosure: L.J. Nagy, Bausch & Lomb, Inc. (F); L. Ding, Bausch & Lomb, Inc. (F); L. Xu, Bausch & Lomb, Inc. (F); W.H. Knox, Bausch & Lomb, Inc. (F); K.R. Huxlin, Bausch & Lomb, Inc. (F)
References
- 1.Krueger RR, Trokel SL, Schubert HD. Interaction of ultraviolet laser light with the cornea. Invest Ophthalmol Vis Sci 1985; 26: 1455–1464 [PubMed] [Google Scholar]
- 2.Pallikaris IG, Saiganos DS. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J Refract Surg 1994; 10: 498–510 [PubMed] [Google Scholar]
- 3.Giguere D, Olivie G, Vidal F, et al. Laser ablation threshold dependence on pulse duration for fused silica and corneal tissues: experiments and modeling. J Opt Soc Am A 2007; 24: 1562–1568 [DOI] [PubMed] [Google Scholar]
- 4.Loesel FH, Niemz MH, Bille JF, Juhasz T. Laser-induced optical breakdown on hard and soft tissue and its dependence on the pulse duration: experiment and model. IEEE J Quant Electr 1996; 32: 1717–1722 [Google Scholar]
- 5.Vogel A, Noack J, Huttman G, Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl Phys B 2005; 81: 1015–1047 [Google Scholar]
- 6.Han M, Giese G, Zickler L, Sun H, Bille JF. Mini-invasive corneal surgery and imaging with femtosecond lasers. Opt Express 2004; 12: 4275–4281 [DOI] [PubMed] [Google Scholar]
- 7.Holzer M, Rabsilber T, Auffarth G. Femtosecond laser-assisted corneal flap cuts: morphology, accuracy and histopathology. Invest Ophthalmol Vis Sci 2006; 47: 2828–2831 [DOI] [PubMed] [Google Scholar]
- 8.Juhasz T, Loesel C, Horvath C, Kurtz RM, Mourou G. Corneal refractive surgery with femtosecond lasers. IEEE J Quant Electr 1999; 5: 902–909 [Google Scholar]
- 9.Kim JY, Kim MJ, Kim TK, Choi H, Pak JH, Tchah H. A femtosecond laser creates a stronger flap than a mechanical microkeratome. Invest Ophthalmol Vis Sci 2006; 47: 599–604 [DOI] [PubMed] [Google Scholar]
- 10.Kurtz RM, Horvath C, Liu HH, Krueger RR, Juhasz T. Lamellar refractive surgery with scanned intrastromal picosecond and femtosecond laser pulses in animal eyes. J Refract Surg 1998; 14: 541–548 [DOI] [PubMed] [Google Scholar]
- 11.Lubatschowski H, Maatz G, Heisterkamp A, et al. Application of ultrafast laser pulses for intrastromal refractive surgery. Graefes Arch Clin Exp Ophthalmol 2000; 238: 33–39 [DOI] [PubMed] [Google Scholar]
- 12.Meltendorf C, Burbach GJ, Bühren J, Bug R, Ohrloff C, Deller T. Corneal femtosecond laser keratotomy results in isolated stromal injury and favorable wound-healing response. Invest Ophthalmol Vis Sci 2007; 48: 2068–2075 [DOI] [PubMed] [Google Scholar]
- 13.Davis KM, Miura K, Sugimoto N, Hirao K. Writing waveguides in glass with a femtosecond laser. Opt Lett 1996; 21: 1729–1731 [DOI] [PubMed] [Google Scholar]
- 14.Kim TN, Campbell K, Groisman A, Kleinfeld D, Schaffer CB. Femtosecond laser-drilled capillary integrated into a microfluidic device. Appl Phys Lett 2005; 86 [Google Scholar]
- 15.Marcinkevicius A, Juokdazis S, Wantanabe W, et al. Femtosecond laser-assisted three-dimensional microfabrication in silica. Opt Lett 2001; 26: 277–279 [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Reimann I, Schubert H, Halbhuber K, Koenig K. In-vivo intratissue ablation by nanojoule near-infrared femtosecond laser pulses. Cell Tissue Res 2007; 328: 515–520 [DOI] [PubMed] [Google Scholar]
- 17.Low DKY, Xie H, Xiong Z, Lim GC. Femtosecond laser direct writing of embedded optical waveguides in luminosilicate glass. Appl Phys A 2005; 81: 1633–1638 [Google Scholar]
- 18.Ding L, Jani D, Linhardt J, et al. Large enhancement of femtosecond laser micromachining speed in dye-doped hydrogel polymers. Opt Express 2008; 16: 21914–21921 [DOI] [PubMed] [Google Scholar]
- 19.Cumpston BH, Ananthavel SP, Barlow S, et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999; 398: 51–54 [Google Scholar]
- 20.Haske W, Chen VW, Hales JM, et al. 65 nm feature sizes using visible wavelength 3-D multiphoton lithography. Opt Express 2007; 15: 3426–3436 [DOI] [PubMed] [Google Scholar]
- 21.Serbin J, Egbert A, Ostendorf A, et al. Femtosecond laser-induced two-photon polymerization of inorganic-organic hybrid materials for applications in photonics. Opt Lett 2003; 28: 301–303 [DOI] [PubMed] [Google Scholar]
- 22.Straub M, Gu M. Near-infrared photonic crystals with higher-order bandgaps generated by two-photon photopolymerization. Opt Lett 2002; 27: 1824–1826 [DOI] [PubMed] [Google Scholar]
- 23.Tetreault N, von Freymann G, Deubel M, et al. New route to three-dimensional photonic bandgap materials: Silicon double inversion of polymer templates. Adv Mater 2006; 18: 457–460 [Google Scholar]
- 24.Yao P, Schneider GJ, Prather DW, Wetzel ED, O'Brien DJ. Fabrication of three-dimensional photonic crystals with multilayer photolithography. Opt Express 2005; 13: 2370–2376 [DOI] [PubMed] [Google Scholar]
- 25.Träger J, Kim H, Hampp N. Two-photon treatment. Nat Photon 2007; 1: 509–511 [Google Scholar]
- 26.Ding L, Knox WH, Bühren J, Nagy LJ, Huxlin KR. Intra-tissue refractive index shaping (IRIS) of the cornea and lens using a low-pulse-energy femtosecond laser oscillator. Invest Ophthalmol Vis Sci 2008; 49: 5332–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice D. The use of fluorescein in ophthalmological research: the Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol Vision Sci 1967; 6: 464–477 [PubMed] [Google Scholar]
- 28.Ding L, Blackwell R, Künzler JF, Knox WH. Large refractive index change in silicone-based and non-silicone-based hydrogel polymers induced by femtosecond laser micro-machining. Opt Express 2006; 14: 11901–11909 [DOI] [PubMed] [Google Scholar]
- 29.Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Hong JW, Lee JS. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma and inflammatory cells. Prog Retin Eye Res 2001; 20: 625–637 [DOI] [PubMed] [Google Scholar]
- 30.Ham AW. Histology 5th ed.London: J.B. Lippincott Co.; 1965: 10–11 [Google Scholar]
- 31.Jeng BH. Preserving the cornea: corneal storage media. Curr Opin Ophthalmol 2006; 17: 332–337 [DOI] [PubMed] [Google Scholar]
- 32.Nelson LR, Hodge DO, Bourne WM. In vitro comparison of Chen Medium and Optisol-GS Medium for human corneal storage. Cornea 2000; 19: 782–787 [DOI] [PubMed] [Google Scholar]
- 33.Arndt C, Reese S, Köstlin R. Preservation of canine and feline corneoscleral tissue in Optisol GS. Vet Ophthalmol 2001; 4: 175–182 [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich P. Contributions to the Theory and Practice of Histological Staining Leipzig, Germany: Inaugural Dissertation (1878) London: Pergamon Press, Inc.; 1956 [Google Scholar]
- 35.Xu C, Webb MW. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J Opt Soc Am B 1996; 13: 481–491 [Google Scholar]
- 36.Wilson SE. Keratocyte apoptosis in refractive surgery: Everett Kinsey Lecture. CLAO J 1998; 24: 181–185 [PubMed] [Google Scholar]
- 37.Wilson SE, He Y-G, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in modulation of corneal tissue organization and wound healing. Exp Eye Res 1996; 62: 325–338 [DOI] [PubMed] [Google Scholar]
- 38.Ding L, Cancado LG, Novotny L, et al. Micro-raman spectroscopic study of silicone-based hydrogel polymers modified by megahertz femtosecond laser pulses. J Opt Soc Am B 2009; 26: 595–602 [Google Scholar]
- 39.Meek KM, Dennis S, Khan S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys J 2003; 85: 2205–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S, Alio JL, Perez-Santonja JJ. Refractive index change in bovine and human corneal stroma before and after LASIK: a study of untreated and re-treated corneas implicating stromal hydration. Invest Ophthalmol Vis Sci 2004; 45: 3523–3530 [DOI] [PubMed] [Google Scholar]
- 41.Heisterkamp A, Ripken T, Mamom T, et al. Nonlinear side effects of fs pulses inside corneal tissue during photodisruption. Appl Phys B 2002; 74: 419–425 [Google Scholar]
- 42.Leonard DW, Meek KM. Estimation of the refractive indexes of collagen fibrils and ground substance of the corneal stroma using data from X-ray diffraction. Biophys J 1997; 72: 1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boote C, Dennis S, Newton RH, Puri H, Meek KM. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest Ophthalmol Vis Sci 2003; 44: 2941–2948 [DOI] [PubMed] [Google Scholar]