Abstract
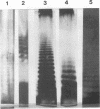
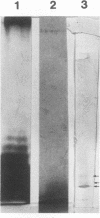
Lipopolysaccharide (LPS) from three human and four monkey isolates of Campylobacter jejuni was extracted in high yields that revealed ladder-like structures in polyacrylamide gels by direct silver staining. These observations demonstrate that C. jejuni possesses LPS with O-chain repeating units typical of the family Enterobacteriaceae. The isolates showed differences in the number and electrophoretic mobility of bands in silver-stained gels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O., McDonald A. G., Pang H. Structures of the O chains from lipopolysaccharides of Campylobacter jejuni serotypes O:23 and O:36. Carbohydr Res. 1992 Jul 2;231:13–30. doi: 10.1016/0008-6215(92)84003-b. [DOI] [PubMed] [Google Scholar]
- Aspinall G. O., McDonald A. G., Raju T. S., Pang H., Mills S. D., Kurjanczyk L. A., Penner J. L. Serological diversity and chemical structures of Campylobacter jejuni low-molecular-weight lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1324–1332. doi: 10.1128/jb.174.4.1324-1332.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker C. R., Alemohammad M. M., Park R. W. A study of factors affecting the sensitivity of the passive haemagglutination method for serotyping Campylobacter jejuni and Campylobacter coli and recommendations for a more rapid procedure. Can J Microbiol. 1987 Jan;33(1):33–39. doi: 10.1139/m87-006. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C., Penner J. L. Basis for serological heterogeneity of thermostable antigens of Campylobacter jejuni. Infect Immun. 1985 Oct;50(1):284–291. doi: 10.1128/iai.50.1.284-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Congi R. V., Hennessy J. N., Penner J. L. Evaluation of a simplified procedure for serotyping Campylobacter jejuni and Campylobacter coli which is based on the O antigen. J Clin Microbiol. 1991 Oct;29(10):2093–2098. doi: 10.1128/jcm.29.10.2093-2098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical composition and biological activity of lipopolysaccharides prepared from type strains of Campylobacter jejuni and Campolybacter coli. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):217–222. doi: 10.1111/j.1699-0463.1984.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical studies of partially hydrolysed lipopolysaccharides from four strains of Campylobacter jejuni and two strains of Campylobacter coli. J Gen Microbiol. 1984 Nov;130(11):2783–2789. doi: 10.1099/00221287-130-11-2783. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Isolation and chemical composition of lipopolysaccharide from Campylobacter jejuni. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):135–139. doi: 10.1111/j.1699-0463.1982.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Hopkins J. A., Blaser M. J. Lipopolysaccharide structures in Enterobacteriaceae, Pseudomonas aeruginosa, and Vibrio cholerae are immunologically related to Campylobacter spp. Infect Immun. 1986 Jan;51(1):204–208. doi: 10.1128/iai.51.1.204-208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Characterization of cross-reacting serotypes of Campylobacter jejuni. Can J Microbiol. 1989 Feb;35(2):265–273. doi: 10.1139/m89-040. [DOI] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987 Aug;55(8):1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Redhead K., Selkirk S., Poole S. Variability in LPS composition, antigenicity and reactogenicity of phase variants of Bordetella pertussis. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):211–217. doi: 10.1016/0378-1097(91)90088-r. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Blaser M. J., Sarmiento J. I., Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989 May;57(5):1438–1444. doi: 10.1128/iai.57.5.1438-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., Sarmiento J. I., Fox J., Panigrahi P. Evidence of reinfection with multiple strains of Campylobacter jejuni and Campylobacter coli in Macaca nemestrina housed under hyperendemic conditions. Infect Immun. 1990 Jul;58(7):2149–2155. doi: 10.1128/iai.58.7.2149-2155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- al-Hendy A., Toivanen P., Skurnik M. Rapid method for isolation and staining of bacterial lipopolysaccharide. Microbiol Immunol. 1991;35(4):331–333. doi: 10.1111/j.1348-0421.1991.tb01562.x. [DOI] [PubMed] [Google Scholar]